Abstract
The 2019 coronavirus disease (COVID-19) has become a global pandemic. Several studies report that ABO blood group polymorphism may be related to COVID-19 susceptibility and clinical outcomes; however, the results are controversial. We conducted a systematic review and meta-analysis to investigate whether ABO blood groups are associated with increased COVID-19 morbidity and mortality. A total of 715 articles were retrieved from seven databases. Ten articles were selected for meta-analysis after removal of duplicates and two levels of screenings. Overall, individuals with blood group A [odds ratio (OR) = 1.33, 95% confidence interval (CI) 1.14 to 1.56] and B (OR = 1.06, 95% CI 1.00 to 1.13) had a substantially higher risk of COVID-19, whereas this was not the case for blood group AB (OR = 1.07, 95% CI 0.88 to 1.30). Individuals with blood group O was not prone to develop the disease (OR = 0.71, 95% CI 0.60 to 0.84). Moreover, the risk of COVID-19 was significantly associated with the Rh-positive blood group (OR = 1.22, 95% CI 0.99 to 1.50). A meta-analysis of 5 studies suggested that blood group A was associated with a significantly increased risk of COVID-19 mortality (OR = 1.25, 95% CI 1.02 to 1.52). Mild publication bias was found in the included studies. This systematic review and meta-analysis indicated that blood groups A and B may be risk factors for COVID-19, whereas the blood group O appears to be protective. Blood group A may be related to unfavourable outcomes. Further rigorous and high-quality research evidence is needed to confirm this association.
Keywords: ABO blood group, 2019 coronavirus disease, Anti-A antibody, Virus
1. Introduction
The 2019 coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first broke out in Wuhan, China, at the end of December 2019 [1]. On March 11, 2020, the World Health Organization declared COVID-19 infection a global pandemic [2]. As of June 24, 2020, it has affected more than 200 countries around the world, resulting in a global burden of over 10.3 million cases and a death toll of more than 506,000 [3]. SARS-COV-2 is a β-coronavirus that is highly homologous to SARS-CoV and uses angiotensin-converting enzyme 2 (ACE2) during transmission [4]. Epidemiology has revealed risk factors for SARS-CoV-2 susceptibility, such as age, gender, and chronic disease [5].
The ABO blood group system was the first human blood group discovered in 1901 [6]. Since then, studies on the relationship between the ABO blood group system and various diseases have never ceased, because it is inherent in humans and easily determinable. ABO blood groups are statistically or biologically related to many chronic diseases, such as vascular disease, coronary heart disease, and tumourigenesis [7]. In recent years, studies of the association between blood groups and certain viral infections have attracted increasing attention. Investigating the contribution of different blood groups to viral infection may be beneficial in determining an individual's susceptibility to a virus. Previous work has determined an association between ABO blood groups and host susceptibility to infectious diseases, including SARS-CoV [8], malignant tumours [9], Helicobacter pylori [10], Norwalk virus [11], and hepatitis B virus [7]. In particular, the association between ABO blood groups and SARS-CoV directly prompted the assumption of a similar susceptibility to COVID-19.
Recently, epidemiological studies have reported that blood group is strongly statistically associated not only with acquisition of SARS-CoV-2 but also with survival following infection [12]. However, there have been conflicting results due to multiple confounding effects. Li et al. [13] compared blood groups between COVID-19 patients and the general population and found that the probability of COVID-19 positivity in blood group A was increased compared with that in the general population, while the probability of COVID-19 positivity in blood group O was decreased. However, Szmuness et al. [14] failed to confirm this association. Thus, controversy remains with respect to whether blood group is related to COVID-19 infection and which antigen is a protective or risk factor. We performed a systematic review and meta-analysis to elucidate the association between ABO blood group and increased COVID-19 morbidity and mortality.
2. Methods
The protocol was registered in PROSPERO (registration number: CRD42020195615). Preferred Reporting Item for Systematic Review and Meta-analysis Protocol (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) recommendations were used to guide this review [15,16].
2.1. Data sources and search strategy
Two independent reviewers (NL and TZ) searched the databases of the China Biology Medicine disc, China National Knowledge Infrastructure, China Science and Technology Periodical Database, Wanfang Database, PubMed, Embase, and Web of Science from the date of inception to June 30, 2020. We used MeSH/Emtree terms combining free-text words, such as ABO blood types (groups), blood group antigens, novel coronavirus-infected pneumonia, COVID-19, and SARS-CoV-2, which were properly adjusted for the different databases. The search words in the Chinese databases were a translation of the above words. We limited the search language to English and Chinese, with no restrictions on country or publication status. To ensure a comprehensive search, the latest research references were manually screened to identify qualified studies.
2.2. Inclusion and exclusion criteria
Inclusion criteria: 1) case-control and cohort studies were included; 2) data relating to ABO blood group distribution, the number of COVID-19 infected and uninfected subjects, and deaths were extracted. Exclusion criteria: 1) results that could not be pooled through calculation; 2) case reports, case series, duplicate reports and in vitro and animal studies; 3) the full text of the study was not be available; 4) the study was not relevant to the subject.
2.3. Study selection
Studies were independently identified by two reviewers (WW and HW). After removal of the duplicates, the two reviewers assessed the studies according to the eligibility criteria by reading the title and abstract. Controversial literature was confirmed by discussion of the two reviewers. A third reviewer (HL) assisted if they were unable to reach an agreement.
2.4. Data extraction and quality assessment
To ensure the completeness and consistency of the data, two independent reviewers (LM and WW) extracted data from the eligible studies using a predesigned template. The template included the following items: general information (first author, corresponding author, contact information, journal, year of publication, country/region, funding source), characteristics of participants (age, gender, race, education level, disease stage, and severity), characteristics of the study (sample size, study design, follow-up time), exposure factor (ABO blood group distribution), and outcomes (morbidity and mortality). Disagreements were resolved by consensus or through consultation with the third reviewer (HL). The Newcastle-Ottawa Scale (NOS) recommended by the Cochrane Collaboration was used to evaluate the methodological quality of the included studies [17]. Content was evaluated on the basis of three parameters: selection, comparability, and exposure/outcome. Quality assessments were performed by two researchers (NL and WW), and if any discrepancies existed, the two authors resolved the issue through discussion to reach a consensus.
