Abstract
Background
One of the most pronounced and poorly understood pathological features of COVID-19 infection has been high risk for venous and arterial thromboembolic complications. An increasing number of thromboembolic events are being reported almost on a daily basis, and the medical community has struggled to predict and mitigate this risk. We aimed to review available literature on the risk and management of COVID-19 related venous thromboembolism (VTE), and provide evidence-based guidance to manage these events.
Methods
A literature review of VTE complications in patients with COVID-19 was performed, in addition to a summary of the societal guidelines and present pathways implemented at our institution for the management of both in- and outpatient COVID-19 related VTE.
Results
Although a significant VTE risk has been confirmed in patients with COVID-19, literature addressing best ways to mitigate this risk is lacking. Furthermore, there has been very limited guidance provided by societal guidelines to help prevent and manage VTE associated with the COVID-19 infection. In light of the available data, we advise that all patients admitted with suspected or confirmed COVID-19 receive pharmacological prophylaxis if bleeding risk is acceptable. For patients with COVID-19 who have been discharged from the emergency department or hospital, we suggest extended thromboprophylaxis (up to 39 days) as long as bleeding risk is low.
Conclusions
We believe that this literature summary along with our center recommendations and algorithms provide valuable guidance to providers caring for patients with COVID-19 related VTE. More research is needed to standardize prophylaxis and management protocols for these patients.
COVID-19 Infection
The 2019-novel coronavirus (COVID-19) first emerged in Wuhan, China as a severe acute respiratory syndrome-coronavirus-2 (SARS-Cov-2) in December 2019. It has since spread to 188 countries causing a major pandemic with a very high death toll.1 Typical symptoms include fever, cough, fatigue, anorexia, diarrhea, and dyspnea.2 Although patients with a history of cardiovascular disease (CVD) are at increased risk of experiencing adverse events from COVID-19, even those without CVD are still at a considerable risk for developing CV events.3 Although direct therapies targeting COVID-19 are being investigated, supportive and intensive care remains key for favorable patient outcomes.
There are currently several specific treatments for COVID-19 under investigation. For patients with life-threatening COVID-19, dexamethasone, remdesivir, and convalescent plasma have shown promising benefits.4, 5, 6, 7 In addition, tocilizumab, an interleukin 6 inhibitor, is currently under investigation for the management of severe cases with COVID-19. Hydroxychloroquine and chloroquine, however, are no longer recommended outside of clinical trials given inconclusive data and lack of clear benefit with potential risks.8 Lopinavir-ritonavir, a protease inhibitor primarily used for HIV infection, has shown little to no promise for the treatment of hospitalized patients with COVID-19.9
Research to develop an effective vaccine for COVID-19 is underway, and there are numerous international studies currently evaluating various vaccine candidates.10
Health Burden of Venous Thromboembolism
Venous thromboembolism (VTE) is an important cause of preventable morbidity and mortality in hospitalized patients. The critical key for delivering optimal VTE care for both out- and inpatient populations includes appropriate prophylaxis, anticoagulation management, and secondary prevention based on thrombosis and bleeding risks.11
Hospitalized medical patients are at higher risk for VTE, with an incidence range of 10–30% in the absence of risk reduction strategies.12 Advanced age, infectious illness, history of cancer, and VTE are associated with higher risk of developing thromboembolic events during hospitalization.13 Anticoagulants have been shown to reduce risk for symptomatic VTE in medically ill patients.14 Traditionally, appropriate prophylaxis is determined by weighing the risk of bleeding with the risk of thrombosis using validated prediction scoring systems (PADUA and The International Medical Prevention Registry on Venous thromboembolism (IMPROVE)).15
