Abstract
This review reported that coronavirus disease 2019 (COVID-19) infected patients with short time bed rest or quarantine and airway inflammation are at more risk of developing hyperglycemia and insulin resistance. This condition can induce oxidative stress, decrease immune system function, impair endothelial function, induce apoptosis, and reduce antioxidant in the lungs. We provide a possible mechanism in severe COVID-19 patients and recommend treatment strategy to reduce mortality rate and prevent adverse outcomes after intensive care unit (ICU).
Keywords: Coronavirus, COVID-19, Diabetes, Hyperglycemia, Oxidative stress, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic that has affected more than 57,000,000 cases and caused about 1,700,000 deaths as of 29 December 2020 [1]. Studies also showed that the rate of hospital admission among COVID-19 infected subjects is very variable ranged from 3% to 100% of confirmed cases. Studies showed that COVID-19 hospitalization rate in United States is above 15% [1]. Among those who are hospitalized, more than one fourth requires intensive care unit (ICU) admission, representing about 5%–10% of the total infected cases [2]. For cases that become critically ill, the mortality rate is approximately 7%–62% [2]. ICU admission rates vary across countries. For instance, in China, ranged from 7 to 26%, in Italyranged from 5% to 12%, and in the United States were 4.9%–11.5% of the total positive cases [3]. The median duration of hospital stay among the COVID-19 patients ranges from 16 to 23 days, and the median length of ICU stay is 7 to 17 days. Furthermore, the average time of mechanical ventilation is about 1–12 days [4]. Immobility from bed rest (especially immobilization in the ICU) is related to various complications, such as bone demineralization, weakness, impaired physical function, neurocognitive problem, muscle atrophy, and metabolic changes. Immobilization, when increased beyond a few hours, is recognized to change vital aspects of metabolic pathways, and consequently increase mortality rate [5]. Therefore, a better understanding of prevention and treatment approaches is essential for minimization of the fatality rate as well as a metabolic alteration in ICU patients and survivors [6]. In this review, the possible mechanisms of hyperglycemia involved in the severity of COVID-19 infection and the efficacy of antidiabetic agents is investigated. Moreover, we showed that short time bed rest (similar to quarantine) and airway inflammation are a risk factor for development of hyperglycemia and insulin resistance (IR). Hence, all patients need primary and secondary care in hospital.
2. Possible adverse effect of bed rest in COVID-19 patients
In critical COVID-19 patients, bed rest is particularly common [7]. In some ICU survivors (due to various type of diseases), poor physical function, reduced mobility, anxious or depressed mood, cognitive defect, sexual dysfunction, fatigue, muscle atrophy, sleep disturbances, weakness, and metabolic alterations can continue for years after discharge from hospital [5,8]. Pneumonia is the main reason for admission to the ICU in COVID-19 subjects. Interestingly, it has been reported that even many years after pneumonia, there is a relationship between the disease, hyperglycemia, and elevated mortality risk [9,10]. Therefore, a better understanding of the mechanism and outcome of bed rest in COVID-19 patients, especially with regard to prevalence and fatality rate, is necessary. Also, a decrease in physical activity in ICU survives, which affects many ICU patients, can induce hyperglycemia and IR [11].
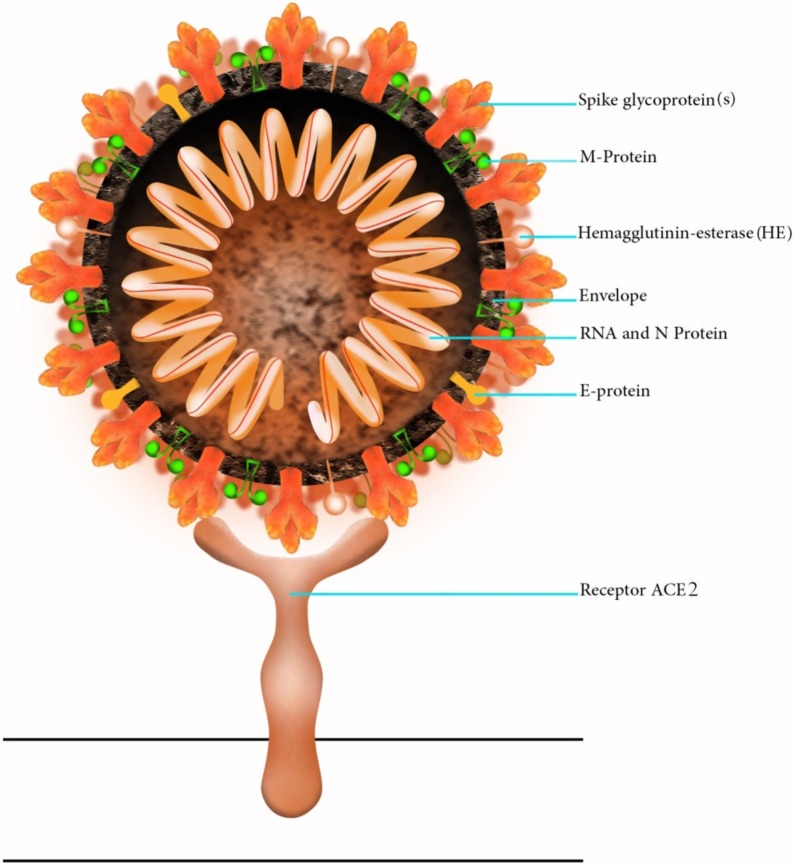
COVID-19, like severe acute respiratory syndrome-related coronavirus (SARS-CoV), enters the cells via angiotensin-converting enzyme 2 (ACE2) receptor (Fig. 1, Fig. 2 ). This protein is expressed in bronchial epithelium, vascular endothelium, and alveolar cells. Hence binding of 2019-novel coronavirus (2019-nCoV) to ACE2 would lead to pulmonary hemorrhage, pulmonary edema, and acute lung injury [12]. In the lung, ACE2 cleaves Ang II to form Ang-(1–7). When the activity of ACE2 suppressed, intact angiotensin II acts via the angiotensin I receptor to stimulate inflammatory markers and motivate aldosterone release; these effects not only lead to hypokalaemia and hypertension, but will also raise vascular permeability, elevating the risk of respiratory distress syndrome [[12], [13], [14]]. Furthermore, Ang-II impairs glucose tolerance and increases blood glucose, by inducing IR and reducing insulin secretion from the pancreas [15].
