Dear Editor:
We read with great interest the recent article published in Clinical Gastroenterology and Hepatology by Fan et al,1 in which they described the characteristics of coronavirus disease 2019 (COVID-19)–related liver damage. Since the outbreak of COVID-19, liver injury has attracted widespread attention, which might be caused by pre-existing liver disease, virus infection of liver cells, and certain medications. The effect of virus infection and antiviral drugs on liver injury has been considered, however, the report and diagnosis on chronic liver disease is insufficient in this emergent situation. In a recent large cohort study, only 1.3% of COVID-19 patients recorded a history of chronic viral hepatitis,2 which was significantly lower than the population prevalence in China.3 Patients with pre-existing liver disease are a high-risk population for COVID-19. In this study, we aimed to report the clinical course of COVID-19 patients with chronic hepatitis B virus (HBV) infection and provide a reference for clinical treatment of patients.
From January 24, 2020, to February 29, 2020, patients with confirmed COVID-19 and chronic HBV infection were admitted to 2 designated hospitals for COVID-19. COVID-19 was confirmed by the detection of severe acute respiratory syndrome coronavirus 2 RNA in throat swabs by reverse-transcription polymerase chain reaction. Patients with abnormal liver enzyme levels at admission or a history of chronic liver diseases might undergo HBV assays. HBV infection was defined by a positive test result for hepatitis B surface antigen. HBV-infected patients are classified as hepatitis B virus carriers, chronic hepatitis B, and hepatitis B cirrhosis.4 We defined liver injury as any parameter exceeding the upper limit of normal value of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL).5 The definition of severity degree and the clinical management of COVID-19 patients were in accordance with the practice guidelines issued by China.6 This study was approved by ethics commissions, and written informed consent was waived.
All of the patients in this study had a COVID-19 exposure history. Of the 23 patients, the mean age was 44.7 ± 11.5 years, and 15 (65.2%) were male. Only 6 (26.1%) patients reported a history of being a HBV carrier, 4 (17.4%) patients reported a history of chronic hepatitis B, but the remaining patients (56.5%) denied a history of HBV infection. The patients reported no other underlying diseases. However, laboratory tests at admission showed that 15 patients were HBV carriers, 7 (30.4%) had chronic hepatitis B, and 1 (4.4%) had hepatitis B cirrhosis. At admission, 6 (26.0%) patients had liver test result abnormalities, of which 2 patients were HBV carriers, 3 patients had chronic hepatitis B, and 1 patient had hepatitis B cirrhosis. Ten patients had increased liver enzyme levels during hospitalization, with AST, ALT, and TBIL ranges of 44 to 277 U/L, 52 to 575.1 U/L, and 17.5 to 309.18 μmol/L, respectively. All patients were mild/moderate on admission, but 3 (13.0%) patients progressed to severe, and 2 (8.7%) progressed to critically ill. The 23 patients were treated with antiviral drugs and 13 were treated with liver-protecting drugs. After treatment, all patients were discharged. The comparison of HBV carriers and patients with chronic hepatitis B/hepatitis B cirrhosis is shown in Table 1 . The results showed no significant differences in all clinical features except for sex, exposure history, activated partial thromboplastin time, AST, ALT, γ-glutamyl transpeptidase (GGT), TBIL, and direct bilirubin. Notably, no differences were found in disease severity or length of hospital stay between these 2 groups.
Table 1.