COVID-19 Infection and Risk of Thromboembolism
Since the beginning of the pandemic, concerns have been raised about the increased risk of both venous and arterial thromboembolic events associated with COVID-19 infection.16 Such events include deep vein thrombosis (DVT), pulmonary embolism (PE), ischemic stroke, myocardial infarction, and peripheral arterial thromboembolism. Early studies have indicated an increased risk for VTE in severe COVID-19 infections compared to non-coronavirus-related pneumonia.17 Likewise, autopsy studies have found pathologic findings associated with higher thrombotic risk in patients with COVID-19, including severe endothelial injury and angiogenesis.18 , 19
Patients with COVID-19 may be predisposed to thrombotic events due to direct and indirect effects of the virus and infection, such as critical illness, hypoxia, and severe inflammatory response.20 Several studies reported hemostatic abnormalities in patients with COVID-19 infection, including increased d-dimer and fibrin degradation product levels, prolonged thrombin and prothrombin times and international normalized ratio, shortened activated partial thromboplastin time, and thrombocytopenia in addition to the other traditional comorbidities.20, 21, 22, 23, 24, 25 Furthermore, positive antiphospholipid syndrome related antibodies have been reported.20 , 21 Klok et al.26 reported an incidence of 31% for venous and arterial thrombotic events (27% were VTE) in 184 patients admitted to the intensive care unit with COVID-19 pneumonia despite appropriate prophylactic anticoagulation. Similarly, Zhang et al. studied 143 inpatients with COVID-19 and reported a lower extremity DVT incidence of 46%. Those with DVT, compared to those without, had higher rates of cardiac injury, worse prognosis, and higher mortality.27
Challenges with Care for Patients at Increased Risk for Venous Thromboembolism during the COVID-19 Era
The COVID-19 pandemic has significantly affected global health care at many levels. Public health interventions implemented by governments around the world, such as social distancing and home quarantine for nonessential workers, were instituted in an attempt to reduce viral transmission and prevent overwhelming health care systems. Staying at home has enabled sedentary lifestyle and decreased daily activity, putting patients at higher risk for VTE, especially those with underlying risk factors. Additionally, patients receiving vitamin K antagonists may be adversely affected by these policies. The requirement for frequent visits for International Normalization Ratio (INR) checks and resultant exposure may increase the risk of infection, but without proper monitoring, these patients may be at greater risk for complication from thromboembolic events or bleeding. INR can also be impacted by dietary changes that may occur due to disruption of daily routines. Specifically, changes in intake of green vegetables, which are a source of vitamin K, can cause INR levels to fluctuate. Additionally, the economic effects of the pandemic may hinder patients from accessing care for thrombotic events. Socioeconomic disadvantage has been associated with increased VTE risk and poorer outcomes.28 , 29 Furthermore, some patients have become noncompliant with anticoagulation therapy due to the misconceptions that antithrombotic agents may increase the risk for contracting COVID-19.
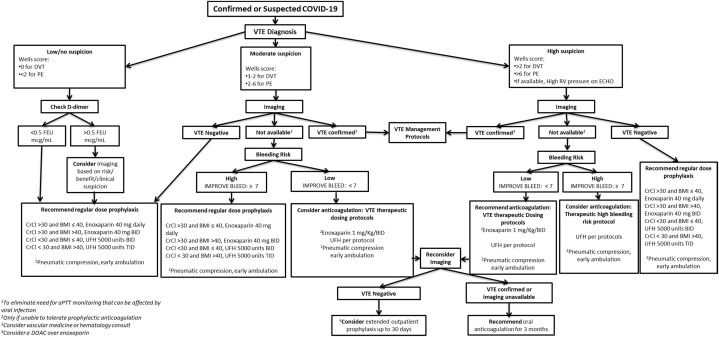
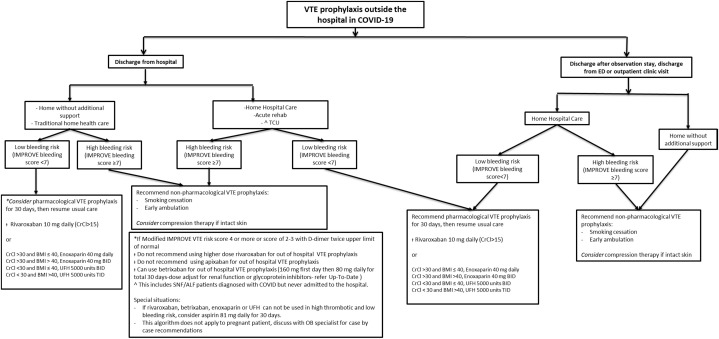
Our institution has created user-friendly recommendation algorithms to provide guidance regarding the diagnosis and management of VTE in patients with confirmed or suspected COVID-19, based on current evidence and multidisciplinary expert discussions (Fig. 1, Fig. 2 ).