2.5. Statistical analysis
Review Manager software (version 5.3.5) and Stata software (version 14.0) were used for statistical analysis. The Mantel-Haenszel model was utilized for dichotomous variables to calculate the odds ratio (OR) and the corresponding 95% confidence interval (CI) to assess the association between ABO blood groups and COVID-19 infection status. Relative risk was measured as the OR in all studies and prioritized the adjusted OR. Heterogeneity between included studies was assessed using the I 2 statistic and P values. I 2 ≤ 50% was considered to indicate little heterogeneity; I 2 > 50% was considered to indicate substantial heterogeneity [18]. A random or fixed-effect model was carried out to assess the data, but only the random effect analyses were reported when the heterogeneity was significant and could not be explained. Publication bias was assessed by using a funnel plot and Egger's regression test. Sensitivity analysis of the primary endpoint was conducted by sequential removal of each trial to assess the impact of individual studies on overall pooled estimates. Forest plots were generated to indicate the pooled results. Subgroup analysis was performed based on pre-set variables (country, race, and study design). According to the main ethnic groups, the study is divided into Caucasians and Asians. A value of P < 0.05 was deemed to be statistically significant.
3. Results
3.1. Literature search
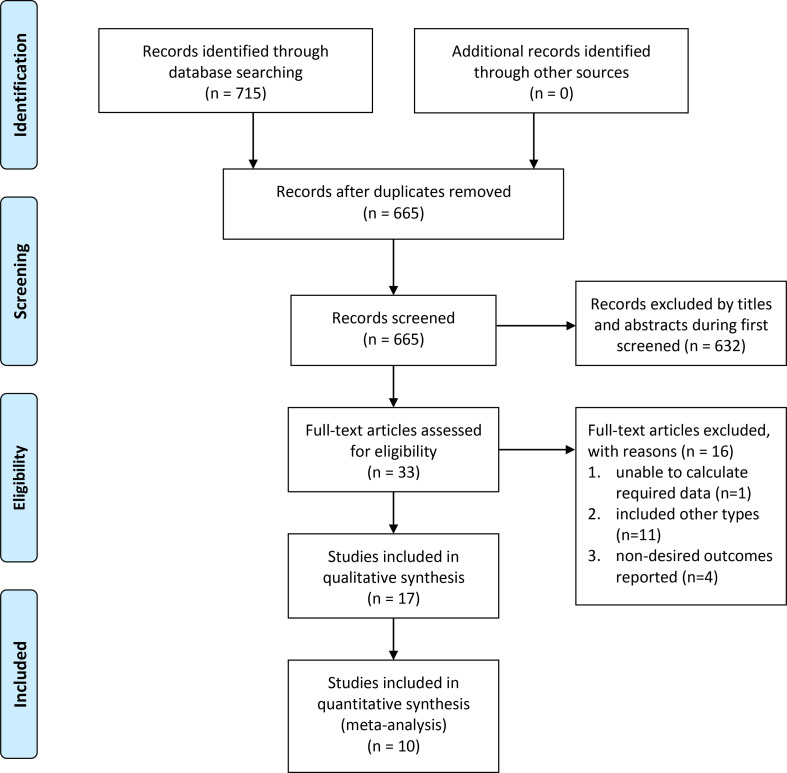
A comprehensive search yielded 715 potentially relevant studies from all databases, of which 50 were excluded after removal of the duplicates. The remaining 665 records were filtered based on the title and abstract, of which 632 were excluded because they addressed unrelated topics. We reviewed the full text of the remaining 33 studies and identified 10 studies that met the inclusion criteria of the meta-analysis: 8 case-control studies [14,[19], [20], [21], [22], [23], [24], [25]] and 2 cohort study [13,26]. The detailed search process is demonstrated in Fig. 1 .
Fig. 1.
Study selection process for the meta-analysis.
3.2. Study characteristics and quality assessment
The 10 studies were published in 2020. Concerning the countries where these studies were performed, 2 were conducted in the United States [14,21], 4 in Europe [20,[24], [25], [26]], and 4 in China [13,19,22,23]. A total of 54,218 subjects were included, with 9383 COVID-19-infected subjects and 44,835 uninfected subjects. The majority of the studies included adults between 13 and 80 years of age. Most of the COVID-19 diagnoses were confirmed by a positive RT-PCR test using nasal and pharyngeal swab specimens. Most participants in the control group were healthy and were blood donors. All studies reported the distribution of the 4 blood groups. Table 1 lists the basic characteristics of the included studies.
Table 1.
Summary characteristics of studies included in the meta-analysis.
| Study year, | Country | Study design | Sample size (+/−) | Age | Gender | Patients | Controls | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Juyi Li 2020 [13] |
China | Retrospective cohort study |
2153/3694 | Case: less than 40 years (n = 342), aged 41–59 years (n = 784), over 60 years (n = 1027); control: NR | Case: male (53.09%) control: NR |
Patients with COVID-19 | The healthy general population | Relationship between ABO blood group and susceptibility, and mortality of COVID-19 patients |
| Sunny Dzik 2020 [14] |
United States | Case-control study | 957/5840 | NR | NR | Patients with COVID-19 infection confirmed by nasal swab PCR. | Patients who were hospitalized without COVID-19 | Relationship between ABO blood group and susceptibility, and mortality of COVID-19 patients |
| Kai Duan 2020 [22] | China | Case-control study | 150/180 | Aged 18–60 years | Case: male (49.33%) control: NR |
COVID-19 survivors. | Blood donors without COVID-19 | Relationship between ABO blood group and susceptibility, and mortality of COVID-19 patients |
| Yuqin Wu 2020 [19] | China | Case-control study | 187/1991 | Case: over 40 years (n = 116), less than 40 years (n = 69); control: NR | Case: male (51.87%) control: NR |
Patients with COVID-19. | The healthy general population | Relationship between ABO blood group and susceptibility of COVID-19 patients |
| Michael Zietz [21] 2020 |
United States | Case-control study | 682/877 | NR | NR | Individuals with a single positive SARS-CoV-2 lab test are considered COVID-19 positive, even if they had previous or subsequent negative tests. | Participants in the same cohort who tested negative for COVID-19 | Morbidity and clinical outcome (intubation or death) |
| Hakan GÖKER 2020 [20] |
Turkish | Case-control study | 186/1882 | Case:42 (19–92) control: NR |
Case: male (53.76%) control: NR |
The 56 COVID-19 infection who were positive for the SARS-CoV-2 RNA test through PCR 57 from the nasopharyngeal swab. | The healthy general population | Relationship between ABO blood group and susceptibility of COVID-19 patients |
| David Ellinghaus 2020 [25] |
Italian and Spanish | Case-control study | 1610/2205 | NR | NR | Patients defined as hospitalization with respiratory failure and a confirmed SARS-CoV-2 viral RNA PCR test from nasopharyngeal swabs or other relevant biologic fluids. | Blood donors without COVID-19 | Morbidity and genomewide analysis |
| Jiao Zhao 2020 [23] | China | Case-control study | 2173/27080 | NR | NR | Patient diagnosis of COVID-19 was confirmed by a positive RT-PCR test on nasal and pharyngeal swab specimens. | The healthy general population | Relationship between ABO blood group and susceptibility, and mortality of COVID-19 patients |
| Marion Kibler 2020 [24] |
France | Case-control study | 22/680 | Mean age:82 ± 6.9 | Male (44.59%) | Patients were considered as COVID-19 in presence of positive RT-PCR testing of a nasopharyngeal swab specimen or with typical symptoms and characteristic imaging findings on chest computed tomography. | Participants in the same cohort who tested negative for COVID-19 | Relationship between blood groups and ARDS, AKI, and mortality, in addition to susceptibility in COVID-19 patients. |
| Boudin 2020 [26] |
France | Retrospective cohort study | 1263/406 | Case: 28 (23–36) a control: 27 (23−33) a |
Male (87%) | SARS-CoV-2–infected subjects were defined as at least one positive RT-PCR (confirmed) and/or crewmembers with clinical symptoms highly suggestive of COVID-19 in this epidemiological context (fever, myalgias, arthralgias, dyspnea, cough, headache, anosmia, ageusia, rhinitis, diarrhea, fatigue, cutaneous signs) |
Participants in the same cohort who tested negative for COVID-19 and no clinical signs. | Relationship between ABO blood groups and SARS-CoV-2 infection |
RT-(PCR) = real-time (polymerase-chain-reaction); COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; +/− = COVID-19 positive / COVID-19 negative; a = Median age (IQR); NR = not report.