Comparison of Clinical Characteristics and Laboratory Findings Between COVID-19 Patients With HBV Carriers and Hepatitis B/Cirrhosis
| Characteristics | Total | HBV carriers (n = 15) | Hepatitis B/cirrhosis (n = 8) | P value |
|---|---|---|---|---|
| Age, y, mean (SD) | 44.7 (11.5) | 47.1 (11.0) | 40.0 (11.6) | .165 |
| Sex, n (%) | ||||
| Male | 15 (65.2) | 7 (46.7) | 8 (100) | .019 |
| Female | 8 (34.8) | 8 (53.3) | 0 | |
| Exposure history, n (%) | ||||
| Contact with confirmed patient | 13 (56.5) | 11 (73.3) | 2 (25.0) | .039 |
| Contact with a resident from Hubei | 10 (43.5) | 4 (26.7) | 6 (75.0) | |
| Disease severity, n (%) | ||||
| Moderate | 18 (78.3) | 12 (80.0) | 6 (75.0) | .088 |
| Severe | 3 (13.0) | 3 (20.0) | 0 | |
| Critically ill | 2 (8.7) | 0 | 2 (25.0) | |
| Blood routine test | ||||
| White blood cell count, ×109/L, mean (SD) | 6.3 (1.9) | 6.7 (2.0) | 5.5 (1.4) | .170 |
| Neutrophil count, ×109/L, mean (SD) | 4.4 (1.7) | 4.6 (1.8) | 4.1 (1.2) | .474 |
| Lymphocyte count, ×109/L, mean (SD) | 1.4 (0.5) | 1.5 (0.5) | 1.2 (0.5) | .140 |
| Lymphocyte count, n (%) | ||||
| <1.1 ×109/L | 7 (30.4) | 4 (26.7) | 3 (37.5) | .657 |
| 1.1–3.2 ×109/L | 16 (69.6) | 11 (73.3) | 5 (62.5) | |
| Hemoglobin level, g/L, mean (SD) | 138.1 (22.5) | 132.8 (22.5) | 148 (20.3) | .126 |
| Platelet count, ×109/L, mean (SD) | 192.8 (53.1) | 201.3 (53.0) | 176.9 (52.9) | .303 |
| Infection-related biomarkers | ||||
| C-reactive protein level, mg/L, mean (SD) | ||||
| C-reactive protein, n (%) | ||||
| <5.0 mg/L | 15 (65.2) | 10 (66.7) | 5 (62.5) | 1.000 |
| ≥5.0 mg/L | 8 (34.8) | 5 (33.3) | 3 (37.5) | |
| Procalcitonin level, ng/mL, mean (SD) | 0.2 (0.3) | 0.1 (0.1) | 0.4 (0.4) | .065 |
| Procalcitonin, n (%) | ||||
| 0–0.1 ng/mL | 12 (52.2) | 10 (66.7) | 2 (25.0) | .074 |
| >0.1 ng/mL | 10 (43.5) | 4 (26.7) | 6 (75.0) | |
| Not available | 1 (4.4) | 1 (6.7) | 0 | |
| Coagulation function | ||||
| Prothrombin time, s, median (interquartile range) | 13.2 (11.8–13.2) | 12.3 (11.6–13.0) | 14.8 (12.3–13.4) | .086 |
| APTT, s, mean (SD) | 33.5 (10.7) | 29.0 (6.6) | 42.6 (11.8) | .003 |
| D-dimer, μg/mL, median (interquartile range) | 0.8 (0.16–0.49) | 0.4 (0.11–0.47) | 1.6 (0.16–0.54) | .408 |
| D-dimer, n (%) | ||||
| 0–0.5 μg/mL | 17 (73.9) | 12 (80.0) | 5 (62.5) | .549 |
| >0.5 μg/mL | 5 (21.7) | 3 (20.0) | 2 (25) | |
| Not available | 1 (4.4) | 0 | 1 (12.5) | |
| Blood biochemistry | ||||
| Lactate dehydrogenase level, U/L, mean (SD) | 192.1 (76.7) | 182 (45.8) | 206.2 (112.4) | .614 |
| Creatine kinase level, U/L, median (interquartile range) | 88.4 (43.3–70.8) | 58.5 (39.0–70.5) | 143.9 (43.0–152.0) | .968 |
| Creatine kinase MB, U/L, mean (SD) | 10.4 (10.5) | 11.3 (9.8) | 8.8 (12.2) | .610 |
| Myoglobin level, ug/L, median (interquartile range) | 48.0 (19.7–38.1) | 29.1 (19.7–37.0) | 83.3 (20.1–48.5) | .317 |