Fig. 1.
Diagnosis and management of venous thromboembolism in hospitalized patients with COVID-19. BID, twice per day; BMI, body mass index; CrCl, creatinine clearance; FEU, fibrinogen equivalent units; TID, three times per day.
Fig. 2.
Out of the hospital DVT prophylaxis for patients with COVID-19 infection. ALF, assisted living facility; BID, twice per day; BMI, body mass index; CrCl, creatinine clearance; ED, emergency department; OB, obstetrician; SNF, skilled nursing facility; TCU, transitional care unit; TID, three times per day.
Our Center Recommendations for the Diagnosis and Management of Venous Thromboembolism in Hospitalized Patients with COVID-19 Infection
It is important to keep a high index of suspicion for VTE in hospitalized patients with COVID-19. A recent study reported that 40% of inpatients with COVID-19 were at high risk of VTE.30 Although one study indicated improved outcomes with prophylactic anticoagulation in patients with severe COVID-19,31 other studies have still reported significant VTEs in COVID-19 inpatients despite receiving prophylactic anticoagulation.32
For COVID-19 inpatients, we recommend obtaining complete blood count and d-dimer on admission. Risk stratification tools, such as the PADUA, IMPROVE, and IMPROVE-bleeding scores, are generally used to predict risk for VTE or bleeding in medically ill patients. However, COVID-19 patients are already considered high risk for thromboembolic events and so we only use IMPROVE-bleeding score to predict bleeding risk in these patients.
In light of the available data, we advise that all patients admitted with suspected or confirmed COVID-19 receive pharmacological prophylaxis if bleeding risk is acceptable (IMPROVE-bleeding score <7). This includes pregnant patients <20 weeks, but if >20 weeks, obstetrician should be contacted for appropriate VTE prevention. Given lack of data, we still do not recommend moderate or therapeutic dose anticoagulation if VTE is not suspected or confirmed (Fig. 1).33
Although no strong evidence supporting the use of mechanical compression devices in COVID-19 patients exists, they are still considered for patients with contraindications to pharmacological prophylaxis. They may be utilized in sedentary as well as ambulatory patients during periods of sleep or bedrest. Use of concurrent pharmacological and mechanical prophylaxis should be avoided.33
The diagnosis and management of VTE in patients with COVID-19 presents a unique challenge as clinicians try to weigh the risk for staff exposure with the benefit of a given test. Furthermore, imaging may not be available or may be difficult to obtain in unstable or critically ill patients. For low risk patients, obtaining imaging may not be worth the risk of transmitting the infection.
For patients with low/no suspicion for VTE (Wells score of 0 for DVT or <2 for PE), we recommend regular dose prophylaxis. Imaging can be considered if d-dimer is elevated (>500 ng/mL) based on risk and benefit.
For patients with moderate suspicion for VTE (Wells score of 1–2 for DVT or 2–6 for PE), we would consider pursuing imaging if available. If imaging is negative, we recommend regular dose pharmacological prophylaxis. If imaging is not available and bleeding risk is high (IMPROVE-bleeding score ≥7), we recommend regular dose pharmacological prophylaxis. If imaging is not available and bleeding risk is low (IMPROVE-bleeding score <7), we consider anticoagulation following the VTE therapeutic dosing protocols. If subsequent imaging becomes available and result is negative, consider extended outpatient prophylaxis up to 30 days. If VTE is confirmed or imaging is still unavailable, we recommend oral anticoagulation for 3 months. We favor direct oral anticoagulants (DOAC) for both VTE prophylaxis and management.