Of the 10 qualified studies, 6 were considered to have high methodological quality (score ≥ 7), while 4 studies were of medium quality (score 4–6). The scores ranged from 4 to 9, with an average score of 7, which indicated that the included studies were generally of high quality ( Supplementary Table S1 ).
3.3. Association between blood group A and COVID-19 infection
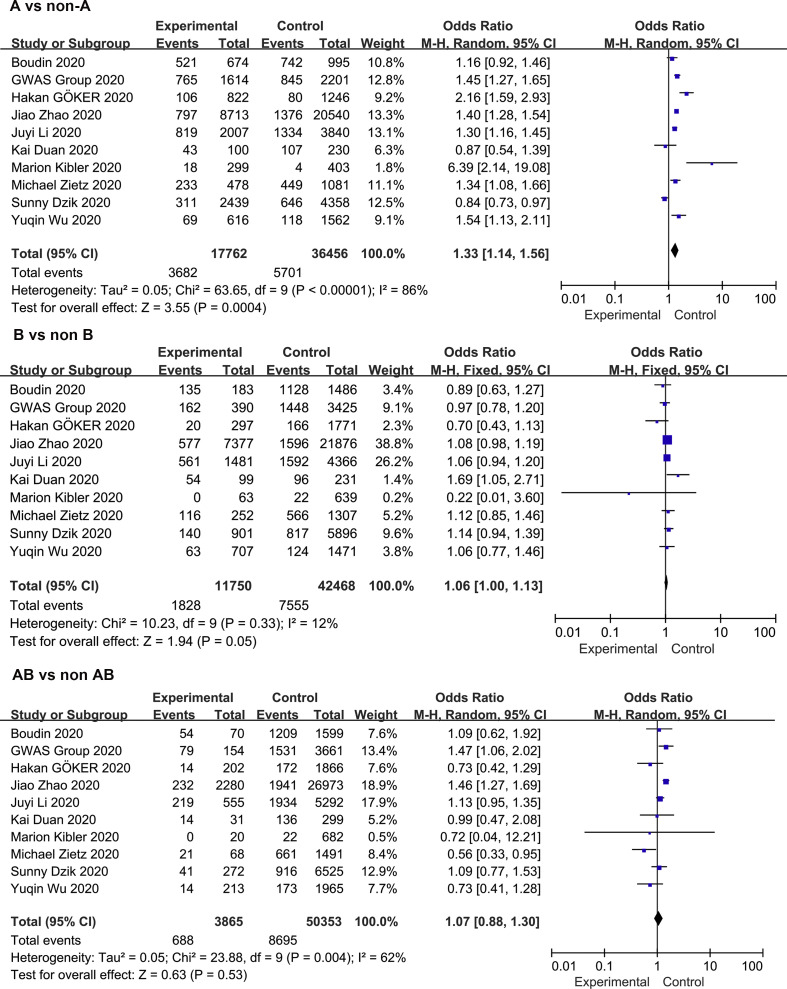
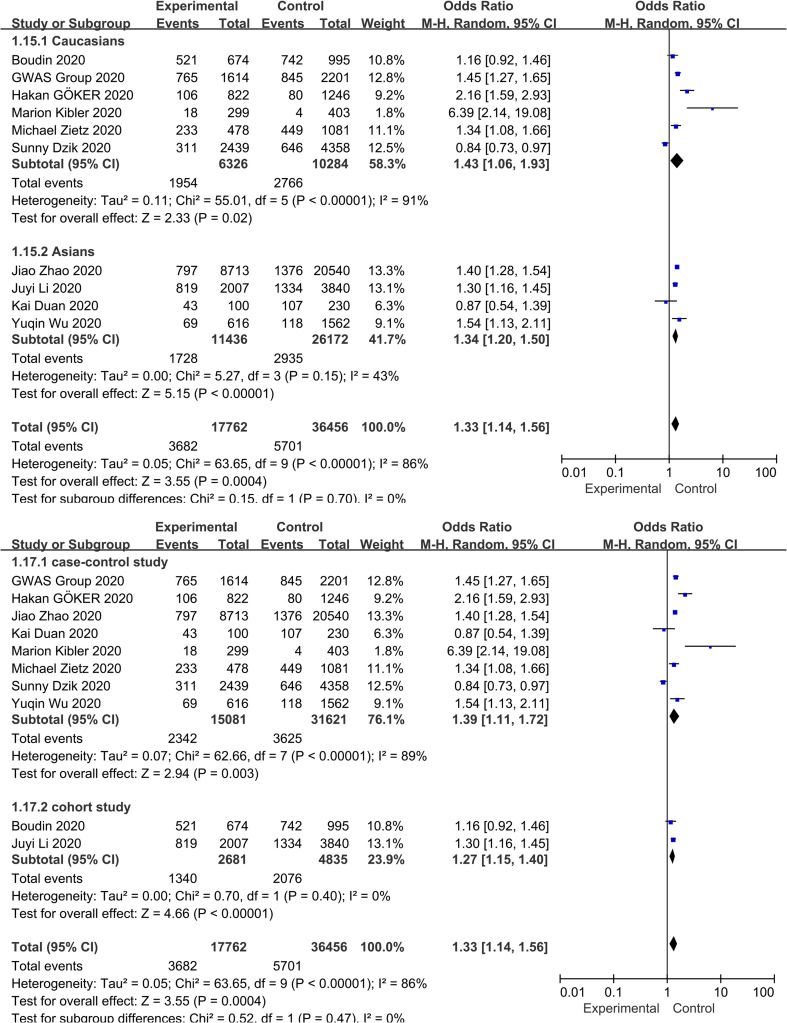
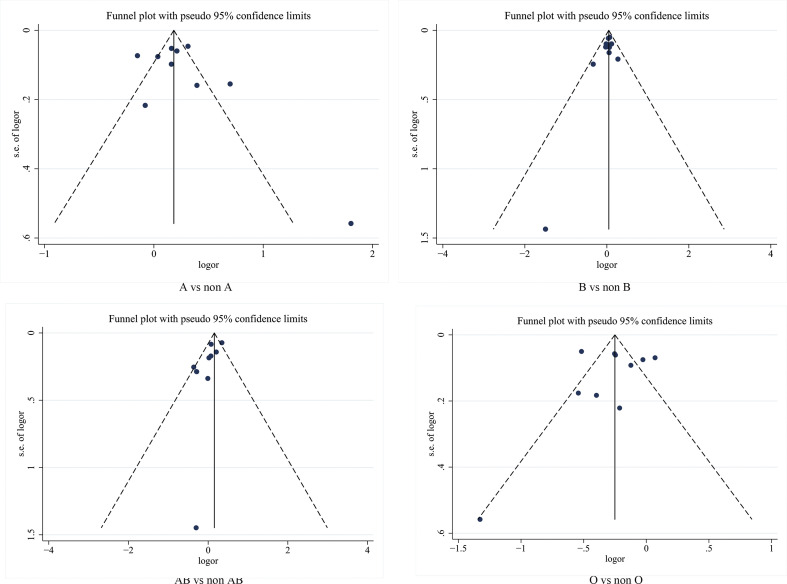
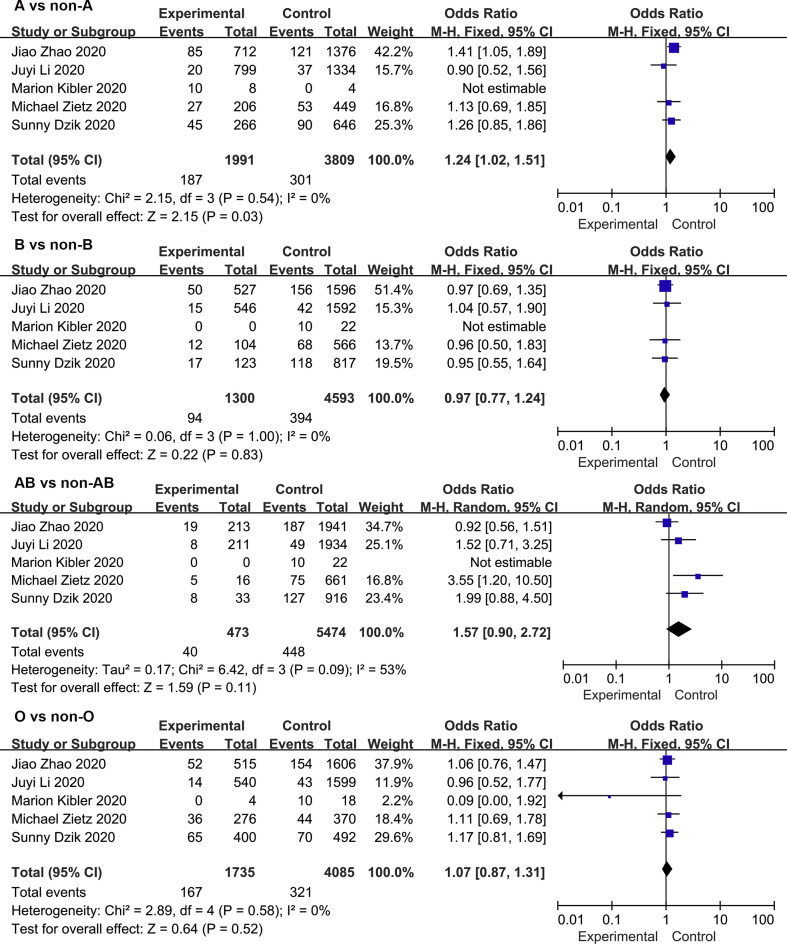
Meta-analysis of the association between blood group A and COVID-19 infection in the 10 studies using a random-effect model demonstrated increased odds of COVID-19 infection compared to non-A blood group participants (OR = 1.33, 95% CI 1.14 to 1.56) ( Fig. 2 ). Although the studies found a positive association between blood group A and COVID-19 susceptibility, there was considerable heterogeneity across studies (I 2 = 86%, P < 0.000). In the subgroup analysis of different races, the relationship between blood group A and COVID-19 infection remained stable (OR = 1.43, 95% CI 1.06 to 1.93 for Caucasians; OR = 1.34, 95% CI 1.20 to 1.50 for Asians) ( Supplementary Fig. S2 ). Further stratified analysis for study design did not substantially change the pooled estimated effect value (OR = 1.39, 95% CI 1.11 to 1.72 for case-control studies; OR = 1.27, 95% CI 1.14 to 1.56 for cohort studies) ( Supplementary Fig. S2 ). Neither the visual inspections of the funnel plot ( Fig. 3 ) nor the Egger's test (P = 0.541) showed evidence of publication bias. Moreover, the sensitivity analysis was completed by deleting each study in turn, providing nearly similar risk estimates ( Supplementary Fig. S3 ).
Fig. 2.
Meta-analysis of the studies that compared the odds of COVID-19 infection among individuals with blood group A, B, and AB vs those with a non-A, non—B, and non-AB blood group.
Supplementary Fig. S2.
Subgroup analysis based on race and study design that compare the odds of COVID-19 infection among individuals with blood group A vs those with a non-A blood group.
Fig. 3.
Assessment of publication bias using a funnel plot. Each circle represents a separate study. The horizontal axis refers to the log odds ratio, and the vertical axis represents the standard error of the log odds ratio.