Fig. 1.
Schematic structure of COVID-19. The spike glycoprotein is essential for virus entry into the cell.
Fig. 2.
A three-dimensional (3D) structure of the novel coronavirus (COVID-19).
On the other hand, ACE2 product, angiotensin 1–7, induces anti-fibrotic and anti-inflammatory pathways that could be favorable to the recovery of COVID-19 cases. Therefore, severe COVID-19 patients have an imbalance in these metabolic pathways, which could be the subject in diabetes, high blood pressure, and IR states [16]. Disturbance of these pathways directly links to diabetes. In the pancreas, binding of the SARS-CoV to ACE2, injuries islet cells, and decrease insulin secretion [12]. The result of one study with a 3-year follow-up showed that more than 50% of the cases became diabetic during hospitalization for the SARS-CoV disease. Of note, these patients had no history of diabetes and steroid therapy. However, later three years of recovery from the SARS-CoV infection, about 5% of cases remained diabetic [12]. It should be noted that, ACE2 has a significant role in glucose homeostasis. Regarding the expression of ACE2 in the pancreas, the coronavirus might enter these cells and lead to acute beta-cell damage, causing severe hyperglycemia and transient diabetes [12].
In the diabetic animal models, pharmacological inhibition of ACE2, aggravate albuminuria and, is associated with kidney damage [15]. Furthermore, in ACE2 knockout (KO) mice, higher fasting blood glucose and impaired glucose tolerance as well as decreased insulin secretion have been established, proposing a significant role of ACE2 in the development of diabetes. ACE2 also attenuates inflammation and fibrosis in the heart, lungs, liver, and pancreatic islet β-cell. Furthermore, ACE2 reduces inflammation, oxidative stress (OS), and fibrosis in the β-cell [15]. This finding proposes that diabetic patients susceptible to a COVID-19 infection. Similarly, diabetes motivates ACE expression in other organs such as the heart, lungs, and liver, which clarifies why diabetes can increase multi-organ dysfunction in coronavirus diseases [12]. Furthermore, the chronic viral infection is a vital risk factor for the development of diabetes, a disease with a high prevalence (9.3%, 463 million people in 2019) is the seventh leading cause of death [17]. Also, inactivity and hyperglycemia during hospitalization and/or ICU also motivate IR and impair glucose homeostasis, even when energy balance is maintained [8]. Hyperglycemia and IR in the hospitalized patients are often multifactorial, including change of glucose and lipid metabolisms, the release of the stress hormones, induced OS, and inflammation [[18], [19], [20], [21]]. In the next section, we will discuss the relationship of hyperglycemia with increased severity of COVID-19 infection.
3. Hyperglycemia and insulin resistance (IR) in ICU patients
Hyperglycemia is common in critically ill subjects, as a result of stress-induced IR and enhanced glucose production. Increased blood glucose is independently related to elevated ICU mortality. Hence strict control of blood glucose levels is considered essential. Among cases admitted to ICU, subjects with recently established hyperglycemia had three times more fatality rate (31%) compared with diabetic subjects (10%) or normoglycemia (11.3%). Of note, the lowest fatality (9.6%), happened in cases with glucose concentration ranged from 80 to 99 mg/dL and elevated progressively as glucose levels augmented, reaching 42.5% among cases with glucose concentration exceeding 300 mg/dL [22].
