Background
Since the onset of the COVID-19 pandemic, it has become evident that, although the respiratory disease is the principal manifestation of this viral infection, COVID-19 presents features of a multisystem syndrome (Remmelink et al., 2020); in this setting, neurologic complications have arisen as an increasingly recognized cause of morbidity and mortality (Mao et al., 2020). These complications include acute cerebrovascular disease (i.e. either ischemic or haemorrhagic strokes), encephalopathy, seizures and/or delirium (Ellul et al., 2020). Besides the complex pathophysiology behind the “neurotropism” of SARS-CoV-2, tissue hypoxia due to respiratory and/or cardiovascular failure, neuroinflammation as a response to systemic organ damage and inflammatory response or a disseminated prothrombotic status with micro-thrombosis are also involved (Remmelink et al., 2020, Pezzini and Padovani, 2020). Therefore, monitor cerebral function in COVID-19 patients appears to be necessary.
Initial assessment
Nurses can grossly assess the neurological status at the bedside using different scales (i.e. Glasgow Coma Scale, GCS; Confusion Assessment Method-Intensive Care Unit, CAM-ICU); moreover, searching for focal motor or sensitive deficit as well as for brainstem impairment (i.e. abnormal pupils or eye movements, absence of cough, irregular breathing) should be routinely performed, although it remains a challenging issue due to the high prevalence of sedated and paralyzed patients. In patients presenting these abnormalities or in those with prolonged altered consciousness despite withdrawal of sedative agents, neuro-imaging should be discussed at the medical round. If cerebral computed tomography (CT) scan detects acute cerebrovascular events (i.e. acute ischemic stroke, intracranial haemorrhage), the use of contrast medium injection for a CT-Angiography (i.e. to detect acute vascular occlusion or cerebral venous thrombosis) or for a CT-Perfusion (i.e. to assess salvageable brain areas in case of acute ischemic stroke) can also be considered; potential contra-indications for these imaging techniques (i.e. contrast allergy or renal failure) should be carefully considered. Magnetic resonance imaging (MRI) is more sensitive to detect brain abnormalities compatible with encephalitis, necrotizing encephalopathy, microbleeds or hypoxic injury in COVID-19 patients (Kremer et al., 2020); however, the patients should not be agitated (i.e. to obtain good quality images) and should tolerate supine position (i.e. it would be difficult to perform MRI in a patient dependent from prone positioning). Finally, contraindications (i.e. metallic materials or ECMO) should be previously excluded. As imaging techniques are not bedside monitoring and require transport of at-risk patients, different non-invasive monitoring techniques can help to refine patients’ evaluation and decide for the optimal timing of CT/MRI.
How nurses can adequately use neuromonitoring at the bedside?
Altered cerebral perfusion can occur during critical illness, also in COVID-19 patients (Sonkaya et al., 2020). The use of cerebral ultrasound could provide an estimation of intracranial pressure (eICP) and cerebral perfusion pressure (eCPP), using a 2-MHz probe to assess mean middle cerebral artery flow velocities (mFV), the corresponding value of mean arterial pressure (MAP) and the following formula: eCPP = MAP × diastolic FV/mFV + 14. In a recent study, high eICP was observed in COVID-19 patients developing neurological complications when compared to others (Battaglini et al., 2020). Although cerebral ultrasound is generally performed by doctors, the training is suitable for anyone, including nurses and technologists, requires few sessions to measure mFV and no prior experience is needed. Together with cerebral ultrasound, non-invasive assessment of brain oxygenation using near-infrared spectroscopy (NIRS) may provide measurement of the prefrontal cortex oxygen saturation (rSO2). In new generation devices, rSO2 < 60% would suggest tissue hypoperfusion; however, contamination from extra-cerebral blood oxygenation is possible and no studies on cerebral oximetry in COVID-19 patients have been reported so far.
Continuous electroencephalography (cEEG) is a non-invasive tool that measures cortical electrical activity, which is generally evaluated by a neurophysiologist. As COVID-19 patients may develop seizures (Antony and Haneef, 2020), the use of quantitative EEG (qEEG), which displays a time-compressed simplified visual display of raw EEG data, could be used by trained nurses to accurately detect recurrent seizure, with a sensitivity and specificity > 85% when compared to experienced neurophysiologists (Kang et al., 2019). Moreover, qEEG can provide information on the frequency and amplitude of EEG background; alterations in some of the EEG-derived parameters (i.e. low spectral edge frequency – SEF; low bispectral index = BIS; low Patient State Index – PSI; high burst-suppression rate - BSR) may therefore avoid over-sedation at the bedside or, in the cases of persistent altered values after interruption of sedation, suggest the presence of diffuse hypoxic injury, COVID-19 related encephalitis or a metabolic encephalopathy in these patients (Sangare et al., 2020, Skorin et al., 2020). Automated pupillometry is a bedside technique able to precisely quantify the pupillary size and pupillary light reflex (PLR). Change in pupillary activity showed a good accuracy to predict delirium in ventilated critically ill patients and to detect unreactive EEG background (Favre et al., 2020, Hasan et al., 2019). As such, automated pupillometry could be also used to titrate the administration of anaesthetics or analgesics, as excessive drug regimens would provide reduced PLR or size, respectively, or identify patients at risk of delirium (i.e. PLR < 25%). Finally, high levels of serum biomarkers for brain injury, such as neuron specific enolase (NSE) and protein S-100β, have reported in COVID-19 patients (DeKosky et al., 2020); their daily measurement could be discussed then at the medical round by the nursing team in patients with suspected brain injury if not prescribed by the attending doctors.