Supplementary Fig. S3.
Sensitivity analysis of the association between ABO blood groups and COVID-19 infection.
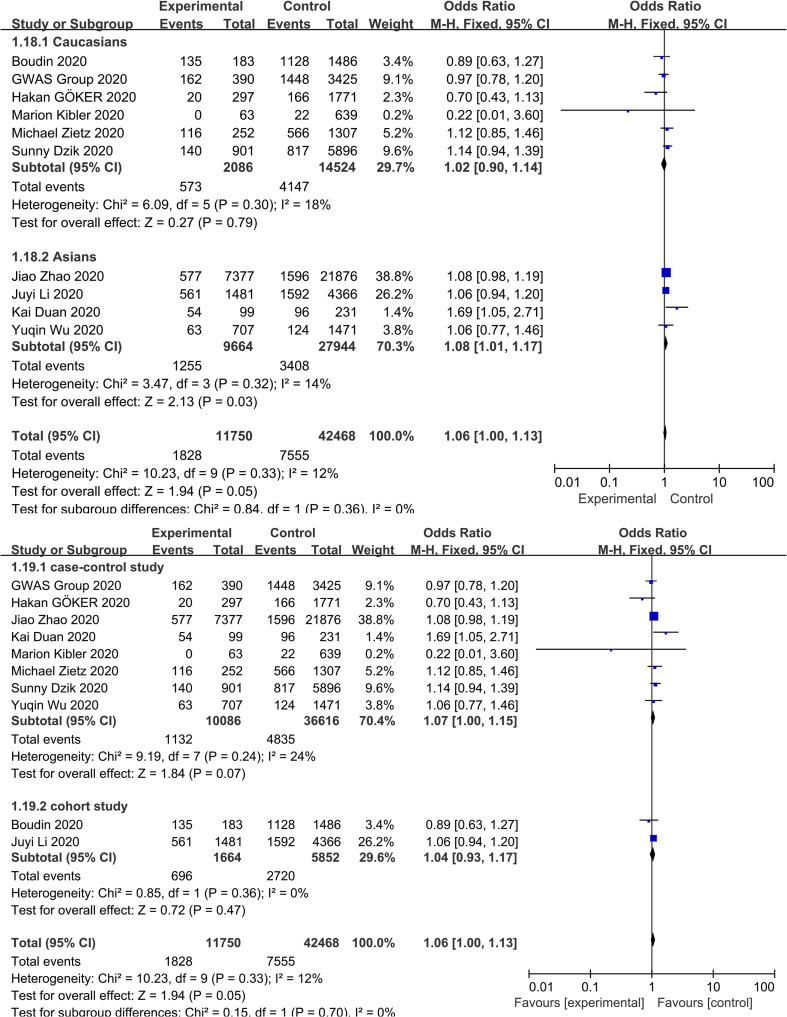
3.4. Association between blood group B and COVID-19 infection
The meta-analysis findings from 10 studies on the association between blood group B and COVID-19 infection showed slightly increased odds of COVID-19 infection compared to non-B blood groups (OR = 1.06, 95% CI 1.00 to 1.13) ( Fig. 2 ), with a mild heterogeneity across studies (I 2 = 12%, P = 0.33). In a subgroup analysis of race, compared with the non-B blood groups, blood group B was associated with an increased risk of COVID-19 infection in the Asian population (OR = 1.08, 95% CI 1.01 to 1.17), while no association was found in the Caucasian population (OR = 1.02, 95% CI 0.90 to 1.14) ( Supplementary Fig. S4 ). Further stratified analysis of study design showed that the cohort study was not relevant (OR = 1.04, 95% CI 0.93 to 1.17) ( Supplementary Fig. S4 ). The robustness of the results was evaluated by deleting each study in turn and reanalysing the data sets, which did not lead to significant changes in the pooled OR estimate ( Supplementary Fig. S3 ). In addition, no significant asymmetry was observed in the funnel plot, indicating that there was no publication bias (Fig. 3). Moreover, the Egger regression asymmetry test (P = 0.232) did not suggest statistical publication bias.
Supplementary Fig. S4.
Subgroup analysis based on race and study design that compare the odds of COVID-19 infection among individuals with blood group B vs those with a non-B blood group.
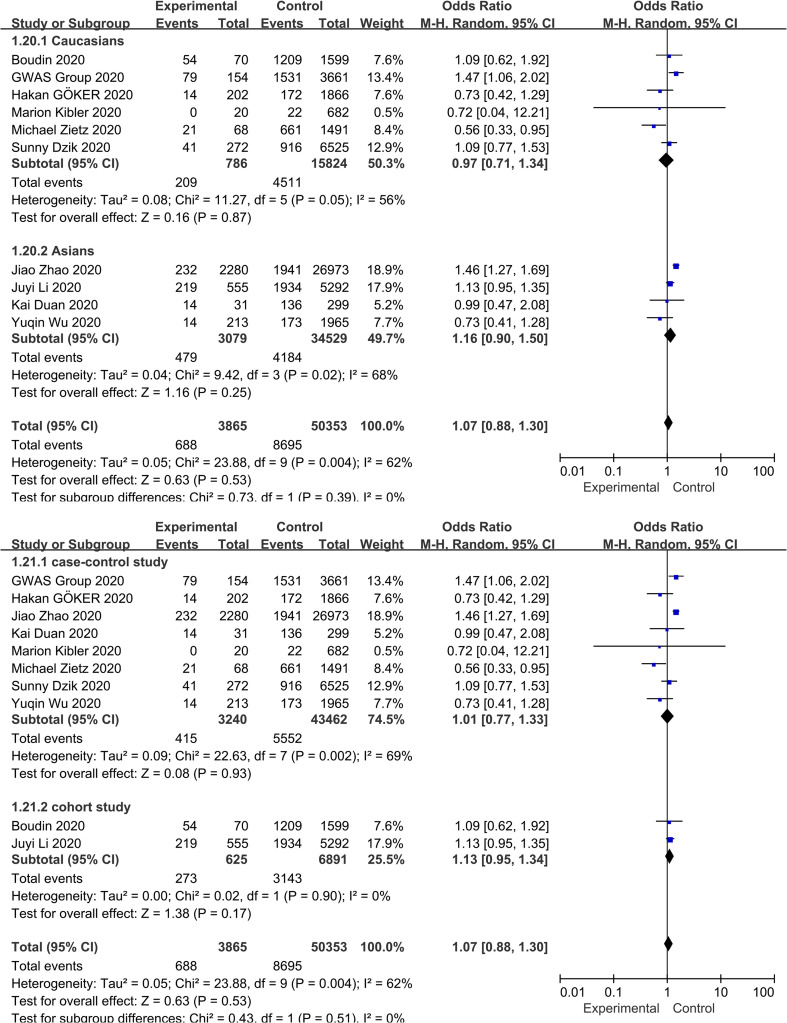
3.5. Association between blood group AB and COVID-19 infection
Fig. 2 shows the overall pooled OR of the association between the blood group AB and COVID-19 susceptibility. Compared with non-AB blood groups, there was no evidence that individuals with blood group A had an increased risk of COVID-19 infection (OR = 1.07, 95% CI 0.88 to 1.30), and there was also evidence of substantial heterogeneity (I 2 = 62%, P = 0.004). The results of the stratified analysis for race and study type did not materially alter the pooled estimate (OR = 0.97, 95% CI 0.71 to 1.34 for Caucasians; OR = 1.16, 95% CI 0.90 to 1.50 for Asians; OR = 1.01, 95% CI 0.77 to 1.33 for case-control studies OR = 1.13, 95% CI 0.95 to 1.34 for cohort studies) ( Supplementary Fig. S5 ). Sensitivity analysis was used to assess the robustness of the results by sequentially removing each study and reanalysing the data sets, resulting in a nearly identical risk estimate ( Supplementary Fig. S3 ). Visual inspection of the funnel plot revealed a mild asymmetry (Fig. 3). Egger's regression asymmetry test (P = 0.020) also suggested publication bias.