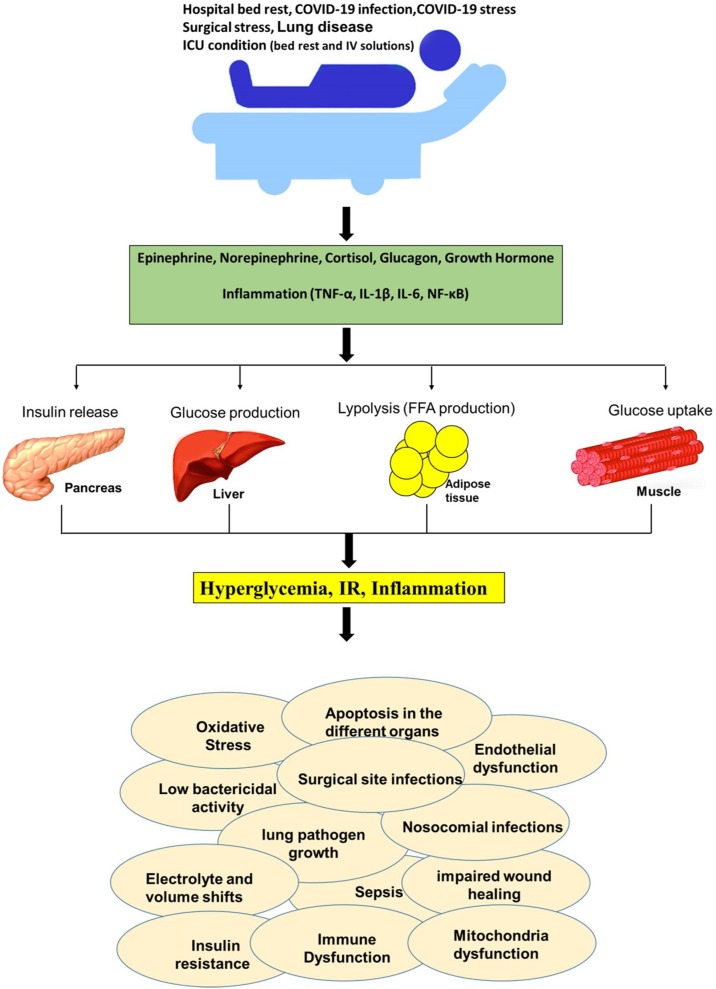
Various factors involved in the hyperglycemia and IR in bed rest, including medications (vasopressors, lithium, exogenous cortisol, and β-blockers), activation of inflammatory pathways and secretion of stress hormones. Furthermore, intravenous dextrose, antibiotic solutions, dialysis solutions, and overfeeding also contribute to hyperglycemia. Inadequate insulin release or volume depletion could lead to hyperglycemia. Hospitalization (bed rest), even in the absence of evident disease, by different mechanisms induce IR, which in turn can aggravate the severity of disease (Fig. 3 ) [18].
Fig. 3.
Effect of different condition that can leads to hyperglycemia. TNF-α: tumor necrosis factor-α, NF-κB: Nuclear factor-κB, IL-: Interleukin- (IL-), IR: Insulin resistance, FFA: Free fatty acid.
High blood glucose concentration and diabetes are accepted as independent predictor of mortality and morbidity in SARS and COVID-19 infected cases [19,23]. It has been shown that one or more pre-existing medical disorders of insulin sensitivity (e.g., high body mass index or obesity) cause susceptibility to stress hyperglycemia in critical illness and may lead to subsequent development of diabetes [11]. Of note, hyperglycemia is related to elevated morbidity and mortality in ICU patients [18], and increased pneumonia risk in COVID-19 patients [19].
Hyperglycemia and IR are existent short period after pulmonary diseases, and before the detection of ventilator-associated pneumonia. The association between hyperglycemia and lung disease is poorly recognized, but it is thought to be because of IR rather than β-cell dysfunction [18,24].
Airway glucose levels become increased if normal homeostasis is disturbed by hyperglycemia or inflammation of the pulmonary epithelium [19]. Hyperglycemia decreased the mobilization of leukocytes and reduced phagocytic activity. Hyperglycemia also impairs endothelial function, induce apoptosis, and reduce antioxidant in the lungs. Increased pulmonary glucose levels are associated with high lung pathogen growth (e.g., viral and bacterial) and reduced intracellular bactericidal activity. Increased airway glucose levels may also aggravate lung disease by inducing inflammation. Furthermore, acute hyperglycemia increases proteolysis and is related to an augmented risk of cardiovascular disease, acute renal failure, and death [18,19]. Severe hyperglycemia is associated with adverse electrolyte and volume shifts [22]. It has been established that even less than one-week bed rest may cause hyperglycemia and IR [25,26]. Alibegovic et al. showed that 9-day bed rest induced substantial IR in healthy young men with or without a recognized predisposition to diabetes [27].
The IR is described by an augmented hepatic glucose production, reduced glucose uptake by muscle, and elevated lipolysis. In this condition, muscles, adipose, and liver don't respond to insulin. IR elevated the risk for hypertension, type 2 diabetes (T2D), and cardiovascular disease (higher risk for severe illness from COVID-19) [28]. Moreover, IR is related to a range of cardiovascular disease risk factors, including hypertension, OS, dyslipidemia, inflammation, and glucose intolerance [18].
Abdelhamid et al., in the meta-analysis study, determined the diabetes risk in ICU patients (2923 patients). They showed that stress-induced hyperglycemia during ICU admission is related to elevated risk for incident diabetes [11]. The risk of diabetes in ICU survivors with increased glucose levels is like the risk in women with gestational diabetes over comparable observation times. Moreover, ICU survivors often experience long-term physical problems, inactivity, impaired insulin action, and consequently might have a unique capacity to profit from screening schedules to identify prediabetes of diabetic cases [11]. Previous studies established that the development of hyperglycemia during critical care is not a normal or physiological situation [18]. Hyperglycemia has been established as a strong proinflammatory mediator, and strict glucose control less than 110 mg/dl with insulin has been reported to show anti-inflammatory effects in the critically ill cases. Glucose administration (75 g, orally) to healthy subjects raises inflammatory markers and reactive oxygen species (ROS) production [29]. Hyperglycemia also induces nuclear factor kappa B (NF-κB) activation, exerts pro-thrombotic properties, and increases OS [29].
