Abstract
Background
Health-care resource constraints in low-income and middle-income countries necessitate the identification of cost-effective public health interventions to address COVID-19. We aimed to develop a dynamic COVID-19 microsimulation model to assess clinical and economic outcomes and cost-effectiveness of epidemic control strategies in KwaZulu-Natal province, South Africa.
Methods
We compared different combinations of five public health interventions: health-care testing alone, where diagnostic testing is done only for individuals presenting to health-care centres; contact tracing in households of cases; isolation centres, for cases not requiring hospital admission; mass symptom screening and molecular testing for symptomatic individuals by community health-care workers; and quarantine centres, for household contacts who test negative. We calibrated infection transmission rates to match effective reproduction number (Re) estimates reported in South Africa. We assessed two main epidemic scenarios for a period of 360 days, with an Re of 1·5 and 1·2. Strategies with incremental cost-effectiveness ratio (ICER) of less than US$3250 per year of life saved were considered cost-effective. We also did sensitivity analyses by varying key parameters (Re values, molecular testing sensitivity, and efficacies and costs of interventions) to determine the effect on clinical and cost projections.
Findings
When Re was 1·5, health-care testing alone resulted in the highest number of COVID-19 deaths during the 360-day period. Compared with health-care testing alone, a combination of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres reduced mortality by 94%, increased health-care costs by 33%, and was cost-effective (ICER $340 per year of life saved). In settings where quarantine centres were not feasible, a combination of health-care testing, contact tracing, use of isolation centres, and mass symptom screening was cost-effective compared with health-care testing alone (ICER $590 per year of life saved). When Re was 1·2, health-care testing, contact tracing, use of isolation centres, and use of quarantine centres was the least costly strategy, and no other strategies were cost-effective. In sensitivity analyses, a combination of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres was generally cost-effective, with the exception of scenarios in which Re was 2·6 and when efficacies of isolation centres and quarantine centres for transmission reduction were reduced.
Interpretation
In South Africa, strategies involving household contact tracing, isolation, mass symptom screening, and quarantining household contacts who test negative would substantially reduce COVID-19 mortality and would be cost-effective. The optimal combination of interventions depends on epidemic growth characteristics and practical implementation considerations.
Funding
US National Institutes of Health, Royal Society, Wellcome Trust.
Introduction
By early September, 2020, 16 countries in sub-Saharan Africa had reported more than 10 000 COVID-19 cases.1 High urban density, few opportunities for physical distancing, and poor access to hygiene interventions increase the risk of severe epidemics in the region.2 The existing public health infrastructure for epidemic responses in sub-Saharan Africa is also of concern because testing capacity, surveillance infrastructure, isolation facilities, and intensive care services are sparse.3, 4
To address the COVID-19 pandemic, low-income and middle-income countries (LMICs) are implementing epidemic control programmes. WHO promotes establishment of disease surveillance platforms, contact tracing, and isolation facilities.5 Epidemiological models of these interventions have indicated that their efficacy is dependent on intervention adherence and transmission dynamics.6, 7 However, few studies have included resource costs to assess their cost-effectiveness and feasibility. Inadequate human resources, public health financing, and availability of health-care facilities necessitate particular attention in LMICs.
Research in context.
Evidence before this study
We searched PubMed and the medRxiv and SSRN preprint servers for original studies that included estimates of the cost-effectiveness of COVID-19 public health intervention strategies, published in English between Jan 1 and June 24, 2020, using the search terms “cost-effectiveness” and “COVID” or “SARS-CoV-2”. We reviewed any original scientific reports that included cost-effectiveness of public health intervention strategies for COVID-19 epidemic control. Our search did not yield any related articles in PubMed. We identified seven related preprint articles. Of these preprint articles, three focused on only one intervention strategy (personal protective equipment for health-care workers, community face mask use, or social distancing), one assessed only home versus hotel isolation, and one did not assess a specific intervention. Two articles compared different interventions via incremental cost-effectiveness ratios but were not country-specific and did not include active case finding strategies such as contact tracing and mass symptom screening. Several published COVID-19 epidemiological modelling analyses have projected the impact of control strategies on epidemic growth, without consideration of costs, cost-effectiveness, or budget impact.
Added value of this study
We used a dynamic microsimulation model to estimate the cost-effectiveness of COVID-19 public health intervention strategies in KwaZulu-Natal province, South Africa, including combinations of interventions currently in use or being considered in South Africa and elsewhere in the region. We projected health-care resource use and health sector budget impact for a period of 360 days for each strategy. We did extensive sensitivity analysis to account for uncertainty in epidemic growth and in the efficacies and costs of different interventions. We found that strategies combining household contact tracing, isolation of individuals with COVID-19, mass symptom screening, and quarantine of household contacts of COVID-19 cases would substantially reduce mortality and would be cost-effective. The optimal combination of interventions was dependent on epidemic growth characteristics and efficacies and costs of interventions.
Implications of all the available evidence
Isolation of individuals with COVID-19 continues to be an important strategy for slowing epidemic growth. Our results show that isolation combined with contact tracing, mass symptom screening, and quarantine of household contacts of cases is a cost-effective strategy for epidemic control, and that upfront expenditures could reduce downstream costs by preventing infections, hospital admissions, and additional resource use. Active case finding led by community health workers, which has been established in public health activities in South Africa and other low-income and middle-income countries, could be leveraged to control the spread of COVID-19 in an economically efficient manner. Where quarantine is not possible due to implementation barriers or poor public support, a combination of the other interventions would be cost-effective.
We used a dynamic microsimulation model to compare medical outcomes and costs for a range of COVID-19 control measures in KwaZulu-Natal province, South Africa. We aimed to inform policy decision making by projecting clinical and economic outcomes, cost-effectiveness, and budget impact of alternative control strategies, focusing on those proposed or currently in use in South Africa. As a frame of reference, epidemic control measures in South Africa in June, 2020, included a combination of health-care testing, contact tracing, isolation centres, and mass symptom screening. Although the first wave of diagnosed COVID-19 cases in South Africa peaked in July, 2020, this analysis could be used to inform preparation for, or response to, a resurgence or subsequent waves.