Supplementary Fig. S5.
Subgroup analysis based on race and study design that compare the odds of COVID-19 infection among individuals with blood group AB vs those with a non-AB blood group.
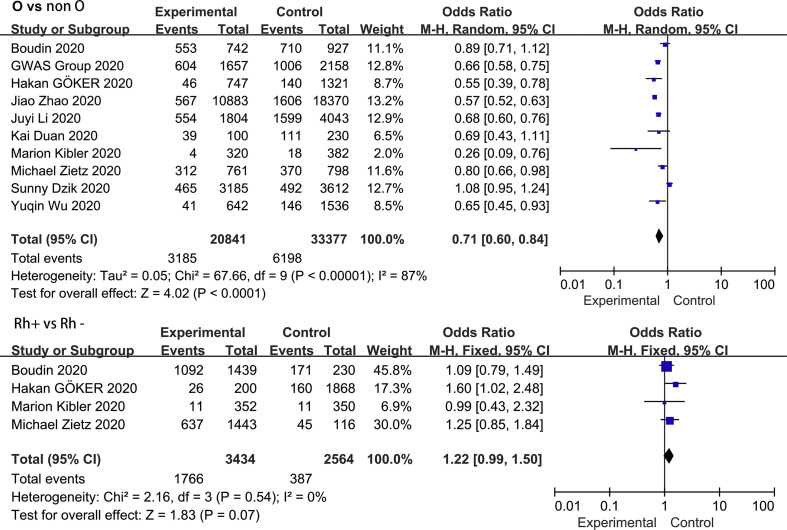
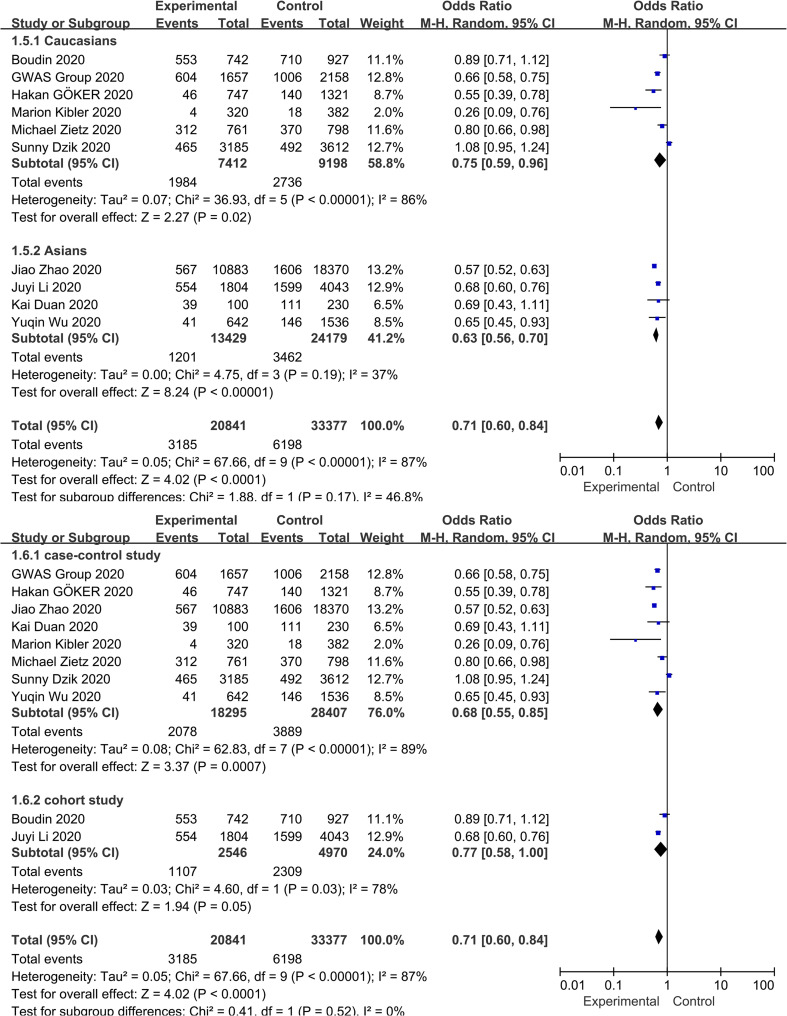
3.6. Association between blood group O and COVID-19 infection
Fig. 4 shows the contribution of blood group O to COVID-19 susceptibility. Seven of the 10 studies reported a negative correlation between the blood group O and COVID-19 infection. Contrary to the results for blood group A, blood group O was found to be protective factor in our analysis, reducing the risk of COVID-19 infection (OR = 0.71, 95% CI 0.60 to 0.84), despite the high heterogeneity (I 2 = 87%, P < 0.000). Subgroup analysis based on race and study type did not substantially alter the pooled effect value (OR = 0.75, 95% CI 0.59 to 0.96 for Caucasians; OR = 0.63, 95% CI 0.56 to 0.70 for Asians; OR = 0.68, 95% CI 0.55 to 0.85 for case-control studies; OR = 0.77, 95% CI 0.58 to 1.00 for cohort studies) ( Supplementary Fig. S6 ). Neither the funnel plot visual test (Fig. 3) nor the Egger's regression asymmetry test (P = 0.908) suggested statistical publication bias.