Bed rest experiments of different periods in healthy subjects have confirmed reduced insulin sensitivity after a short period (even after 3–5 days) of inactivity [26,30]. Hamburg et al. have demonstrated that only 5 days of bed rest in healthy volunteers leads to the development of IR, raises total cholesterol, triglyceride, blood pressure, and reduces microvascular performance [31]. Longer (9-day) bed rest studies have also revealed reduced insulin sensitivity and consequently IR in healthy subjects [27]. Moreover, the development of IR has been associated with only 3 days of bed rest [25].
It has been reported that 6–7 days of bed rest in healthy subjects, significantly reduced glucose tolerance and glucose uptake in muscle and associated with IR [25]. These results are consistent with those reported by Bienso et al., who found decreased muscle glucose transporter type 4 (GLUT-4), insulin-dependent glucose transporter, and beta serine/threonine-protein kinase (AKT2) expression after one-week bed rest in healthy subjects [32]. Hirose et al. [33] experiment has demonstrated that 1-week bed rest decreases phosphorylation of insulin receptor substrate 1 (IRS-1) and phosphoinositide 3-kinase (PI3K), which induce skeletal muscle IR. As mentioned above, the median duration of ICU stay among COVID-19 patients is ranged from 7 to 17 days. Therefore, COVID-19 patients have a high risk of hyperglycemia and IR that aggravate the severity of the disease.
4. Stress hyperglycemia in COVID-19 patient
The COVID-19 patient experienced high stress, particularly as this virus does not have a vaccine at present. Stress in the patient is related to some critical disease by stimulation of the hypothalamic-pituitary-adrenal (HPA) axis. The release of stress hormones, including catecholamines, cortisol, growth hormone (GH), and glucagon is known as the main components of the general adaptation to critical illness. Blood concentration of these hormones can elevate 2–5 folds during stress in humans. The acute response to critical disease is usually accepted as a suitable and adaptive response that happens in the first few days after illness. Hyperglycemia induced by mild-to-moderate stress is protective since it provides a source of energy for the brain and the immune system in stress conditions. Nevertheless, many of the stress hormone responses result in persistent hyperglycemia and IR, which can be strongly deleterious in the long run [21]. Epinephrine, by suppression of glucose uptake by skeletal muscle, can induce IR [18]. Moreover, the secretion of stress hormones, can activate lipolysis, increase free fatty acids (FFA) levels, and inhibit glucose uptake by peripheral tissues [18]. Increased FFA levels decrease insulin sensitivity and inhibit insulin signaling. Moreover, elevated FFA concentration can lead to mitochondrial dysfunction, increased superoxide radical production, and reduced antioxidant enzyme activity [28].
A meta-analysis study shows that stress hyperglycemia classifies patients at elevated risk of incident diabetes [11]. Previous studies reported that stress-induced hyperglycemia of critical disease had been attributed to IR [18,21]. Also, these stress hormones motivate gluconeogenesis and glycogenolysis in the liver, consequently increase blood glucose levels. Catecholamines induce IR by inhibiting tyrosine kinase activity, insulin binding, and glucose uptake (inhibit GLUT-4). Cortisol induces gluconeogenesis and impairs glucose uptake in skeletal muscle. GH inhibits the inhibiting tyrosine kinase activity and decreases insulin receptors [29]. Bed rest studies indicate that muscle tyrosine kinase activity and the expression of GLUT-4 are reduced in this condition [25]. All of these changes can lead to IR, and subsequently, increased mortality risk in ICU patients as well as increased diabetes risk in survivors.
5. Inflammation in COVID-19 patient
Systemic inflammation in ICU patients may be involved in the development of IR. C-reactive protein (CRP) and inflammatory cytokines are significantly elevated in COVID-19 patients [7]. Cytokine storm (significant increase of inflammatory cytokines) has been associated with multi-organ failure in COVID-19 patients with severe illness. Cytokines have a vital role in inflammation which, in turn, can lead to IR [34]. Lung disease induces inflammation and is associated with hyperglycemia. This hyperglycemia elevates the risk of poor outcomes in ICU cases with lung damages. The severity of inflammation throughout the pulmonary disease is associated with diminished insulin action [18,22].
Cyphert et al. [24] showed that lung inflammation, even without other comorbidities, induces IR, inflammation, and elevates diabetes risk. In patients with lung disease, systemic inflammation, along with elevated liver tumor necrosis factor-α (TNF-α) and interleukin 5 (IL-5), involved the elevated risk of diabetes [24]. Lung inflammation also impaired glucose uptake by the skeletal muscle, and consequently lead to hyperglycemia [24]. On the other hand, the combination of COVID-19 infection and diabetes significantly induce inflammation, subsequent increased fatality rate. Furthermore, the inflammatory response cause reduced GLUT-4 translocation, indicating inhibition of glucose uptake. Increased TNF-α in COVID-19 patients probably interferes with insulin signaling cascade [34].