| Serum creatinine level, μmol/L, mean (SD) | 74.6 (16.3) | 70.4 (13.7) | 82.4 (18.8) | .093 |
| Serum uric acid level, μmol/L, mean (SD) | 300.2 (87.7) | 303.9 (90.5) | 293.1 (87.7) | .785 |
| Serum uric acid level, n (%) | ||||
| Male, <208.3 μmol/L; female, <149 μmol/L | 2 (8.7) | 1 (6.7) | 1 (12.5) | 1.000 |
| Male, 208.3–428.4 μmol/L; female, 149–369 μmol/L | 18 (78.3) | 12 (80.0) | 6 (75.0) | |
| Male, >428.4 μmol/L; female, >369 μmol/L | 3 (13.0) | 2 (13.3) | 1 (12.5) | |
| Glucose level, mmol/L, mean (SD) | 7.1 (3.0) | 7.5 (3.6) | 6.5 (1.4) | .443 |
| Glucose, n (%) | ||||
| 4.3–5.9 mmol/L | 9 (39.1) | 7 (46.7) | 2 (25.0) | .596 |
| >5.9 mmol/L | 13 (56.5) | 7 (46.7) | 6 (75.0) | |
| Not available | 1 (4.4) | 1 (6.7) | 0 | |
| HBV serum markers | ||||
| HBsAg level, n (%) | ||||
| <0.05 IU/mL | 1 (4.3) | 0 | 1 (12.5) | .585 |
| ≥0.05 IU/mL | 21 (91.3) | 14 (93.3) | 7 (87.5) | |
| Not available | 1 (4.3) | 1 (6.7) | 0 | |
| HBsAb level, n (%) | ||||
| <10.00 mIU/mL | 20 (87.0) | 12 (80.0) | 8 (100.0) | .684 |
| ≥10.00 mIU/mL | 2 (8.7) | 2 (13.3) | 0 | |
| Not available | 1 (4.3) | 1 (6.7) | 0 | |
| HBeAg level, signal-to-cutoff, n (%) | ||||
| <1.00 | 18 (78.3) | 13 (86.7) | 5 (62.5) | .168 |
| ≥1.00 | 4 (17.4) | 1 (6.7) | 3 (37.5) | |
| Not available | 1 (4.3) | 1 (6.7) | 0 | |
| HBeAb level, signal-to-cutoff, n (%) | ||||
| >1.00 | 6 (26.1) | 2 (13.3) | 4 (50.0) | .131 |
| ≤1.00 | 16 (69.6) | 12 (80.0) | 4 (50.0) | |
| Not available | 1 (4.3) | 1 (6.7) | 0 | |
| HBcAb level, signal-to-cutoff, n (%) | ||||
| <1.00 | 2 (8.7) | 0 | 2 (25.0) | .111 |
| ≥1.00 | 20 (87.0) | 14 (93.3) | 6 (75.0) | |
| Not available | 1 (4.3) | 1 (6.7) | 0 | |
| Liver function test | ||||
| AST level, U/L, median (interquartile range) | 31.6 (15.0–36.8) | 22.0 (14.5–30.0) | 51.0 (20.6–69.1) | .028 |
| AST level, n (%) | ||||
| Male, 14–40 U/L; female, 13–35 U/L | 19 (82.6) | 15 (100) | 4 (50.0) | .008 |
| Male, >40 U/L; female, >35 U/L | 4 (17.4) | 0 | 4 (50.0) | |
| ALT level, U/L, median (interquartile range) | 38.6 (17.0–42.0) | 23.4 (13.0–25.0) | 67.1 (20.3–106.9) | .030 |
| ALT level, n (%) | ||||
| Male, 9–50 U/L; female, 7–40 U/L | 19 (82.6) | 14 (93.3) | 5 (62.5) | .103 |
| Male U/L, >50; female, >40 U/L | 4 (17.4) | 1 (6.7) | 3 (37.5) | |
| GGT level, U/L, median (interquartile range) | 32.3 (13.5–41.0) | 23.1 (9.8–25.8) | 48.4 (27.5–67.3) | .024 |
| GGT level, n (%) | ||||
| Male, 10–60 U/L; female, 7–45 U/L | 17 (73.9) | 12 (80) | 5 (62.5) | .549 |
| Male, >60 U/L; female, >45 U/L | 5 (21.7) | 2 (13.3) | 3 (37.5) | |
| Not available | 1 (4.4) | 1 (6.7) | 0 | |
| ALP level, U/L, mean (SD) | 73.0 (24.7) | 70.0 (23.2) | 78.1 (27.9) | .471 |
| ALP, n (%) | ||||
| 40–129 U/L | 20 (87.0) | 13 (86.7) | 7 (87.5) | 1.000 |
| >129 U/L | 2 (8.7) | 1 (6.7) | 1 (12.5) | |
| Not available | 1 (4.4) | 1 (6.7) | 0 | |