Methods
Model overview
We developed the Clinical and Economic Analysis of COVID Interventions (CEACOV) dynamic state-transition Monte Carlo microsimulation model to reflect the natural history, diagnosis, and treatment of COVID-19. We compared six public health intervention strategies (appendix pp 28–31). For all control strategies, the basic assumptions were: testing consists of PCR for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a nasopharyngeal specimen; individuals awaiting test results are instructed to self-isolate; individuals who become severely ill (with dyspnoea or hypoxaemia), regardless of test result, are admitted to hospital until hospital capacity is reached; individuals with a negative test result are advised to practise physical distancing and hand hygiene; individuals with an initial negative test result can present for repeat testing if they develop new or worsening symptoms; and individuals not initially admitted to hospital can be admitted later if they develop severe illness. Each modelled strategy also had unique assumptions. For the health-care testing alone strategy, approximately 30% of people with mild or moderate COVID-19-like symptoms and all with severe symptoms self-present to a health-care centre for testing, and those with a positive result who are not severely ill are instructed to self-isolate at home. For the health-care testing and contact tracing strategy, in addition to health-care testing, household contacts of COVID-19 cases are tested, and individuals with a positive result are instructed to self-isolate at home. For the health-care testing, contact tracing, and isolation centre strategy, in addition to health-care testing and contact tracing, cases who are not severely ill are referred to an isolation centre offering food, shelter, and basic medical care without supplemental oxygen, and are discharged after 14 days. For the health-care testing, contact tracing, isolation centre, and mass symptom screening strategy, in addition to health-care testing, contact tracing, and use of isolation centres, community health-care workers screen the entire population for COVID-19 symptoms every 6 months and refer those with symptoms for PCR testing. Individuals with a positive PCR test who are not severely ill are referred to an isolation centre. For the health-care testing, contact tracing, isolation centre, and quarantine centre strategy, in addition to health-care testing, contact tracing, and use of isolation centres, household contacts with a negative test result who cannot safely quarantine at home are referred to a quarantine centre where they receive food and shelter. Quarantined individuals are discharged after 14 days, unless they develop COVID-19-like symptoms, in which case they are referred to isolation centres or hospitals, as available. For the health-care testing, contact tracing, isolation centre, mass symptom screening, and quarantine centre strategy, all five interventions are combined.
We initiated our model with a SARS-CoV-2 infection prevalence of 0·1%, which corresponds to approximately 11 000 cases in KwaZulu-Natal (based on empirical data; appendix p 4). We simulated COVID-19-specific outcomes for a period of 360 days, including daily and cumulative infections (detected and undetected), deaths, resource use, and health-care costs from the health sector perspective without discounting. Outside the model, we calculated the mean lifetime years of life saved (YLS) from each averted COVID-19 death during the 360-day time period, which equated to 16·8 life-years without discounting, or 12·5 life-years when discounted 3% per year (appendix pp 5–6). The primary outcome for this analysis was the incremental cost-effectiveness ratio (ICER), which was calculated as the difference between two strategies in health-care costs (2019 US$) divided by the difference in undiscounted life-years. We did not include costs beyond the 360-day model horizon. Non-HIV public health expenditure in South Africa is approximately $600 per year per capita;8, 9 including those annual costs over a lifetime yields a lifetime ICER approaching $600 per YLS. Therefore, our ICER estimates include health-care costs during the 360-day model and YLS over a lifetime from averted COVID-19 deaths during the 360-day model. We defined an ICER cutoff of less than $3250 per YLS as cost-effective, on the basis of an opportunity cost approach (appendix p 2).10
Model structure
At simulation initiation, each individual is either susceptible to, or infected with, SARS-CoV-2 according to age-stratified probabilities (0–19, 20–59, ≥60 years). Once infected, an individual transitions to the preinfectious latency state. Each individual faces an age-dependent probability of developing asymptomatic, mild or moderate, severe, or critical disease (appendix pp 2, 11, 32). Individuals with critical disease have a daily probability of death. If individuals with critical disease survive, they pass through a recuperation state (remaining infectious) before entering the recovery state. Individuals in other disease states can transition directly to the recovery state. Recovered individuals pose no risk of transmission and are assumed immune from repeat infection for the simulation duration. All simulated individuals advance through the model simultaneously to capture infection transmission dynamics. To validate our natural history assumptions, we compared model-projected COVID-19 deaths with those reported in KwaZulu-Natal between May 6 and Aug 30, 2020 (appendix p 4).
Individuals in asymptomatic, mild or moderate, severe, critical, or recuperation states of COVID-19 can transmit infection to susceptible individuals at state-dependent daily rates. The number of daily infections is a function of the proportion of susceptible people in the population, the distribution of disease states among those with COVID-19, and interventions that influence transmission (appendix p 3). PCR testing specifications include sensitivity, specificity, time from testing to result, and cost. Interventions influence testing probability (eg, contact tracing and mass symptom screening), infection transmission rate (eg, isolation centre and quarantine centre), and costs.
Individuals with severe disease are referred to hospitals and those with critical disease are referred to intensive care units. In the event that hospital or intensive care unit resources are not available, the individual receives the next lower available intervention, which is associated with a different mortality risk and cost (eg, if a person needs intensive care when no intensive care beds are available, they receive non-intensive hospital care).
Model calibration
We populated CEACOV with COVID-19 natural history data from published literature (table 1 ). We used estimates of the basic reproduction number and viral shedding duration in various disease states to calculate transmission rates. We then calibrated transmission rates to construct an effective reproduction number (R e) corresponding to South African estimates in May, 2020, after implementation of physical distancing and lockdown policies (appendix p 4).12
Table 1.