It has been found that contracting muscles during ICU, increased IL-6 levels, suggesting that this cytokine’s release may be affected by the critically ill. The expression of IL-6, NF-κB, TNF-α, and toll-like receptor 4 (TLR4) are elevated during bed rest in different organs. These inflammatory markers have been associated with IR and diabetes [35]. Drummond et al. have shown that after one week of bed rest, the expression of TLR, IL-6, NF-κB1, IL-1, and IL-15 mRNA was significantly enhanced in bed rest subjects as compared with controls [30]. They found that inflammation reduces insulin sensitivity in part through the TLR activation. TLR4 activation, in turn, impairs insulin action by inducing proinflammatory factors and OS as well as by stimulation of cytokine signaling pathways: insulin-desensitizing markers. Upon TLR4 activation, NF-κB raises IL-6, TNF-α, and IL-1β expression. The role of the NF-κB cascade in the pathogenesis of IR is well established [35].
6. Mitochondrial dysfunction and oxidative stress (OS) in COVID-19 patient
It has been shown that mitochondrial dysfunction contributes to organ failure in the critical ill. Treatment of mitochondrial dysfunction in ICU cases may potentially improve the clinical outcome. In some conditions such as severe disease, patient genotypes, or some drug may reduced mitochondrial biogenesis and elevate susceptibility to oxidative damage [20]. Hyperglycemia (e.g., in critically ill) can leads to mitochondrial dysfunction, activating ROS production, and increasing inflammatory factors [20]. Elevated inflammatory markers and ROS is assumed to interfere with the insulin signaling pathway, thus causing anabolic resistance [35].
A significant decrease in mitochondrial content and function with a concomitant reduction in antioxidant enzymes was reported after a short time of bed rest (less than one week) in ICU patients [20,26]. Mitochondrial dysfunction, particularly the production of mitochondrial free radicals, has been accepted as the main factor in the development of IR [36].
Bed rest studies have revealed a reduction in mitochondrial volume density, function, and capacity [26]. It has been reported that the development of IR is attributed to a decrease in the content and function of mitochondria. Defective mitochondrial oxidative phosphorylation may raise diabetes risk [36].
A study on bed rest by Alibegovic et al. [37] has revealed a change in the expression of more than 4500 genes and downregulation of 34 pathways, mostly those involving factors related to mitochondrial function, such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), which controls mitochondrial oxidative phosphorylation. They have shown that the expression of PGC1α and the oxidative phosphorylation (OXPHOS) pathway is significantly reduced, indicating decreased oxidative capacity during bed rest [37]. Brocca et al. study has demonstrated a significant reduction in PGC-1α expression after 35 days of day bed rest [38], contributing to the development of IR. PGC-1 is a prominent bidirectional regulator of mitochondrial biogenesis and insulin signaling and contributes to metabolic disorders, such as IR and diabetes. IR subjects, compared with healthy subjects, have fewer mitochondria in their tissues (e.g., muscle), probably owing to reduced PGC-1α expression [39]. Reduced mitochondrial function (e.g., in muscle), which can occur as a primary defect, can slow down fat oxidation; this change eventually elevates lipid metabolites in muscle, and consequently causes IR [39].
There is evidence demonstrating that 5 days of strict bed rest in healthy subjects was associated with the development of IR, dyslipidemia, hypertension, and lead to microvascular dysfunction in healthy volunteers [31].
7. Control and treatment of hyperglycemia in COVID-19 patients
Currently, there are few options for the treatment or prevention of ICU-acquired complications in COVID-19 patients. In addition to current drugs, tight control of hyperglycemia using insulin therapy may reduce physiological abnormalities in cases mechanically ventilated for one week or more [8]. As mentioned above, hyperglycemia has been established that has a direct relationship with inflammation, OS, pneumonia, and consequently, death. Therefore, strict glucose control less than 110 mg/dl with insulin has been reported to exert anti-inflammatory effects and reduced morbidity and mortality among critically ill patients in the ICU [40].
One of the therapeutic options in ICU patients, including control of hyperglycemia and local manipulation of respiratory glucose homeostasis, as well as inflammation, should be considered [19]. The American Diabetes Association has increased the treatment threshold to levels of more than 180 mg/dL and target glucose concentration between 140 and 180 mg/dL for ICU patients [19,40]. It has been proposed that the early blood glucose concentration is measured every one or two hours till the infusion speed has been set. Later blood glucose should check every 4 h once the levels have been stabilized [41]. In critical patients, intravenous insulin infusion is recommended. Strict insulin treatment reduces the risks of pneumonia, multiple organ failure, inflammation, and reduces mechanical ventilation and ICU dependence [19].