Fig. 4.
Meta-analysis of the studies that compared the odds of COVID-19 infection among individuals with blood group O and who were Rh-positive vs those with a non-O blood group and were Rh-negative.
Supplementary Fig. S6.
Subgroup analysis based on race and study design that compare the odds of COVID-19 infection among individuals with blood group O vs those with a non-O blood group.
3.7. Association between Rh blood group and COVID-19 infection
A total of 4 studies involving 5998 patients reported an association between Rh blood group and COVID-19 infection. As depicted in Fig. 4, the pooled OR in a fixed-effect model suggested that the risk of COVID-19 was significantly associated with the Rh-positive blood group (OR = 1.22, 95% CI 0.99 to 1.50) compared with the Rh-negative blood group. In the meantime, no heterogeneity between these studies was revealed (I 2 = 0.0%, P = 0.54).
3.8. Association between blood groups and COVID-19 mortality
Five of the 10 eligible studies explored the relationship between blood groups and COVID-19 mortality. Overall, blood group A was associated with a significantly increased risk of COVID-19 mortality (OR = 1.25, 95% CI 1.02 to 1.52) compared with non-A blood groups. Additionally, the pooled data did not demonstrate statistical heterogeneity (I 2 = 0.0%, P = 0.54). No statistical evidence of this association was found in the other three groups ( Fig. 5 ).
Fig. 5.
Meta-analysis of 5 studies that compared the odds of COVID-19 mortality among individuals with ABO blood groups.
4. Discussion
To the best of our knowledge, this is the first meta-analysis that investigates the relationship between ABO blood groups and COVID-19 infection. Our meta-analysis of 10 eligible studies consisting of 54,218 subjects suggested that blood groups A and B are associated with an estimated increase in the probability of COVID-19 infection compared with non-A and non-B blood groups, which was statistically significant. However, compared with the non-O blood groups, individuals with blood group O had a significantly lower predisposition to COVID-19, which was highly statistically significant. No statistical evidence was found for an association between blood group AB and COVID-19 infection. In terms of Rh, patients who were Rh positive were more vulnerable to COVID-19 than those who were Rh negative. Furthermore, we found a contribution of ABO blood groups to the clinical outcome of patients with COVID-19. Compared with non-A blood groups, higher mortality was observed in patients with blood group A, suggesting that blood group A may be related to unfavourable outcomes.
At present, there is no research reporting the impact of race differences in blood group distribution on COVID-19 infection. Herein, we considered race to be a confounding factor in the subgroup analysis to investigate whether race differences affect the contribution of blood group to infection. Interestingly, we only found that the overall pooled results of COVID-19 infection in Caucasians with blood group B were altered. However, the alteration was limited, which indicates that more researche is needed to confirm this evidence. In addition, when independently evaluating the study design, the pooled results were not significantly different from the overall association. Mild publication bias was found across studies through visual inspection of the funnel plot and Egger's test. However, there was moderate to substantial heterogeneity across studies that cannot be ignored, which may be related to the individual study factor (eg., study quality and study design) and participant characteristics (eg., race and age).
Although the mechanisms underlying this association are still unclear, several hypotheses might be raised. The ABO blood group system involves A and B antigens and their corresponding antibodies. The antigen-encoding gene is located on chromosome 9q34.1–34.2 [27] and is composed of A, B, and O alleles, with a total of 4 genetic phenotypes. Differences in blood group antigen expression can increase or decrease the sensitivity of the host to pathogen infection, which may partially explain the blood group difference affecting host predisposition to COVID-19 [28,29]. ACE2 was reported to be a receptor for SARS-COV [30], a virus that caused acute severe respiratory syndrome in 2003. Comparison of the spike (S) protein-coding sequences of SARS-COV-2 and SARS-COV revealed 76%–78% similarity, suggesting that they may share a common receptor [31]. Studies on various genetic factors for SARS susceptibility have found that monoclonal or human natural anti-A antibodies can specifically inhibit the interaction between S protein and ACE2, indicating that anti-A antibodies in individuals with blood group O may block the interaction of SARS-CoV virus S protein with the ACE2 receptor to provide protection [32]. This explained why individuals with blood group A were vulnerable to SARS-CoV, while individuals with blood group O were not [8]. It can be speculated that the decreased sensitivity of individuals with blood group O to COVID-19 and the increased sensitivity of individuals with blood group A to COVID-19 may be related to the presence of natural anti-blood group antibodies, especially anti-A antibodies [33]. Other mechanisms that might explain this association must be further studied.
It has been reported that ABO blood groups are significantly associated with not only susceptibility to SARS-CoV-2 but also with unfavourable outcomes following infection [[34], [35], [36]]. However, we found inconsistent conclusions regarding the relationship between ABO blood groups and COVID-19 mortality. Zhao et al. [23] reported that among 206 COVID-19 patients who died, those with blood group A had a higher mortality rate than those with non-A blood groups, while patients with blood group O had a lower mortality rate than those with non-O blood groups. Zietz et al. [21] did not provide strong evidence of associations between blood groups and intubation or death among COVID-19 patients. Our meta-analysis included 5 studies demonstrating that blood group A was associated with a significantly increased risk of COVID-19 mortality compared with other blood groups. However, it is worth noting that the data reported in the study by Dzik et al. [14] did not support the association between ABO blood group polymorphism and COVID-19 fatality because they compared the ABO blood group distribution between infected survivors and infection-related deaths. They believed this comparison was the most informative way to understand if a particular ABO blood group was associated with mortality within a cohort of infected individuals.
Our findings have some limitations. First, due to the significant heterogeneity, caution should be exercised in interpreting the overall estimates of this meta-analysis. We lack any explanation for this heterogeneity apart from the study type, the method of outcome assessments, and the possible differences in the study population. However, although the unexplained significant heterogeneity limits the interpretation of the pooled estimates, subgroup analysis and sensitivity analysis still indicate that the results of our meta-analysis were reliable. Second, potential confounding factors, such as age, gender, vascular disease, and diabetes, could not be ruled out, which may lead to deviations in study conclusions because those factors may affect the vulnerability of an individual to COVID-19 and the severity of the disease. Third, we limited the study language to English and Chinese, which may have led to exclusion of studies in other languages that are suitable, potentially resulting in publication bias.