Model input parameters for analysis of COVID-19 intervention strategies in KwaZulu-Natal province, South Africa
| Base case value | Data source | |||
|---|---|---|---|---|
| Cohort age groups, years | ||||
| 0–19 | 40·26% | 11 | ||
| 20–59 | 51·48% | 11 | ||
| ≥60 | 8·26% | 11 | ||
| Proportion of individuals in each health state at model start, % | ||||
| Susceptible | 99·900% | Assumption | ||
| Infected | ||||
| Preinfectious latency stage | 0·030% | Assumption | ||
| Asymptomatic | 0·030% | Assumption | ||
| Mild or moderate disease | 0·030% | Assumption | ||
| Severe disease | 0·005% | Assumption | ||
| Critical disease | 0·005% | Assumption | ||
| Recuperation after critical disease | 0·000% | Assumption | ||
| Recovered | 0·000% | Assumption | ||
| Re | 1·5 (1·1–2·6) | 12 | ||
| Daily probability of onward transmission by health state* | ||||
| Asymptomatic | 0·1556 | Appendix (pp 3, 6) | ||
| Mild or moderate disease | 0·1266 | Appendix (pp 3, 6) | ||
| Severe disease | 0·0088 | Appendix (pp 3, 6) | ||
| Critical disease | 0·0070 | Appendix (pp 3, 6) | ||
| Recuperation after critical disease | 0·0088 | Appendix (pp 3, 6) | ||
| PCR testing† | ||||
| Sensitivity‡, % | 70 (50–90) | 13, 14 | ||
| Specificity, % | 100 | Assumption | ||
| Cost, 2019 US$ | 26 (13–52) | Appendix (pp 6, 7) | ||
| Time to result return and action, days | 5 (1–7) | Appendix (pp 6, 7) | ||
| Resource availability per 11 000 000 people, n | ||||
| Hospital beds | 26 220 | 15 | ||
| Intensive care unit beds | 748 | 15 | ||
| Isolation centre beds | As needed, no capacity limitation | Assumption | ||
| Quarantine centre beds | As needed, no capacity limitation | Assumption | ||
| Cost per person, 2019 US$ | ||||
| Hospital bed, daily | 165 (83–330) | 16 | ||
| Intensive care unit bed, daily | 2059 (1030–4118) | 17 | ||
| Contact tracing/mass symptom screen, per instance | 3 (2–6) | Appendix (pp 24–27) | ||
| Isolation centre bed, daily | 44 (22–88) | Appendix (pp 23, 25–27) | ||
| Quarantine centre bed, daily | 37 (19–74) | Appendix (pp 23, 25–27) | ||
Data in parentheses are ranges used in sensitivity analysis. Re=effective reproductive number.
Transmission probabilities in a scenario where Re is 1·5.
Nasopharangeal specimens.
Test sensitivity does not vary by disease stage, with the exception of the preinfectious latency phase in which it is 0%.
Input parameters
We defined cohort demographic characteristics using 2019 population estimates (table 1).11 In 2019, 40·26% of the population in KwaZulu-Natal were aged 0–19 years, 51·48% were aged 20–59 years, and 8·26% were older than 60 years. Day 0 of the model represents a provincial 0·1% prevalence of active SARS-CoV-2 infection (approximately 11 000 individuals), with the remainder of the population susceptible to infection.
For individuals newly infected with SARS-CoV-2, mean preinfectious latency was 2·6 days. The estimated duration of time individuals spend in each health state, age-dependent probabilities of developing severe or critical disease, and age-dependent mortality for individuals with critical disease are shown in the appendix (p 11).
We stratified transmission rates by disease state (table 1). We adjusted transmission rates to reflect a scenario in which R e was 1·5.12 Considering uncertainty with regard to R e, we also simulated alternative epidemic growth scenarios with lower (1·1 or 1·2) or higher (2·6) R e values (appendix p 4).
In the base case, we assumed PCR had 70% sensitivity and 100% specificity, and time to PCR result return and action was 5 days for all active infection states.18 We defined the probability of being tested based on the health state and intervention strategy in place (appendix p 7). Considering the scarcity of data about the precise efficacy of each intervention for reducing SARS-CoV-2 transmission rates (eg, isolation centre), we made assumptions about efficacies and varied them in sensitivity analysis. We assumed ongoing transmission after diagnosis was reduced by 50% as a result of health-care testing alone and by 95% when health-care testing was combined with the use of isolation or quarantine centres (appendix p 12).
On the basis of the estimated population of KwaZulu-Natal province in 2019 of 11 million, the maximum capacity of hospital beds in the province was 26 220 and the maximum capacity of intensive care unit beds was 748 (table 1).15 We assumed that isolation centre and quarantine centre beds were available to all who needed them. We applied costs of PCR testing, contact tracing, and mass symptom screening, and daily costs of hospital stays, intensive care unit stays, and isolation centre or quarantine centre stays on the basis of published estimates or cost quotes obtained in KwaZulu-Natal (appendix p 6).
Resource use and budget impact analysis
We did resource use and budget impact analysis from a combined health sector perspective (public and private) for the population of KwaZulu-Natal. We projected the total resources (daily, peak daily, and cumulative), including testing, hospital and intensive care unit beds, and isolation centre and quarantine centre beds, which would be used in each intervention strategy. Peak daily use of each resource refers to the maximum number of that resource used in a single day over the 360-day period. Isolation centre and quarantine centre beds are offered to individuals who meet criteria (ie, individuals with a positive PCR test who are not severely ill are offered isolation centre beds, and household contacts with a negative test result are offered quarantine centre beds), and we assumed in the base case that all beds offered would be used. In budget impact analysis, we projected total and component health-care costs associated with each strategy during the 360-day period and compared them with the 2019 KwaZulu-Natal Department of Health budget ($3·12 billion).19 Because intensive care is relatively expensive and mostly provided in the private sector, we also considered costs exclusive of intensive care.
Sensitivity analysis
We did one-way sensitivity analysis by varying key parameters across plausible ranges to determine the effect on clinical and cost projections (table 1; appendix p 12). To extrapolate to other settings, we restricted hospital and intensive care unit bed availability to the mean number of beds available in sub-Saharan Africa countries (22 275 hospital beds and 371 intensive care unit beds per 11 million people).20 We did multiway sensitivity analysis in which we varied parameters that had relatively large effects in one-way sensitivity analysis, including reducing isolation centre or quarantine centre efficacy and costs to reflect the impact of home-based isolation and quarantine strategies.
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the paper for publication.