OS has been associated with the severity of the disease, depletion of body antioxidants, systemic inflammation, and mitochondrial dysfunction. Therefore, the depletion of body antioxidant elevated the fatality rate of ICU patients [42]. There is evidence that administrations of vitamins A, C, and E in critically ill patients decrease the incidence of organ injury and reduces the ICU length of stay. These antioxidants reduce OS and inflammation in critical care patients and markedly affected their clinical outcomes [42]. Recent studies have documented that moderate to high doses of vitamin C (orally, 6 g daily) may prevent respiratory tract infection in COVD-19 patients [43]. The reduction of vitamin D and E levels in cattle could increase the risk of coronavirus infection. The administration of vitamin C can potentially reduce the incidence of pneumonia. Vitamin C potentially alleviates severe pneumonia in the most severely ill patients by inhibiting OS and inflammation [7]. Vitamin D, due to its anti-viral, antimicrobial anti-inflammatory, and antioxidant activities can reduce the severity and risk of pneumonia in COVID-19 patients. Serum vitamin D levels tend to decrease with age and severe disease (e.g., diabetes and hypertension), which may be vital for COVID-19 infection since mortality rates are significantly elevated with age and comorbidity disorders. This vitamin showed potential anti-viral, anti-inflammatory, antimicrobial, and antioxidant effects. Therefore, it has been recommended to prevent and treatment of COVID-19 infection [44]. Furthermore, this vitamin can positively influence hospital-acquired infections. Recent studies suggested using vitamin D loading doses of 200,000–300,000 IU in 50,000-IU capsules to reduce the risk and severity of COVID-19 disease [44]. These findings propose that appropriate supplementation of these vitamins may increase our resistance to COVID-19 infection. Antioxidant supplementation is potentially recommended in COVID-19 patients with primary underlying diseases, such as hypertension, diabetes, coronary heart disease, and cancer [7,[45], [46], [47]].
Zinc is required for more than 300 enzymes in the body and participates in immune system function. Zinc deficiency can increase the risk of lower respiratory tract infections. It has been reported that the combination of this ion plus low doses of pyrithione can inhibit SARS‐CoV replication [48]. Zinc nanoparticle, also due to the antioxidant and anti-inflammatory effects [49], suggested for the treatment of COVID-19 infection [50]. Hence, zinc supplements may alleviate Covid‐19 disease. Zinc also can inhibit the SARS-coronavirus replication [48].
Early rehabilitation and mobilization of COVID-19 patients who are critically ill may prevent improved strength, reduce OS and inflammation and improve patient outcomes [8].
Glucagon-like peptide-1 (GLP-1) analogues and dipeptidyl peptidase-4 (DPP4) inhibitors improve hyperglycemia through various mechanisms, including an enhancement of insulin release, a glucose-dependent decrease of postprandial glucagon as well as delayed gastric emptying. The use of DPP4 inhibitors alone or in combination with insulin is a safe and effective approach to control of blood glucose levels without elevating the risk of hypoglycemia or excessive weight gain [51]. DPP4 inhibitors show anti-inflammatory effects and do not potentiate ACE levels in animal models, consequently they may be considered useful in diabetic patients with COVID-19 [52]. All COVID-19 diabetic patients on GLP-1 analogues treatment should be carefully checked and provided with adequate fluid intake and regular meals to avoid the risk of dehydration [53]. Moreover, the prescription of the two medicines with a single daily injection prevents unnecessary exposure to COVID-19 subjects, minimizing the infection transmission risk.
Sodium glucose transporter (SGLT)-2 inhibitors increase ACE2 in renal and therefore, may show poor outcomes. Osmotic diuresis and potentially dehydration as well as euglycemic diabetic ketoacidosis (EDKA) are known as limiting factors to the use of these agents [52]. Because of poor caloric intake in acute disease (e.g., COVID-19 infection), the use of sulfonylureas may motivate hypoglycemia. Thus, these agents are not ideal in controlling high blood glucose levels in COVID-19, especially in ICU admissions [52].
Metformin can inhibit the coronavirus entry into the cell by disruption of binding capacity. Metformin also exerts antioxidant and anti-inflammatory effects as well as modulates dysbiosis. Hence, it can be used in subjects with diabetes who are hospitalised for COVID-19, as long as they have not developed concomitant liver and renal failure. Liver failure decreases lactate elimination, and renal disease leads to metformin accumulation, elevating the risk of lactic acidosis. Nevertheless, due to the risk of lactic acidosis, this agent should be used with caution in critically ill patients [53].
Thiazolidinediones can lead to fluid retention, worsening heart failure, and induces weight gain and edema, hence make these agents unsuitable in subjects with both diabetes and COVID-19 [52] (Table 1 ).
Table 1.
Antidiabetic agents in hospitalized patients with type 2 diabetes and COVID-19 [53].
| Hospitalized moderate disease | Hospitalized severe disease (ICU patients) | |
|---|---|---|
| Recommended | ✓ Insulin | ✓ Insulin |
| ✓ DPP4 inhibitor | ✓ DPP4 inhibitor | |
| ✓ GLP-1 analogues | ||
| ✓ Metformin | ||
| Not recommended | ✓ Thiazolidinedione | ✓ Thiazolidinedione |
| ✓ SGLT2 inhibitors | ✓ SGLT2 inhibitors | |
| ✓ Sulfonylurea | ||
| Use with caution | ✓ Sulfonylurea | ✓ Metformin |
| ✓ α-Glucosidase inhibitors | ✓ α-Glucosidase inhibitors | |
| ✓ GLP-1 analogues |
[DPP4: Dipeptidyl peptidase-4, SGLT2: Sodium glucose transporter 2, GLP-1: Glucagon-like peptide-1].