For patients with high suspicion of VTE (Wells score of >2 for DVT or >6 for PE or high right ventricle pressure on echocardiogram, if available), we recommend pursuing imaging. If imaging results are negative, we recommend regular dose pharmacological prophylaxis. If imaging is not available and bleeding risk is high (IMPROVE-bleeding risk score ≥7), we consider anticoagulation using the therapeutic high bleeding risk protocol. If imaging is not available and bleeding risk is low (IMPROVE-bleeding risk score <7), we recommend anticoagulation following the VTE therapeutic dosing protocols. If subsequent imaging is negative, we consider extended outpatient prophylaxis up to 30 days. If VTE is confirmed or imaging is still unavailable, we recommend oral anticoagulation for 3 months. If possible, we still favor DOACs over other anticoagulation therapies.
Please refer to Figure 1 for more information regarding anticoagulation agents and dosing.
For therapeutic dosing protocol, we favor using enoxaparin 1 mg/kg twice daily over unfractionated heparin (UFH) to eliminate the need for active pro-thrombin time (aPTT) monitoring that can be affected by viral infection and increase risk for exposure. Furthermore, low molecular weight heparin (LMWH) has a lower risk of heparin-induced thrombocytopenia than UFH. The protocol for therapeutic high bleeding risk includes the use of UFH aiming for an aPTT target of 74–89 sec. For patients unable to tolerate anticoagulation, we suggest early ambulation and using pneumatic compression. Consider a pharmacy managed protocol if a patient is morbidly obese or has renal impairment. Transition from LMWH/UFH to warfarin may be challenging due to restricted access to outpatient care for frequent monitoring. DOACs may be considered in patients who do not require procedures. Further research is needed to standardize management recommendations for patients with VTE in the era of COVID-19.
Out of the Hospital Deep Vein Thrombosis Prophylaxis for Patients with COVID-19 Infection
Another important question is how to manage thrombotic risk in patients with COVID-19 not requiring hospitalization or have been discharged from the hospital or emergency department. At this point, a wide variety of practices exist for outpatient prophylaxis in medically ill patients (Fig. 2).33
In major trials supporting VTE prophylaxis in medically ill patients, 6–14 days has been used as the standard duration for prophylaxis.34 In practice, average hospitalization duration is 3–5 days, and post discharge VTE prophylaxis is uncommonly used. It is well established that significant VTE risk exists after discharge from the hospital.35 Extended VTE prophylaxis after discharge has been studied using different anticoagulants including enoxaparin and DOACs yielding different outcomes.33
The extended prophylaxis for VTE in acutely ill medical patients with prolonged immobilization (Extended Prophylaxis for Venous Thromboembolism in Acutely Ill Medical Patients (EXCLAIM)) trial evaluated the role of extended duration of anticoagulation (28 ± 4 days) with enoxaparin versus placebo after an initial enoxaparin administration for 6–14 days. In a subgroup of patients who were female, older than 75 years of age, and immobile, extended prophylaxis with enoxaparin demonstrated 37% relative risk reduction in symptomatic VTE or VTE-related mortality with the expense of increased major bleeding.36
In the Multi-center, Randomized, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalized Acutely Ill Medical Patients Comparing Rivaroxaban with Enoxaparin (MAGELLAN) trial, rivaroxaban 10 mg for 10 (noninferiority analysis) or 35 ± 4 days (superiority analysis) was compared with enoxaparin 40 mg once daily for 10 ± 4 days followed by placebo. Although rivaroxaban was noninferior at 10 days, it was associated with 23% relative risk reduction of primary outcomes (asymptomatic proximal or symptomatic VTE) at 35 ± 4 days, albeit at slightly increased major bleeding risk. However, the significant difference in major bleeding between the 2 groups was mitigated by excluding patients at high risk.37
Due to heightened concern of thromboembolic events in these patients, we suggest extended thromboprophylaxis (preferably with rivaroxaban 10 mg daily up to 39 days) after discharge as long as bleeding risk is low.
Most studies looking at thromboprophylaxis in outpatient settings involved patients with cancer.38, 39, 40 In a study evaluating rivaroxaban in ambulatory patients with cancer receiving systemic therapy, there was no significant reduction of thromboembolism.41 A similar study with apixaban showed significant reduction of VTEs (number needed to treat-17) albeit with increased risk for bleeding (number needed to harm-59).42 The cumulative analysis of these 2 studies showed reduced VTE rate with acceptable bleeding risk in these patients.