Results
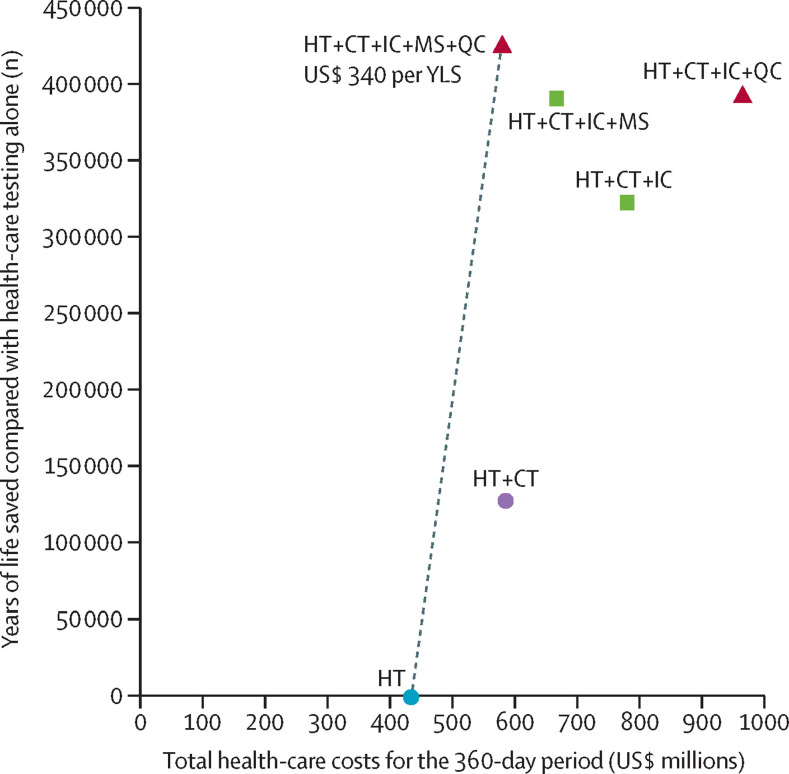
When R e was 1·5, we projected that health-care testing alone would result in the highest number of COVID-19 infections and deaths, most life-years lost, and lowest costs during the 360-day period (table 2 ; appendix pp 33–34). A combination of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres provided the greatest clinical benefit and was cost-effective (ICER $340 per YLS; figure 1 ). Compared with health-care testing alone, the combined strategy of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres decreased life-years lost by 94% and increased costs by 33%. All other strategies resulted in higher costs and provided less clinical benefit than the combined strategy of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres. In settings where quarantine centres cannot be implemented, a strategy of health-care testing, contact tracing, use of isolation centres, and mass symptom screening was the cost-effective strategy (ICER $590 per YLS compared with health-care testing alone).
Table 2.
Model-projected life-years lost, health-care costs, and cost-effectiveness of COVID-19 intervention strategies in KwaZulu-Natal province, South Africa
| Total life-years lost,*n | Health-care costs over 360-day period, US$† | ICER, US$ per YLS‡ | |
|---|---|---|---|
| Re 1·5 | |||
| Health-care testing alone | 450 940 | 437 000 000 | .. |
| Health-care testing, contact tracing, isolation centres, mass symptom screening, and quarantine centres | 27 220 | 581 000 000 | 340 |
| Health-care testing and contact tracing | 322 970 | 588 000 000 | Dominated |
| Health-care testing, contact tracing, isolation centres, and mass symptom screening | 60 930 | 668 000 000 | Dominated |
| Health-care testing, contact tracing, and isolation centres | 128 890 | 780 000 000 | Dominated |
| Health-care testing, contact tracing, isolation centres, and quarantine centres | 60 190 | 965 000 000 | Dominated |
| Re 1·2 | |||
| Health-care testing, contact tracing, isolation centres, and quarantine centres | 3890 | 139 000 000 | .. |
| Health-care testing, contact tracing, and isolation centres | 6850 | 141 000 000 | Dominated |
| Health-care testing, contact tracing, isolation centres, and mass symptom screening | 4260 | 183 000 000 | Dominated |
| Health-care testing, contact tracing, isolation centres, mass symptom screening, and quarantine centres | 2040 | 190 000 000 | 27 590 |
| Health-care testing and contact tracing | 32 040 | 276 000 000 | Dominated |
| Health-care testing alone | 97 600 | 393 000 000 | Dominated |
Strategies are listed in order of ascending costs. Life-years and costs were rounded, but the ICER was calculated using non-rounded values for life-years and costs. ICER=incremental cost-effectiveness ratio. YLS=years of life saved. Re=effective reproductive number. Dominated=strong dominance, resulting in more life-years lost and higher costs than an alternative strategy.
We assumed that each death results in 16·8 life-years lost, on average, based on our derivation (appendix pp 5–6).
This reflects costs to the health-care sector.
The ICER is the difference between two strategies in costs divided by the difference in undiscounted life-years (16·8 YLS per averted COVID-19 death; appendix pp 5–6); a strategy was considered cost-effective when the ICER was less than US$3250 per YLS.10
Figure 1.
Cost-effectiveness of COVID-19 intervention strategies in KwaZulu-Natal province, South Africa
Model results are shown for the scenario in which the effective reproduction number was 1·5. Strategies to the right of the dotted line were not cost-effective. For non-dominated strategies, ICERs are shown below the strategy label. HT=health-care testing. CT=contact tracing within households. IC=isolation centres. MS=mass symptom screening. QC=quarantine centres. YLS=years of life saved. ICER=incremental cost-effectiveness ratio.
When R e was 1·2, health-care testing, contact tracing, use of isolation centres, and use of quarantine centres resulted in lower health-care costs than health-care testing alone (table 2). Compared with the strategy of health-care testing, contact tracing, and use of isolation centres and quarantine centres, the addition of mass symptom screening resulted in 48% fewer life-years lost but was not cost-effective (ICER $27 590 per YLS). Health-care testing, contact tracing, and use of isolation centres was the least costly strategy in settings where quarantine centres were not feasible, and other strategies were not cost-effective compared with health-care testing, contact tracing, and use of isolation centres. When we used life-years discounted 3% per year (12·5 discounted YLS per averted COVID-19 death), cost-effectiveness interpretations were unchanged.