8. Conclusion
Among COVID-19 patients who are hospitalized, more than one fourth requires ICU admission, representing about 5%–10% of the total infected cases. Subjects with a chronic viral infection, pneumonia, lung inflammation, and short time bed rest (e.g., due to hospitalization) are at more risk of developing hyperglycemia and IR. This condition can induce oxidative stress, decrease immune system function, impair endothelial function, induce apoptosis, and reduce antioxidant in the lungs. Hyperglycemia also reduces intracellular bactericidal activity, increases inflammation, and is associated with an elevated risk of lung disease, cardiovascular disorders, kidney failure, and death. Furthermore, the developments of IR and hyperglycemia have been reported in ICU survivors. In surgical cases, perioperative hyperglycemia elevates the postoperative morbidity and mortality rate. Hyperglycemia in surgical patients is related to the risk of atrial fibrillation, infection, cardiovascular disease, myocardial infarction, pericarditis, cerebral ischemia, impaired wound healing, as well as respiratory and neurologic complications. Careful management of hyperglycemia in COVID-19 severe patients (with or without operation) can reduce mortality and morbidity risk.
The current manuscript has some limitations. Our study was that it only included articles published in English and scientific published papers. Hence we did not any internal or external secondary research. Moreover, we did not present all variables, including advanced glycation end products (AGEs), hemoglobin A1c (HbA1c) levels, and hormones. We did not include gestational diabetes, so these findings may not generalize to all diabetic subjects. Further studies are needed to determine the possible mechanisms of hyperglycemia in pediatrics. Furthermore, there is no sufficient available data at the moment that show any significant benefit or harm effects of non-insulin anti-diabetic agents. The short and long-time effects of these agents on COVID-19 subjects need further investigation. No comprehensive findings are currently available about the anti-DPP4 vaccine. This vaccine may represent another potential approach for COVID-19 patients. Further preclinical studies are needed to evaluate the effects of different types of vaccines in the diabetic patients.
Contribution statement
EAO, FM and AV wrote the manuscript with support from FF, IK, and HT. FM and EAO designed the experiments, and revised the manuscript. EAO revised the manuscript. All authors read and approved the final version.
Funding source declaration
Self-funded.
Ethics approval
Not applicable.
Conflict of interests
The author declared no conflict of interest.
References
- 1.COVID-19 statistics, Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports, COVID-19 Weekly Epidemiological Update. (updated 2020; (Accessed 11 May 2020)).
- 2.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020;21:1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 3.Coronavirus disease 2019 (COVID-19): Critical care issues. Available from: https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-critical-care-and-airway-management-issues. (updated 2020; (Accessed 1 October 2020)).
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region-case series. N. Engl. J. Med. 2020;21:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens R.D., Hart N., de Jonghe B. Weakness in the ICU: a call to action. Crit. Care. 2009;13:1002. doi: 10.1186/cc8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry S.M., Puthucheary Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem. Physiol. Med. 2015;9:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L.S., Wang Y.R., Ye D.W. Review of the 2019 novel coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents. 2020;55:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truong A.D., Fan E., Brower R.G. Bench-to-bedside review: mobilizing patients in the intensive care unit–from pathophysiology to clinical trials. Crit. Care. 2009;13:216. doi: 10.1186/cc7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koskela H.O., Salonen P.H., Romppanen J. Long-term mortality after community-acquired pneumonia–impacts of diabetes and newly discovered hyperglycaemia: a prospective, observational cohort study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farahani F., Mirzaei F., Khodadadi I. Importance of hyperglycemia in preoperative, intraoperative and postoperative periods in COVID-19 patients. Int. J. Surg. 2020;8:1–2. doi: 10.1016/j.ijsu.2020.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali Abdelhamid Y., Kar P., Finnis M.E. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and meta-analysis. Crit. Care. 2016;27:301. doi: 10.1186/s13054-016-1471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein S.R., Dalan R., Hopkins D. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020;16:297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshaghi Oshaghi E., Farahani F., Khodadadi I. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): laboratory, PCR, and chest CT imaging findings. Int. J. Surg. 2020;79:143–153. doi: 10.1016/j.ijsu.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra K.H., Chodavarapu H., Lazartigues E. Angiotensin converting enzyme 2: a new important player in the regulation of glycemia. IUBMB Life. 2013;65:731–738. doi: 10.1002/iub.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbasi-Oshaghi E., Mirzaei F., Khodadadi I. Letter to the Editor regarding ‘COVID-19 and diabetes: what does the clinician need to know?’. Prim. Care Diabetes. 2021;15:30. doi: 10.1016/j.pcd.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez-Grobe Y., Ponciano-Rodriguez G., Mendez-Sanchez N. Viral hepatitis infection and insulin resistance: a review of the pathophysiological mechanisms. Salud Publica Mex. 2011;53:S46–S51. PMID: 21877073. [PubMed] [Google Scholar]
- 18.Hsu C.W. Glycemic control in critically ill patients. World J. Crit. Care Med. 2012;4:31–39. doi: 10.5492/wjccm.v1.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker E.H., Wood D.M., Brennan A.L. Hyperglycaemia and pulmonary infection. Proc. Nutr. Soc. 2006;65:227–235. doi: 10.1079/pns2006499. [DOI] [PubMed] [Google Scholar]