Deep Vein Thrombosis Prophylaxis Algorithm for Patients with Suspected or Confirmed COVID-19 after Discharged from the Hospital
For patients discharged from the hospital without need for additional support, if bleeding risk is high (IMPROVE-bleeding score ≥7), we recommend nonpharmacological VTE prophylaxis. However, if bleeding risk is low (IMPROVE-bleeding score <7), we consider extended pharmacological VTE prophylaxis favoring rivaroxaban 10 mg daily for 30 days over enoxaparin or UFH.
For patients discharged from the hospital requiring additional support (rehab unit), if bleeding risk is high (IMPROVE-bleeding score ≥7), we recommend nonpharmacological VTE prophylaxis. However, if bleeding risk is low (IMPROVE-bleeding score <7), we recommend extended pharmacological VTE prophylaxis favoring rivaroxaban 10 mg daily for 30 days over enoxaparin or UFH.
Deep Vein Thrombosis Prophylaxis Algorithm for Patients with Suspected or Confirmed COVID-19 after Discharged from Observation Unit, Emergency Department or Outpatient Clinic
For patients assigned to home hospital care, if bleeding risk is low (IMPROVE-bleeding score <7), we recommend extended pharmacological VTE prophylaxis favoring rivaroxaban over enoxaparin or UFH. If bleeding risk is high (IMPROVE-bleeding score ≥7), we recommend nonpharmacological VTE prophylaxis.
If patients were discharged home without additional support, we recommend nonpharmacological VTE prophylaxis regardless of bleeding risk.
For further information regarding medication options and dosing please refer to Figure 2.
Nonpharmacological measures include smoking cessation and early ambulation. Compression therapy can be considered if skin is intact.
Guideline Recommendations
There have been limited guideline recommendations published by societal guidelines addressing the management of VTE in patients with COVID-19.
American College of Cardiology Guidelines
It is reasonable to employ individualized risk stratification based on thrombotic and hemorrhagic risk. Extended prophylaxis (for up to 45 days) may be considered for patients with increased risk for VTE (e.g., reduced mobility, active cancer, d-dimer >2 × upper limit of normal) and low risk for bleeding.20
Anticoagulation Forum
Extended VTE prophylaxis is not necessary for all patients with COVID-19 who are being discharged from the hospital. Post-hospital VTE prophylaxis in patients with COVID-19 may be considered on a case-by-case basis for those at low bleeding risk (IMPROVE-bleeding score <7).43
CHEST Guidelines
Thromboprophylaxis is recommended only during hospitalization. Extended thromboprophylaxis can be considered based on the net benefit determined by thrombotic and bleeding risk.44
National Institute of Health Guidelines
Anticoagulation and antiplatelet therapy should not be initiated for venous or arterial thrombosis unless there are other indications. There are currently insufficient data to recommend for or against routine DVT screening in COVID-19 patients without signs or symptoms of VTE.45
Conclusion
Although a significant VTE risk has been confirmed in patients with COVID-19, literature addressing best ways to mitigate this risk is lacking. Furthermore, there has been very limited guidance provided by societal guidelines to help prevent and manage VTE associated with the COVID-19 infection. We believe that this literature summary along with our center recommendations and algorithms provide valuable guidance to providers caring for these patients. More research is needed to standardize prophylaxis and management protocols for patients with COVID-19 related VTE.
Footnotes
Disclosures: None.
Funding: None.