Regarding resource use, when R e was 1·5, health-care testing alone resulted in the highest peak daily use of hospital beds (table 3 ). Compared with health-care testing alone, the combined strategy of health-care testing, contact tracing, isolation centres, mass symptom screening, and quarantine centres increased cumulative PCR test usage by 2·6 times (with lower peak daily PCR use) and reduced peak daily hospital bed use by 86% (due to fewer cumulative infections), with 12 380 isolation centre beds and 18 140 quarantine centre beds required at peak daily use. Only the health-care testing, contact tracing, use of isolation centres, and mass symptom screening strategy; health-care testing, contact tracing, use of isolation centres, and use of quarantine centre strategy; and the health-care testing, contact tracing, use of isolation centres, mass symptom screening, and quarantine centre strategy maintained peak daily intensive care unit bed demand below provincial capacity.
Table 3.
Model-projected resource use of COVID-19 intervention strategies in KwaZulu-Natal province, South Africa
| Cumulative PCR tests in 360-day period, n |
Highest daily resource use during the 360-day period, n |
|||||
|---|---|---|---|---|---|---|
| PCR tests | Hospital beds (non-ICU) | ICU beds* | Isolation centre beds | Quarantine centre beds | ||
| Re 1·5 | ||||||
| Health-care testing alone | 1 527 450 | 14 820 | 4690 | 748 | .. | .. |
| Health-care testing, contact tracing, isolation centres, mass symptom screening, and quarantine centres† | 3 904 230 | 12 900 | 640 | 341 | 12 380 | 18 140 |
| Health-care testing and contact tracing | 5 951 180 | 31 050 | 3440 | 748 | .. | .. |
| Health-care testing, contact tracing, isolation centres, and mass symptom screening | 4 639 280 | 16 930 | 1320 | 715 | 21 260 | .. |
| Health-care testing, contact tracing, and isolation centres | 4 904 010 | 19 340 | 1930 | 748 | 30 510 | .. |
| Health-care testing, contact tracing, isolation centres, and quarantine centres | 4 478 770 | 16 710 | 1380 | 737 | 26 710 | 39 470 |
| Re 1·2 | ||||||
| Health-care testing, contact tracing, isolation centres, and quarantine centres† | 2 963 280 | 9870 | 590 | 363 | 1840 | 3110 |
| Health-care testing, contact tracing, and isolation centres | 3 025 260 | 9870 | 590 | 363 | 1620 | .. |
| Health-care testing, contact tracing, isolation centres, and mass symptom screening | 3 159 950 | 10 520 | 570 | 396 | 1510 | .. |
| Health-care testing, contact tracing, isolation centres, mass symptom screening, and quarantine centres | 3 120 800 | 10 520 | 570 | 396 | 1860 | 3480 |
| Health-care testing and contact tracing | 3 647 570 | 12 450 | 770 | 506 | .. | .. |
| Health-care testing alone | 766 140 | 4440 | 1680 | 748 | .. | .. |
Strategies are listed in order of ascending costs. ICU=intensive care unit. Re=effective reproductive number.
748 ICU beds available in total.
The cost-effective strategy in this scenario.
When R e was 1·2, compared with health-care testing alone, the combination of all five interventions increased cumulative PCR test usage by 4·1 times and reduced the peak daily hospital bed use by 66%, with 1860 isolation centre beds and 3480 quarantine centre beds required at peak daily use. All strategies with the exception of health-care testing alone maintained peak daily intensive care unit bed demand below capacity.
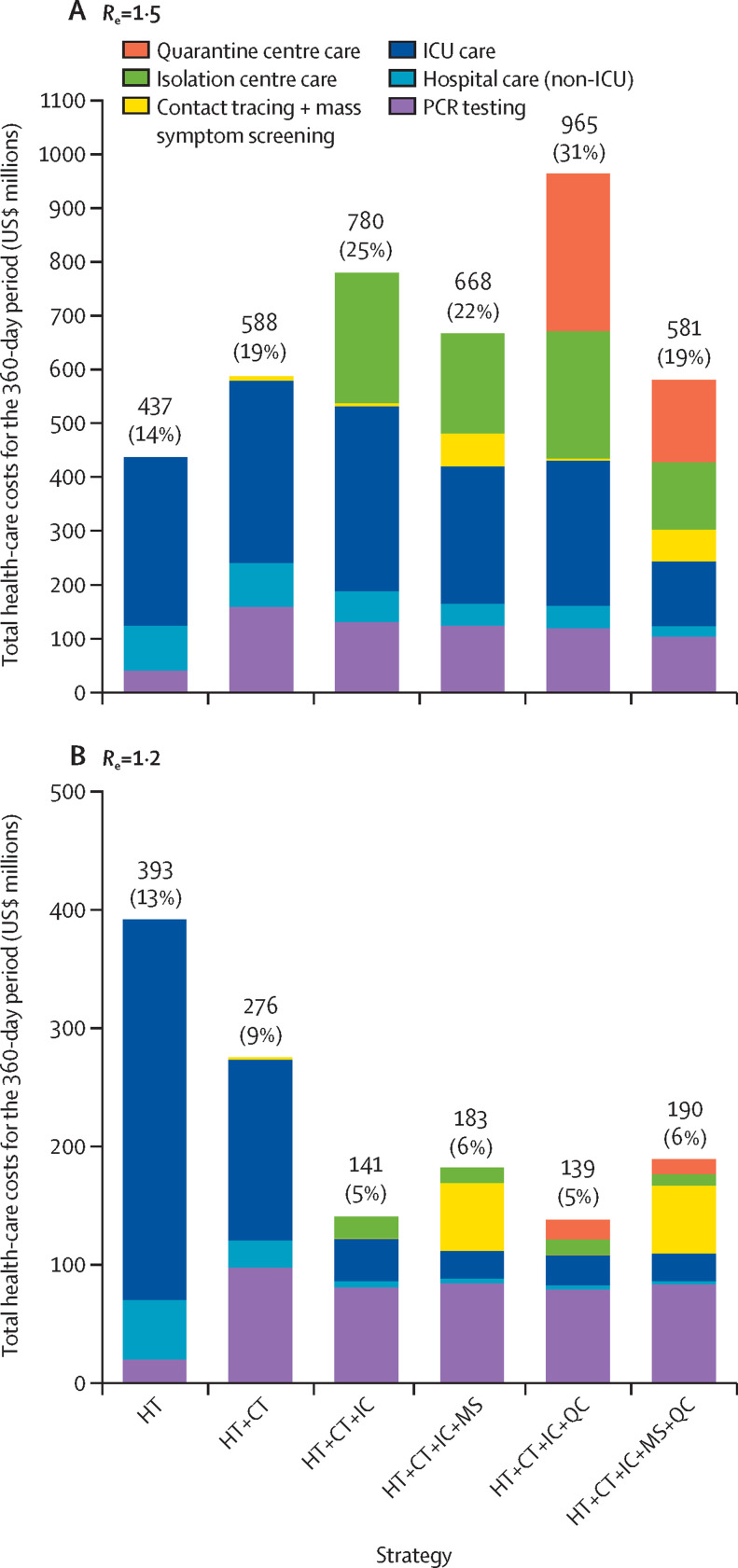
Over the 360-day modelled period, when R e was 1·5, PCR testing contributed 9–27% to overall costs, depending on the strategy (figure 2 ). In strategies that included use of quarantine centres, these centres contributed 26–30% to overall costs. In strategies without quarantine centres, intensive care was the largest contributor to costs, ranging from 38–71%. Costs exclusive of intensive care were $125 million for the health-care testing alone strategy, $413 million for the health-care testing, contact tracing, use of isolation centres, and mass symptom screening strategy, and $461 million for all five interventions combined, equating to approximately 4%, 13%, and 15% of the 2019 KwaZulu-Natal Department of Health budget, respectively. Contact tracing and mass symptom screening combined contributed to 10% or less of overall costs and isolation centres contributed 22–31% to overall costs in strategies in which they were used. When R e was 1·2, costs exclusive of intensive care were $71 million for the health-care testing alone strategy, $159 million for the health-care testing, contact tracing, use of isolation centres, and mass symptom screening strategy, and $167 million for all five interventions combined, reflecting 2%, 5%, and 5% of the 2019 KwaZulu-Natal Department of Health budget, respectively.
Figure 2.