- 20.van Niekerk G., Davis T., Engelbrecht A.M. Hyperglycaemia in critically ill patients: the immune system’s sweet tooth. Crit. Care. 2017;21:202. doi: 10.1186/s13054-017-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiu F., Stanojcic M. Stress hyperglycemia, insulin treatment, and innate immune cells. Int. J. Endocrinol. 2014;2014:486403. doi: 10.1155/2014/486403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pakhetra R., Garg M.K., Suryanarayana K.M. Management of hyperglycemia in critical illness: review of targets and strategies. Med. J. Armed Forces India. 2011;67:53–57. doi: 10.1016/S0377-1237(11)80015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang L., Shao M., Guo Q. Diabetes mellitus is associated with severe infection and mortality in patients with COVID-19: a systematic review and meta-analysis. Arch. Med. Res. 2020;51:700–709. doi: 10.1016/j.arcmed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cyphert T.J., Morris R.T., House L.M. NF-kappaB-dependent airway inflammation triggers systemic insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R1144–R1152. doi: 10.1152/ajpregu.00442.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart C.A., Shangraw R.E., Prince M.J. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37:802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 26.Larsen S., Lundby A.M., Dandanell S. Four days of bed rest increases intrinsic mitochondrial respiratory capacity in young healthy males. Physiol. Rep. 2018;6 doi: 10.14814/phy2.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alibegovic A.C., Hojbjerre L., Sonne M.P. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes. 2009;58:2749–2756. doi: 10.2337/db09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sears B., Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marik P.E., Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–756. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 30.Drummond M.J., Timmerman K.L., Markofski M.M. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R216–R223. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamburg N.M., McMackin C.J., Huang A.L. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers, Arterioscler. Thromb. Vasc. Biol. 2007;27:2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bienso R.S., Ringholm S., Kiilerich K. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes. 2012;61:1090–1099. doi: 10.2337/db11-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirose M., Kaneki M., Sugita H. Immobilization depresses insulin signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000;279:E1235–E1241. doi: 10.1152/ajpendo.2000.279.6.E1235. [DOI] [PubMed] [Google Scholar]
- 34.Marik P.E., Bellomo R. Stress hyperglycemia: an essential survival response! Crit. Care. 2013;17:305. doi: 10.1097/CCM.0b013e318283d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin R.L., Rune A., Bouzakri K. siRNA-mediated reduction of inhibitor of nuclear factor-κb kinase prevents tumor necrosis factor-α–induced insulin resistance in human skeletal muscle. Diabetes. 2008;57:2066–2073. doi: 10.2337/db07-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen K.F., Befroy D., Dufour S. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alibegovic A.C., Sonne M.P., Hojbjerre L. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am. J. Physiol. Endocrinol. Metab. 2010;299:E752–E763. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- 38.Brocca L., Cannavino J., Coletto L. The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J. Physiol. 2012;590:5211–5230. doi: 10.1113/jphysiol.2012.240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagel-Langenickel I., Bao J., Joseph J.J. PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J. Biol. Chem. 2008;283:22464–22472. doi: 10.1074/jbc.M800842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Or D., Rael L.T., Madayag R.M. Stress hyperglycemia in critically ill patients: insight into possible molecular pathways. Front. Med (Lausanne) 2019;6:54. doi: 10.3389/fmed.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Calatayud A.A., Guillen-Vidana A., Fraire-Felix I.S. Metabolic control in the critically ill patient an update: hyperglycemia, glucose variability hypoglycemia and relative hypoglycemia. Cir. Cir. 2017;85:93–100. doi: 10.1016/j.circir.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Abiles J., de la Cruz A.P., Castano J. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Crit. Care. 2006;10:R146. doi: 10.1186/cc5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug. Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant W.B., Lahore H., McDonnell S.L. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;2:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kassaee S.M., Taghi Goodarzi M., Abbasi E. Antioxidant, antiglycation and anti-hyperlipidemic effects of Trigonella foenum and Cinnamon in type 2 diabetic rats. Jundishapur J. Nat. Pharm. Prod. 2018;13(1) doi: 10.8512/jjnpp.38414. [DOI] [Google Scholar]
- 46.Abbasi-Oshaghi E., Khodadadi I., Mirzaei F., Anethum graveolens L. Alleviates sperm damage by limiting oxidative stress and insulin resistance in diabetic rats. Open Med. Chem. J. 2020;14:35–44. doi: 10.2174/1874104502014010035. [DOI] [Google Scholar]
- 47.Arshad M.S., Khan U., Sadiq A. Coronavirus disease (COVID-19) and immunity booster green foods: a mini review. Food Sci. Nutr. 2020;8:3971–3976. doi: 10.1002/fsn3.1719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.te Velthuis A.J., van den Worm S.H., Sims A.C. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbasi-Oshaghi E., Mirzaei F., Mirzaei A. Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine Lond. (Lond) 2018;13:2791–2816. doi: 10.2217/nnm-2018-0202. [DOI] [PubMed] [Google Scholar]
- 50.Ghaffari H., Tavakoli A., Moradi A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Peralta F., Abreu C., Gomez-Rodriguez S. Safety and efficacy of DPP4 inhibitor and basal insulin in type 2 diabetes: an updated review and challenging clinical scenarios. Diabetes Ther. 2018;9:1775–1789. doi: 10.1007/s13300-018-0488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gheblawi M., Wang K., Viveiros A. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim S., Bae J.H. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]