In conclusion, this meta-analysis provides evidence that blood groups A and B are associated with an increased risk of COVID-19, whereas blood group O appears to be protective. Rh-positive individuals are more susceptible to COVID-19 than Rh-negative individuals. Moreover, individuals with blood group A are not only prone to developing the disease but also show unfavourable outcomes. However, considering the heterogeneity and the limited number of included studies, more rigorous and high-quality research evidence is needed to confirm this association. Although our study confirmed the association between ABO blood group polymorphism and COVID-19 infection, and prognosis, this does not mean that individuals with blood group O should take the virus lightly. Moreover, individuals with blood groups A and B should not panic, but protective measures are still critical.
5. Future considerations
Due to the few number of articles included in this study, as new evidence emerges, we will continue to follow this topic to provide more convincing evidence.
Practice points
-
•
Individuals with blood groups A and B are more susceptible to COVID-19 infection, whereas the blood group O appears to be protective.
-
•
Individuals with blood group A are not only prone to developing the disease but also shows unfavourable outcomes.
-
•
Individuals with blood group O should not take the virus lightly, and individuals with blood groups A and B should not panic; protective measures are still critical.
Research agenda
-
•
Investigation of the potential mechanisms by the which ABO blood groups affects COVID-19 infection.
-
•
While maintaining ABO matching compatibility, for convalescent plasma therapy, it might be wise to prefer COVID-19 rehabilitation donors with blood group O.
The following are the supplementary data related to this article.
Assessment of the quality of all the studies included in the review according to the Newcastle-Ottawa Scale.
Funding
This work was supported by the China National Science and Technology Major Project for “Essential new drug research and development” (No.2018ZX09301038-003). The funding source had no role in the study.
Declaration of Competing Interest
No relevant conflicts of interest to declare.
Acknowledgements
We would like to thank all the authors who contributed to this systematic review.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. Ther. 2020;12(1):69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Director-General's opening remarks at the media briefing on COVID-19 - 24 June. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---24-june-2020 2020; 14: 69–71.
- 4.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesky E. Viennese serological research about the year 1900: its contribution to the development of clinical medicine. Bull. N. Y. Acad. Med. 1973;49:100–111. [PMC free article] [PubMed] [Google Scholar]
- 7.Jing W., Zhao S., Liu J., Liu M. ABO blood groups and hepatitis B virus infection: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y., Cheng G., Chui C.H., Lau F.Y., Chan P.K., Ng M.H., et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 9.Wang D.S., Chen D.L., Ren C., Wang Z.Q., Qiu M.Z., Luo H.Y., et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int. J. Cancer. 2012;131:461–468. doi: 10.1002/ijc.26376. [DOI] [PubMed] [Google Scholar]
- 10.Borén T., Falk P., Roth K.A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 11.Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L., et al. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 12.Leaf R.K., Al Samkari H., Brenner S.K., Gupta S., Leaf D.E. ABO phenotype and death in critically ill patients with COVID-19. Br. J. Haematol. 2020 doi: 10.1111/bjh.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Wang X., Chen J., Cai Y., Deng A., Yang M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br. J. Haematol. 2020;190:24–27. doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzik S., Eliason K., Morris E.B., Kaufman R.M., North C.M. COVID-19 and ABO blood groups. Transfusion. 2020 doi: 10.1111/trf.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.DLATJ Moher. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y., Feng Z., Li P., Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta. 2020;509:220–223. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Göker H., Aladağ K.E., Demiroğlu H., Ayaz C.Ç., Büyükaşik Y., Inkaya A.Ç., et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J. Med. Sci. 2020;50(4):679–683. doi: 10.3906/sag-2005-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zietz M., Tatonetti N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. medRxiv. 2020 doi: 10.1101/2020.04.08.20058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan K., Di X., Hu F., Wu P., Liu B. The relationship between ABO blood group distribution of COVID-19 convalescents and the plasma antibody titer against SARS-CoV-2. Chin. J. Emerg. Med. 2020;29:629–633. [Google Scholar]
- 23.Jiao Z., Yan Y., Han H., Dong L., Dong G., Xiang L., et al. Relationship between the ABO Blood group and the COVID-19 susceptibility. medRxiv. 2020 doi: 10.1101/2020.03.11.20031096. [DOI] [Google Scholar]
- 24.Kibler M., Carmona A., Benjamin Matsushita, Trimaille A., Mohamad, et al. Risk and severity of COVID-19 and ABO blood group in transcatheter. medRxiv. 2020 doi: 10.1101/2020.06.13.20130211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., et al. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudin L., Janvier F., Bylicki O., Dutasta F. ABO blood groups are not associated with risk of acquiring the SARS-CoV-2 infection in young adults. Haematologica. 2020 doi: 10.3324/haematol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasan S.K., Rostgaard K., Majeed A., Ullum H., Titlestad K.E., Pedersen O.B., et al. ABO blood group and risk of thromboembolic and arterial disease: A study of 1.5 million blood donors. Circulation. 2016;133:1449–1457. doi: 10.1161/CIRCULATIONAHA.115.017563. [DOI] [PubMed] [Google Scholar]
- 28.Cooling L. Blood groups in infection and host susceptibility. Clin. Microbiol. Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amundadottir L., Kraft P., Stolzenberg-Solomon R.Z., Fuchs C.S., Petersen G.M., Arslan A.A., et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat. Genet. 2009;41:986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur. J. Prev. Cardiol. 2020;27(13):1436–1437. doi: 10.1177/2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillon P., Clément M., Sébille V., Rivain J., Chou C., Ruvoën-Clouet N., et al. Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Focosi D. Anti-A Isohemagglutinin titers and SARS-CoV2 neutralization: implications for children and convalescent plasma selection. Br. J. Haematol. 2020;190(3):e148–e150. doi: 10.1111/bjh.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaidi F.Z., Zaidi A., Abdullah S.M., Zaidi S. COVID-19 and the ABO blood group connection. Transfus. Apher. Sci. 2020 doi: 10.1016/j.transci.2020.102838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gérard C., Maggipinto G., Minon J.M. COVID-19 and ABO blood group: another viewpoint. Br. J. Haematol. 2020;190(2):e93–e94. doi: 10.1111/bjh.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Sullivan J.M., Ward S., Fogarty H., O’Donnell J.S. More on ‘Association between ABO blood groups and risk of SARS-CoV-2 pneumonia’. Br. J. Haematol. 2020;190(1):27–28. doi: 10.1111/bjh.16845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of the quality of all the studies included in the review according to the Newcastle-Ottawa Scale.