Budget impact analysis of contributors to health-care costs of COVID-19 intervention strategies applied to the population of KwaZulu-Natal province, South Africa (11 million people)
Total and component COVID-19-related health-care costs, from a health sector perspective, associated with different intervention strategies when applied to the population of KwaZulu-Natal (11 million people) for an epidemic with an Re of 1·5 (A) and 1·2 (B). The costs are derived from model-generated results. Percentages in parentheses represent the proportion of the 2019 KwaZulu-Natal Department of Health budget. Re=effective reproductive number. HT=health-care testing. CT=contact tracing within households. IC=isolation centres. MS=mass symptom screening. QC=quarantine centres. ICU=intensive care unit.
In sensitivity analyses, results were similar to the base case, whereby a combination of all five interventions was cost-effective, with varying costs of contact tracing and mass symptom screening (appendix p 13) and hospitalisation (appendix p 14); varying PCR sensitivity, time to result, and PCR cost (appendix pp 16–17); and varying availability of hospital and intensive care unit beds (appendix p 18). When PCR sensitivity increased to 90%, both the health-care testing, contact tracing, use of isolation centres, and mass symptom screening strategy (ICER $440 per YLS) and all five interventions combined (ICER $1660 per YLS) used resources efficiently.
Conversely, the projected ICERs changed meaningfully when R e was 2·6. Resource requirements increased substantially, which resulted in the combination of all five interventions no longer being cost-effective (ICER $25 040 per YLS), and all strategies had ICERs above the cost-effectiveness cutoff when compared with the health-care testing alone strategy (appendix p 19). Similar to when R e was 1·2, when R e was 1·1 no other strategy was cost-effective compared with the health-care testing, contact tracing, use of isolation centres, and use of quarantine centres strategy (appendix p 19). When the efficacies of contact tracing and mass symptom screening for the detection of COVID-19 were halved from the base case values, the combination of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres was no longer cost-effective (ICER $5930 per YLS; appendix p 20). When the efficacy of isolation centres and quarantine centres for transmission reduction was decreased from 95% to 75%, the combination of health-care testing, contact tracing, use of isolation centres, mass symptom screening, and use of quarantine centres was not cost-effective (ICER $12 490 per YLS; appendix p 21). When the isolation centre and quarantine centre costs decreased, the health-care testing, contact tracing, use of isolation centre, mass symptom screening, and use of quarantine centres strategy became more favourable in terms of cost-effectiveness, and it remained cost-effective when isolation centre and quarantine centre costs were double those of the base case (appendix p 22).
In a multiway sensitivity analysis, we varied the efficacy of contact tracing and mass symptom screening and reduced the efficacy and cost of isolation centres and quarantine centres to assess lower cost but potentially lower efficacy home-based isolation and quarantine programmes. This analysis showed that a combination of all five interventions and the health-care testing, contact tracing, use of isolation centres, and use of quarantine centres strategy were cost-effective in nearly all scenarios in which contact tracing and mass symptom screening efficacy for case detection was double that of the base case efficacy (appendix p 35). When contact tracing and mass symptom screening efficacy for case detection was half that of the base case efficacy, strategies involving quarantine centres were less likely to be cost-effective. In scenarios where quarantine centres were not feasible, the health-care testing, contact tracing, use of isolation centres, and mass symptom screening strategy was cost-effective in most scenarios (appendix p 36).
Discussion
In KwaZulu-Natal, public health strategies that combine contact tracing, isolation of individuals with confirmed COVID-19, community-based mass symptom screening, and quarantine of household contacts of confirmed cases will substantially reduce infections, hospital admissions, and deaths while efficiently using health-care resources. We estimate that a strategy combining all interventions would cost an additional $340 per YLS, which compares favourably with the cost-effectiveness of many established public health interventions in South Africa, including tuberculosis diagnostic testing21 and cervical cancer screening.22 In scenarios in which implementation of quarantine centres is not possible, a strategy of contact tracing, isolation centres, and mass symptom screening would be cost-effective.