| TBIL level, μmol/L, median (interquartile range) | 24.9 (7.2–13.9) | 10.2 (6.7–11.8) | 50.6 (10.0–18.3) | .014 |
| TBIL level, n (%) | ||||
| 3.4–17.1 μmol/L | 18 (78.3) | 13 (86.7) | 5 (62.5) | .168 |
| >17.1 μmol/L | 4 (17.4) | 1 (6.7) | 3 (37.5) | |
| Not available | 1 (4.4) | 1 (6.7) | 0 | |
| DBIL level, μmol/L, median (interquartile range) | 12.9 (2.8–5.4) | 3.2 (2.0–4.0) | 29.8 (3.6–8.5) | .014 |
| DBIL level, n (%) | ||||
| 0–5 μmol/L | 17 (73.9) | 13 (86.7) | 4 (50.0) | .033 |
| >5 μmol/L | 5 (21.7) | 1 (6.7) | 4 (50.0) | |
| Not available | 1 (4.4) | 1 (6.7) | 0 | |
| Albumin level, g/L, mean (SD) | 42.3 (8.7) | 42.1 (7.0) | 42.5 (11.9) | .921 |
| Globulin level, g/L, mean (SD) | 26.4 (4.7) | 25.8 (4.1) | 27.4 (5.8) | .448 |
| Treatment | ||||
| Antiviral drug | 23 (100.0) | 18 (100.0) | 5 (100.0) | |
| Interferon | 20 (87.0) | 12 (80.0) | 8 (100.0) | .526 |
| Arbidol (Jiangsu, China) | 10 (43.5) | 5 (33.3) | 5 (62.5) | .221 |
| Peramivir | 8 (34.8) | 5 (33.3) | 3 (37.5) | 1.000 |
| Lopinavir/ritonavir | 7 (30.4) | 3 (20) | 4 (50.0) | .182 |
| Chloroquine | 6 (26.1) | 4 (26.7) | 2 (25.0) | 1.000 |
| Ribavirin | 6 (26.1) | 4 (26.7) | 2 (25.0) | 1.000 |
| Oseltamivir | 2 (8.7) | 1 (6.7) | 1 (12.5) | 1.000 |
| Liver-protecting drug | 13 (56.5) | 7 (46.7) | 6 (75.0) | .379 |
| Diammonium glycyrrhizinate | 8 (34.8) | 5 (33.3) | 3 (37.5) | 1.000 |
| Reduced glutathione | 6 (26.1) | 2 (13.3) | 4 (50.0) | .131 |
| Compound glycyrrhizin | 3 (13.0) | 2 (13.3) | 1 (12.5) | 1.000 |
| Magnesium isoglycyrrhizinate | 3 (13.0) | 1 (6.7) | 2 (25.0) | .269 |
| Glucurolactone | 1 (4.3) | 0 | 1 (12.5) | .348 |
| Antibiotics | 20 (87.0) | 13 (86.7) | 7 (87.5) | 1.000 |
| Herbal medicine | 20 (87.0) | 13 (86.7) | 7 (87.5) | 1.000 |
| Glucocorticoid | 6 (26.1) | 5 (33.3) | 1 (12.5) | .369 |
| Noninvasive ventilation | 3 (13.0) | 2 (13.3) | 1 (12.5) | 1.000 |
| Admission to intensive care unit | 5 (21.7) | 3 (20.0) | 2 (25.0) | 1.000 |
| Comorbidities | ||||
| ARDS | 2 (8.7) | 1 (6.7) | 1 (12.5) | 1.000 |
| Deep venous thrombosis | 1 (4.3) | 1 (6.7) | 0 | 1.000 |
| Upper gastrointestinal hemorrhage | 1 (4.3) | 0 | 1 (12.5) | .348 |
| Liver failure | 1 (4.3) | 0 | 1 (12.5) | .348 |
| Renal insufficiency | 1 (4.3) | 0 | 1 (12.5) | .348 |
| Prognosis | ||||
| Discharged | 23 (100.0) | 15 (100.0) | 8 (100.0) | .911 |
| Length of hospitalization, d, mean (SD) | 15.1 (7.4) | 15.0 (7.8) | 15.4 (7.0) |
NOTE. P values were calculated by t test, Mann–Whitney U test, chi-squared test, or the Fisher exact test as appropriate.
ALP, alkaline phosphatase; ALT, alanine transaminase; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; HBcAb, hepatitis b core antigen; HBeAb, hepatitis be antibody; HBeAg, hepatitis be antigen; HBsAb, hepatitis b surface antibody; HBsAg, hepatitis b surface antigen; HBV, hepatitis B virus; MB, isoenzyme; TBIL, total bilirubin.