Conclusions
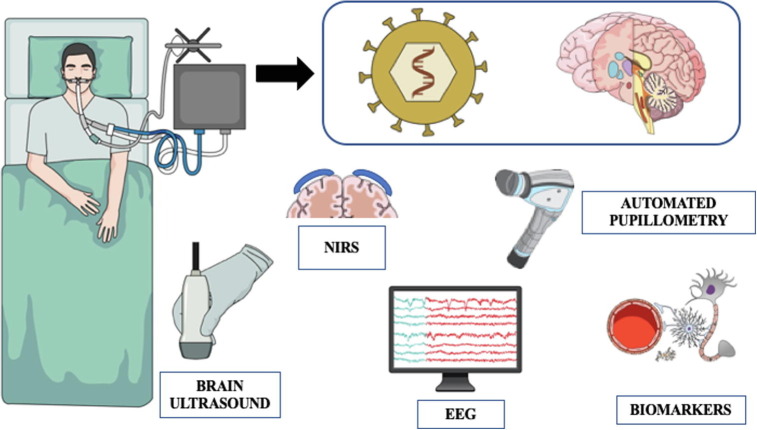
Due to the complexity of neurological complications in COVID-19 patients, the role of nurses in routine and repeated clinical examination is mandatory. Neuro-imaging techniques can provide relevant diagnostic information and should be discussed on a daily basis. As clinical examination can have a limited role in critically ill COVID-19 ventilated patients, a multimodal neuromonitoring approach would be recommended (Fig. 1 ). Trained nurses can interpret most of the findings from different monitoring tools and should integrate these observations to the ongoing therapies and the discussion at the medical round in order to assess their clinical relevance. Future studies should address the therapeutic and prognostic role of this monitoring strategy in COVID-19 patients, to support its potential routine use in this setting.
Fig. 1.
A multimodal approach for neuromonitoring in COVID-19 patients. NIRS = near-infrared spectroscopy; EEG = electroencephalography.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antony A.R., Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglini D., Santori G., Chandraptham K. Neurological complications and noninvasive multimodal neuromonitoring in critically ill COVID-19 patients. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.602114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S.T., Kochanek P.M. Blood biomarkers for detection of brain Injury in COVID -19 patients. J. Neurotrauma. 2020 doi: 10.1089/neu.2020.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre E., Bernini A., Morelli P. Neuromonitoring of delirium with quantitative pupillometry In sedated mechanically ventilated critically ill patients. Crit. Care. 2020;24:66. doi: 10.1186/s13054-020-2796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S., Peluso L., Ferlini L., Legros B. Correlation between electroencephalography an automated pupillometry in critically Ill patients: a pilot study. J Neurosurg. Anesthesiol. 2019 doi: 10.1097/ANA.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Sherill G.C., Sinha S.R., Swisher C.B. A trial of real-time electrographic seizure detection by neuro-ICU nurses using a panel of quantitative EEG trends. Neurocrit. Care. 2019;31(2):312–320. doi: 10.1007/s12028-019-00673-z. [DOI] [PubMed] [Google Scholar]
- Kremer S., Lersy F., Anheim M., Merdji H., Schenck M., Oesterlé H., Bolognini F., Messie J., Khalil A., Gaudemer A., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Jager L., Nesser P., Mba Y.T., Hmeydia G., Benzakoun J., Oppenheim C., Ferré J.-C., Maamar A., Carsin-Nicol B., Comby P.-O., Ricolfi F., Thouant P., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Constans J.-M., Zorn P.-E., Mathieu M., Baloglu S., Ardellier F.-D., Willaume T., Brisset J.-C., Caillard S., Collange O., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., Meyer N., Helms J., Cotton F. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology. 2020;95(13):e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y.u., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B.O. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmelink M., De Mendonça R., D’Haene N., De Clercq S., Verocq C., Lebrun L., Lavis P., Racu M.-L., Trépant A.-L., Maris C., Rorive S., Goffard J.-C., De Witte O., Peluso L., Vincent J.-L., Decaestecker C., Taccone F.S., Salmon I. Unspecific post-mortem findings despite Multiorgan viral spread in COVID-19 patients. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare A., Dong A., Valente M., Pyatigorskaya N., Cao A., Altmayer V., Zyss J., Lambrecq V., Roux D., Morlon Q., Perez P., Ben Salah A., Virolle S., Puybasset L., Sitt J.D., Rohaut B., Naccache L. Neuroprognostication of consciousness recovery in a patient with COVID-19 related encephalitis: preliminary findings from a multimodal approach. Brain Sci. 2020;10(11):845. doi: 10.3390/brainsci10110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorin I., Carrillo R., Perez C.P. EEG findings and clinical prognostic factors associated with Mortality in a prospective cohort of inpatients with COVID-19. Seizure. 2020;83:1–4. doi: 10.1016/j.seizure.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkaya A.R., ÖztÜrk B., KaradaŞ Ö. Cerebral hemodynamic alterations in patients with Covid-19. Turk. J. Med. Sci. 2020 doi: 10.3906/sag-2006-203. [DOI] [PMC free article] [PubMed] [Google Scholar]