The cost-effectiveness of strategies was sensitive to epidemic growth conditions. We did sensitivity analyses to enable generalisation of results to other settings with resource constraints, to epidemics at varying degrees of acceleration (including published estimates for South Africa12, 23), and with varying intervention costs.24 With low epidemic growth (R e 1·1–1·2), a combination of health-care testing, contact tracing, and use of isolation and quarantine centres was the optimal strategy; quarantine centres remained cost-effective but the addition of mass symptom screening was not cost-effective. With high epidemic growth (R e of 2·6), when the epidemic outpaced control measures and costs increased substantially, no combination of the modelled interventions was cost-effective compared with health-care testing alone.
Our model parameters and specifications were selected for their relevance to LMICs. Our estimates are based on the population structure of KwaZulu-Natal, which has a median age of 25 years (compared with 38 years in the USA), and thus are likely to reflect epidemic scenarios in LMICs with similarly young age structures. We chose intervention scenarios based on previous research supporting their efficacy for epidemic control, WHO recommendations, and particular relevance to settings with limitations in formal health-care infrastructure.5, 6, 7 To enable estimation of the total number of PCR tests needed and associated costs, we did not restrict PCR testing availability: peak PCR use reached approximately 10 000–15 000 tests per day for the optimal strategies, which was marginally higher than the established capacity in KwaZulu-Natal during the recent surge in COVID-19 cases in July, 2020.25 We specified the model to reflect the number of available hospital and intensive care unit beds in KwaZulu-Natal,15 and results were similar when we further restricted bed availability to that of other settings in sub-Saharan Africa.20 Contact tracing and community-based screening have been frequently used for case-finding in LMICs.26 Many sub-Saharan African countries are thus theoretically poised to implement such interventions through established networks of community health workers. Isolation centres, which are likely to require the greatest investment in new infrastructure, have been implemented successfully in response to Ebola epidemics in West Africa and the Democratic Republic of Congo, where the availability of health-care resources is among the lowest in the world.27 South Africa rapidly implemented and expanded COVID-19 related services between April and June, 2020, but further scale-up would be required to meet demand in some of our modelled scenarios.25, 28
In our model, isolation centres are designed as housing facilities for people with confirmed COVID-19 who do not require hospital care but cannot safely isolate at home. We estimated that use of these centres reduces ongoing transmission after a confirmed diagnosis from 50% (in the health-care testing alone strategy) to 5%. Isolation centres are likely to be most effective in areas with high household density and little capacity for in-home isolation, which is the case for many urban centres in sub-Saharan Africa. Quarantine centres, which include optional housing for contacts who test negative and cannot safely distance during the latency period, have also been proposed for interrupting epidemic spread and were implemented in the early phases of the COVID-19 response in China. They were effective in our model at reducing the deleterious impact of the epidemic and were cost-effective in many modelled scenarios.
Important social and human rights require consideration with regard to the implementation of isolation and quarantine in many settings, due to trade-offs between public health benefits and civil liberties.29 In our model, both interventions are provided optionally for those who cannot do so safely at home, but we conservatively included costs to reflect needs should they be used. We also considered the use of home-based isolation and quarantine in a multi-way analysis that reduced efficacies and costs of both interventions. We found that isolation and quarantine remained cost-effective in some lower efficacy scenarios, particularly if their costs were also reduced. From a public health perspective, our findings support use of quarantine centres in areas with individual and community support for their use.
Our model should be interpreted within the context of several limitations. We did not account for heterogeneous mixing within the population. Instead, we assumed that contact patterns were random, as commonly done in infectious disease models. We assumed that the age-adjusted prevalence of non-communicable comorbidities in South Africa would be similar to that in in the USA and that age would be the primary driver of COVID-19 outcomes as previously reported in multiple settings.30, 31, 32 Consistent with most published studies,32, 33 we conservatively assumed no modifying effect of HIV on the severity of COVID-19, although additional data are needed from HIV-endemic countries to support this assumption. Considering a scenario in which a high prevalence of non-communicable diseases and HIV in South Africa did worsen COVID-19 outcomes compared with resource-rich settings, then the benefits of public health interventions in terms of YLS and cost-effectiveness are likely to be greater than our estimates. We accounted for mortality rates specific to South Africa in our calculations of life expectancy and years of life lost. Consideration of how resources and interventions implemented in response to COVID-19 will impact available resources for other regional health-care priorities will be crucial. We did not include lifetime costs of health care beyond COVID-19 or of sequelae among individuals who recovered, and we did not account for the effect of COVID-19 interventions on other economic sectors. Consistent with all modelling exercises, our estimates were determined by assumptions of input parameters. We selected COVID-19 clinical parameters based on published literature, which are largely derived from high-income settings. Intervention efficacy estimates were hypothesised based on other model parameters, existing literature where available, or expert opinion if no data were available. Recognising a paucity of empirical data for some of these estimates, we focused our sensitivity analyses on varying those for which data were scarce. Costing data for hospital and intensive care were derived from the literature, whereas personnel, medical supply, transportation, and other related costs for contact tracing, isolation centres, mass symptom screening, and quarantine centres were invoiced from local vendors in KwaZulu-Natal and therefore might not reflect costs in other contexts nor full implementation and scale-up costs. However, our primary findings and policy conclusions were largely consistent across a range of costing estimates.
We recommend that policy makers consider a combined strategy of health-care testing, contact tracing, isolation of confirmed cases, mass symptom screening, and quarantine of household contacts of cases to address COVID-19 epidemic control efficiently. Where quarantine centres are not feasible—for example, due to budget constraints or absence of public support—a strategy that includes the other interventions would still provide clinical benefit in an economically efficient manner.
Data sharing
This modelling study used published or publicly available data. The data used and the sources are described in this Article and the appendix. No primary data were collected for this study. Model flowcharts are in the appendix. The model code is in the process of being made publicly available.