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Available at:
- 3.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020;383:993–994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [published correction appears in Lancet. 2020 May 30;395(10238):1694] Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joyner M.J., Wright R.S., Fairweather D., et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US F.D.A. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and Available at:
- 9.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization https://www.who.int/publications-detail/an-international-randomised-trial-of-candidate-vaccines-against-covid-19 Available at:
- 11.Elias P., Khanna R., Dudley A., et al. Automating venous thromboembolism risk calculation using electronic health record data upon hospital admission: the automated Padua prediction score. J Hosp Med. 2017;12:231–237. doi: 10.12788/jhm.2714. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A.T., Alikhan R., Arcelus J.I., et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost. 2005;94:750–759. [PubMed] [Google Scholar]
- 13.Alikhan R., Cohen A.T., Combe S., et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963–968. doi: 10.1001/archinte.164.9.963. [DOI] [PubMed] [Google Scholar]
- 14.Dentali F., Douketis J.D., Gianni M., et al. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 15.Germini F., Agnelli G., Fedele M., et al. Padua prediction score or clinical judgment for decision making on antithrombotic prophylaxis: a quasi-randomized controlled trial. J Thromb Thrombolysis. 2016;42:336–339. doi: 10.1007/s11239-016-1358-z. [DOI] [PubMed] [Google Scholar]
- 16.Poggiali E., Bastoni D., Ioannilli E., et al. Deep vein thrombosis and pulmonary embolism: two complications of COVID-19 pneumonia? Eur J Case Rep Intern Med. 2020;7:001646. doi: 10.12890/2020_001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui S., Chen S., Li X., et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020 doi: 10.7326/M20-2566. M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skeik N., Mirza A., Manunga J. Management of venous thromboembolism during the COVID-19 pandemic. J Vasc Surg Venous Lymphat Disord. 2020;8:897–898. doi: 10.1016/j.jvsv.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 Available at:
- 23.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y., Li T., Han M., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Xiao M., Zhang S., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Feng X., Zhang D., et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome [published correction appears in Circulation. 2020 Jul 14;142(2):e33] Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 28.Kort D., van Rein N., van der Meer F.J., et al. Relationship between neighborhood socioeconomic status and venous thromboembolism: results from a population-based study. J Thromb Haemost. 2017;15:2352–2360. doi: 10.1111/jth.13868. [DOI] [PubMed] [Google Scholar]
- 29.Isma N., Merlo J., Ohlsson H., et al. Socioeconomic factors and concomitant diseases are related to the risk for venous thromboembolism during long time follow-up. J Thromb Thrombolysis. 2013;36:58–64. doi: 10.1007/s11239-012-0858-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang T., Chen R., Liu C., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang N., Bai H., Chen X., et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skeik N., Westergard E. Recommendations for VTE prophylaxis in medically ill patients. Ann Vasc Dis. 2020;13:38–44. doi: 10.3400/avd.ra.19-00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobesh P. The importance of appropriate prophylaxis for the prevention of venous thromboembolism in at-risk medical patients. Int J Clin Pract. 2010;64:1554–1562. doi: 10.1111/j.1742-1241.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 35.Amin A.N., Varker H., Princic N., et al. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med. 2012;7:231–238. doi: 10.1002/jhm.1002. [DOI] [PubMed] [Google Scholar]
- 36.Hull R.D., Schellong S.M., Tapson V.F., et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. [DOI] [PubMed] [Google Scholar]
- 37.Cohen A.T., Spiro T.E., Büller H.R., et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 38.Austin K., George J., Robinson E.J., et al. Retrospective cohort study of venous thromboembolism rates in ambulatory cancer patients: association with Khorana score and other risk factors. J Hematol. 2019;8:17–25. doi: 10.14740/jh471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes C.E., Ades S., Gilchrist S., et al. Successful model for guideline implementation to prevent cancer-associated thrombosis: venous thromboembolism prevention in the ambulatory cancer clinic. JCO Oncol Pract. 2020;16:e868–e874. doi: 10.1200/JOP.19.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cella C.A., Di Minno G., Carlomagno C., et al. Preventing venous thromboembolism in ambulatory cancer patients: the ONKOTEV study. Oncologist. 2017;22:601–608. doi: 10.1634/theoncologist.2016-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khorana A.A., Soff G.A., Kakkar A.K., et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 42.Carrier M., Abou-Nassar K., Mallick R., et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 43.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moores L.K., Tritschler T., Brosnahan S., et al. Prevention, diagnosis, and treatment of VTE in patients with COVID-19: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.COVID-19 Treatment Guidelines Panel Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ Available at: [PubMed]