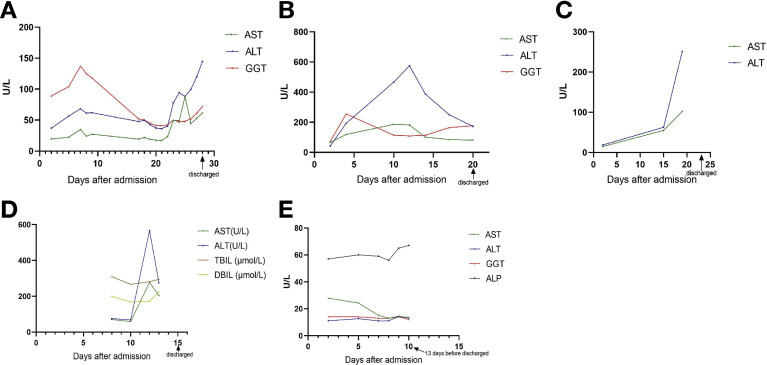
The 5 severe/critically ill patients (cases A–E) all were male. These 5 patients were admitted to the intensive care unit, 3 required noninvasive mechanical ventilation and all were treated with corticosteroids. During intensive care unit treatment, case A developed deep venous thrombosis, cases B and C developed acute respiratory distress syndrome, and case D developed upper gastrointestinal hemorrhage, liver failure, and renal insufficiency. Figure 1 shows follow-up liver function tests of the 5 patients during hospitalization. The increase in ALT level was significantly higher than AST. The ALT increase is the primary indicator of liver injury in COVID-19.7 , 8 The GGT increase was not significant. Before discharge, liver function of the 5 patients could not return to baseline.
Figure 1.
Temporal liver function test of cases A–E during hospitalization. (A) In patient A (male, 51 y; hepatitis B virus carrier, severe), AST and ALT levels increased to 3.2 times baseline in the first week and decreased to near normal on day 14 after admission with the use of liver-protecting drugs. However, 5 days later, AST and ALT levels increased again to 3.2 and 4.0 times baseline, respectively. (B) In case B (male, 29 y; chronic hepatitis B, critically ill), AST and ALT levels increased to 2.7 and 13.7 times baseline at day 12 after admission, and then decreased with the use of liver-protecting drugs. In the last test, AST and ALT levels were 1.2 and 4.1 times baseline, respectively. (C) In case C (male, 64 y; hepatitis B virus carrier, severe), AST and ALT levels increased continuously to 6.9 and 13.6 times baseline, respectively. (D) In case D (male, 44 y; hepatitis B cirrhosis, critically ill), AST and ALT levels increased to 4.0 and 7.5 times baseline at day 12 after admission, respectively, and then decreased to 1.7 and 1.9 times baseline owing to the use of liver-protecting drugs. TBIL and DBIL stabilized at a higher level, exceeding the upper limit of normal value. (E) In case E (female, 47 y; hepatitis B virus carrier, severe), liver function tests of the patient were normal. ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin.
Because of the emergence of COVID-19, the report and diagnosis on chronic HBV infection is insufficient, therefore, the impact of HBV infection on liver injury might be underestimated. Most HBV carriers with COVID-19 will not progress to becoming severely or critically ill. However, 26% of patients had abnormal liver function test results at admission, 19% of whom progressed to being severely or critically ill, which was not associated with HBV infection status. Accordingly, we recommend dynamic monitoring of liver function in COVID-19 patients with liver test abnormalities at admission. ALT and AST levels, especially ALT levels, are preferred parameters that should be used to monitor liver function during hospitalization.
Acknowledgment
The authors thank all the medical workers for fighting against COVID-19, and to the people of the country and the world for their contributions to this campaign.
Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.Fan Z. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei F. Hepatology. 2020 Epub ahead of print. [Google Scholar]
- 3.Dore G.J. Clin Infect Dis. 2020 Epub ahead of print. [Google Scholar]
- 4.Chinese Society of Infectious Diseases Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Qi X. J Hepatol. 2020 Epub ahead of print. [Google Scholar]
- 6.National Health Commission, State Administration of Traditional Chinese Medicine . 2020. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) [Google Scholar]
- 7.Liaw Y.F. Liver. 1988;8:231–235. doi: 10.1111/j.1600-0676.1988.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 8.Chau T.N. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]