Acknowledgments
Acknowledgments
This study was supported by the National Institutes of Health (R37 AI058736, K24 AR057827, and T32 AI007433) and by a fellowship from the Royal Society and Wellcome Trust (210479/Z/18/Z). The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the US National Institutes of Health, Royal Society, or Wellcome Trust. We thank Nicole McCann for technical assistance.
Contributors
All authors contributed to study and model design. KPR, FMS, JHAF, GH, KPF, KAF, PK, and MJS analysed data. All authors interpreted results, KPR and MJS drafted the manuscript, and all authors critically revised the manuscript and approved the final submitted version of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Xu B, Kraemer MUG, Xu B. Open access epidemiological data from the COVID-19 outbreak. Lancet Infect Dis. 2020;20:534. doi: 10.1016/S1473-3099(20)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone-Robertson SP, Mark D, Morrow C. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;174:1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siedner MJ, Gostin LO, Cranmer HH, Kraemer JD. Strengthening the detection of and early response to public health emergencies: lessons from the West African Ebola epidemic. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert M, Pullano G, Pinotti F. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO COVID-19 strategic preparedness and response plan. Operational planning guidelines to support country preparedness and response. Feb 12, 2020. https://www.who.int/docs/default-source/coronaviruse/covid-19-sprp-unct-guidelines.pdf?sfvrsn=81ff43d8_4
- 6.Hellewell J, Abbott S, Gimma A. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peak CM, Childs LM, Grad YH, Buckee CO. Comparing nonpharmaceutical interventions for containing emerging epidemics. Proc Natl Acad Sci USA. 2017;114:4023–4028. doi: 10.1073/pnas.1616438114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu S, Wagner RG, Sewpaul R, Reddy P, Davies J. Implications of scaling up cardiovascular disease treatment in South Africa: a microsimulation and cost-effectiveness analysis. Lancet Glob Health. 2019;7:e270–e280. doi: 10.1016/S2214-109X(18)30450-9. [DOI] [PubMed] [Google Scholar]
- 9.Dieleman JL, Haakenstad A, Micah A. Spending on health and HIV/AIDS: domestic health spending and development assistance in 188 countries, 1995-2015. Lancet. 2018;391:1799–1829. doi: 10.1016/S0140-6736(18)30698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edoka IP, Stacey NK. Estimating a cost-effectiveness threshold for health care decision-making in South Africa. Health Policy Plan. 2020;35:546–555. doi: 10.1093/heapol/czz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statistics South Africa Mid-year population estimates. 2019. http://www.statssa.gov.za/publications/P0302/P03022019.pdf
- 12.National Institute for Communicable Diseases The initial and daily COVID-19 effective reproductive number (R) in South Africa. May 27, 2020. https://www.nicd.ac.za/wp-content/uploads/2020/05/The-Initial-and-Daily-COVID-19-Effective-Reproductive-Number-R-in-South-Africa-002.pdf
- 13.Yang Y, Yang M, Shen C. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020 doi: 10.1101/2020.02.11.20021493. published Feb 17. (preprint) [DOI] [Google Scholar]
- 14.Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Department of Health South Africa COVID-19 public health response. April 10, 2020. https://sacoronavirus.co.za/2020/04/11/covid-19-public-health-response/
- 16.Netcare Hospitals Netcare Tariffs. 2019. https://www.netcarehospitals.co.za/Portals/3/Images/Content-images/PDF/2019-Private-Paying-Patients.pdf
- 17.Mahomed S, Mahomed OH. Cost of intensive care services at a central hospital in South Africa. S Afr Med J. 2018;109:35–39. doi: 10.7196/SAMJ.2018.v109i1.13268. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Communicable Diseases COVID-19 testing summary. May 23, 2020. https://www.nicd.ac.za/wp-content/uploads/2020/05/NICD-COVID-19-Testing-Summary_-Week-21-2020-007.pdf
- 19.Department of Health Province of KwaZulu-Natal Annual performance plan 2019/20–2021/22. 2019. http://www.kznhealth.gov.za/app/APP-2019-20.pdf
- 20.Craig J, Kalanxhi E, Hauck S. National estimates of critical care capacity in 54 African countries. medRxiv. 2020 doi: 10.1101/2020.05.13.20100727. published online July 6. (preprint) [DOI] [Google Scholar]
- 21.Reddy KP, Gupta-Wright A, Fielding KL. Cost-effectiveness of urine-based tuberculosis screening in hospitalised patients with HIV in Africa: a microsimulation modelling study. Lancet Glob Health. 2019;7:e200–e208. doi: 10.1016/S2214-109X(18)30436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 23.Centre for the Mathematical Modelling of Infectious Diseases. London School of Hygiene and Tropical Medicine COVID-19 estimates for South Africa. June 22, 2020. https://epiforecasts.io/covid/posts/national/south-africa/
- 24.Siedner MJ, Harling G, Reynolds Z. Social distancing to slow the US COVID-19 epidemic: longitudinal pretest-posttest comparison group study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute for Communicable Diseases COVID-19 testing summary. March 2, 2020. https://www.nicd.ac.za/wp-content/uploads/2020/08/COVID-19-Testing-Summary-Week-34-2020.pdf
- 26.Shapiro AE, Variava E, Rakgokong MH. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med. 2012;185:1110–1116. doi: 10.1164/rccm.201111-1941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legrand J, Grais RF, Boelle PY, Valleron AJ, Flahault A. Understanding the dynamics of Ebola epidemics. Epidemiol Infect. 2007;135:610–621. doi: 10.1017/S0950268806007217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdool Karim SS. The South African response to the pandemic. N Engl J Med. 2020;382:e95. doi: 10.1056/NEJMc2014960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blendon RJ, Koonin LM, Benson JM. Public response to community mitigation measures for pandemic influenza. Emerg Infect Dis. 2008;14:778–786. doi: 10.3201/eid1405.071437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewnard JA, Liu VX, Jackson ML. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Docherty AB, Harrison EM, Green CA. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulle A, Davies M-A, Hussey H. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. published online Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Rio C. COVID-19 in persons living with HIV—what do we know today? [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This modelling study used published or publicly available data. The data used and the sources are described in this Article and the appendix. No primary data were collected for this study. Model flowcharts are in the appendix. The model code is in the process of being made publicly available.