Abstract
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a new virus that has never been identified in humans before. COVID-19 caused at the time of writing of this article, 2.5 million cases of infections in 193 countries with 165,000 deaths, including two-third in Europe. In this context, Oncology Departments of the affected countries had to adapt quickly their health system care and establish new organizations and priorities. Thus, numerous recommendations and therapeutic options have been reported to optimize therapy delivery to patients with chronic disease and cancer.
Obviously, while these cancer care recommendations are immediately applicable in Europe, they may not be applicable in certain emerging and low- and middle-income countries (LMICs). In this review, we aimed to summarize these international guidelines in accordance with cancer types, making a synthesis for daily practice to protect patients, staff and tailor anti-cancer therapy delivery taking into account patients/tumour criteria and tools availability. Thus, we will discuss their applicability in the LMICs with different organizations, limited means and different constraints.
Keywords: Cancer, COVID-19, Guidelines, Recommendations, LMICs, Health care, TRONE, AROME
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a new virus that has never been identified in humans before. This virus causes respiratory illness with symptoms like cough, fever and, in most severe cases, pneumonia. At the time of writing of this article, 2.5 million cases of infections have been reported in 193 countries with 165,000 deaths, including Two-third in Europe in accordance with the applied case definitions and testing strategies in the affected countries. This pandemic surprised the whole world by its contagiousness, with a high speed of diffusion in all subpopulations and its violence in terms of deaths.
The Oncology Departments of the affected countries had to adapt quickly and establish new organizations with a practical definition of priorities. Thus, numerous recommendations and therapeutic options have been developed to allow the optimization of departments’ organization and function in order to provide and continue to deliver optimal therapy to all patients with cancer.
In these exceptional circumstances, many groups and scientific societies have made practical recommendations. In Europe, the first recommendations concerned mainly the way of protecting patients with cancer [1]. Since then, many guidelines have been proposed for different types of cancers. Obviously, this type of recommendation immediately applicable in Europe may not be applicable in certain emerging countries or low- and middle-income countries (LMICs). Thus, it seemed fundamental to us to summarize these international guidelines according to cancer types and to make a synthesis for daily practice. In addition, we aimed to discuss their applicability in the LMICs with different organizations, limited means and different constraints.
2. Material and methods
To collect the French data, we sent an e-mail to the French Oncology Societies that had not reported guidelines for COVID-19 crisis between March 1st and April 30th. The responses are summarized in Table 1 .
Table 1.
Survey from the French oncology societies and clinical research groups.
| Type of cancer | Name of the society/group(references) | Response | International scientific societies/Clinical research groups |
|---|---|---|---|
| Head and neck | GORTEC [30,31] | Yes | – |
| Lung | GOLF [32,33] | ESTRO, ASTRO [31] | |
| Breast | Saint Paul-de-Vence [35] | Yes | ASBS [27] |
| Gynaecological | GINECO | No | Yale University [37] |
| SCGP and FRANCOGY [36] | Yes | ||
| GU | GETUG [38] | Yes | – |
| CCAFU [12] | Yes | ||
| GI | GERCOR SNFGE [40] AP-HP [41] |
Yes | ESMO [46,47] |
| Yes | |||
| Radiation oncology | SFRO [39] | Yes | International radiation therapy network [22] |
GI, gastrointestinal; GORTEC, Groupe d'Oncologie Radiothérapie Tête Et Cou; GOLF, Groupe d'Oncologie de Langue Française; ASBS, American Society of Breast Surgeons; SCGP, Société de Chrurgie Gynécologique et Pelvienn; GETUG, Groupe d'Etude des Tumeur genito-Urinaires; CCAFU, Comité de Cancérologie de l'Association Françasie d'Urologie; GERCOR, groupe coopérateur multidisciplinaire en oncologie; SNFGE, société nationale francaise de gastro-enterologie; APHP, Assistance Publique Hopitaux de Paris; SFRO, Société Francais d'Oncologie Radiothérapie. ESMO, European Society of Medical Oncology.
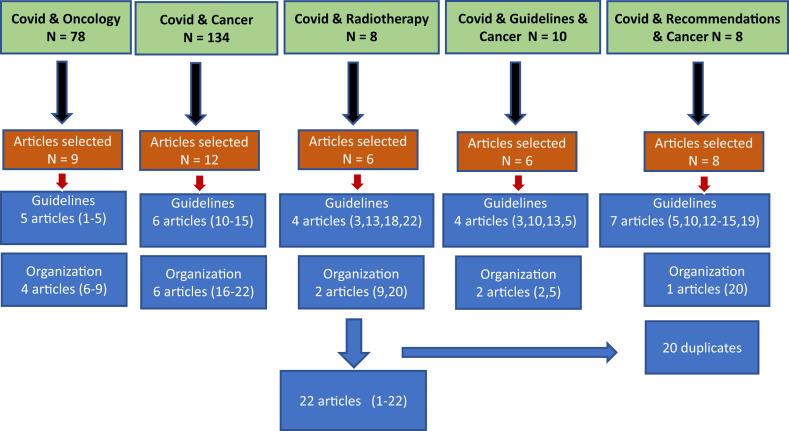
For the international literature data, a search in PubMed was performed using the following key words: ‘COVID-19 and oncology’, ‘COVID-19 and cancer’, ‘COVID-19 and radiotherapy’ (RT), ‘COVID-19 and guidelines for cancer’, ‘COVID-19 and recommendations for cancer’. All abstracts and articles in English were collected. Articles in Chinese without English abstracts were not included in the review. In Fig. 1 , we summarized the literature search and references that are included in this review. In summary, articles related to oncology guidelines and organization were selected based on the search using the following keywords:
-
-
For ‘COVID-19 and oncology’: Among the 78 articles, 9 were related to either guidelines/recommendations (n = 5) [[1], [2], [3], [4], [5]] or medical and health system organization (n = 4) [[6], [7], [8], [9]].
-
-
For ‘COVID-19 and cancer’: Among the 134 articles, 12 were related to either guidelines/recommendations (n = 6) [[10], [11], [12], [13], [14], [15]] or medical and health system organization (n = 6) [[16], [17], [18], [19], [20], [21], [22]].
-
-
For the last 3 key words, namely ‘COVID-19 and radiotherapy’ (n = 13) [[23], [24], [25], [26], [27], [28], [29]], ‘COVID-19 and guidelines for cancer’ and ‘COVID-19 and recommendations for cancer’, the 21 additional references found were redundant and were found with the first two searches (Fig. 1).
Fig. 1.
Results of the literature screening, selection process of articles and keywords.
All recommendations, including guidelines for RT practice (23-29), have been finally summarized in tables and discussed via e-mails with the AROME and TRONE networks members regarding their applicability in LMICs.
3. Results
3.1. Patient visits, staff and departments organization (Table 2)
Table 2.
Recommendations for departments organization and procedures for patients, visitors and staff.
| Patients and visitors | Patients visits | Staff | Departments organization | Treatments scheduling | References |
|---|---|---|---|---|---|
| Minimal presence at hospital | Telemedicine and phone calls | Dedicated areas in oncology and radiotherapy departments | Switch IV treatment to oral drugs when possible | You B et al. [1] | |
| Management at home encouraged | Open space out patients centres | Prioritization according to life expectancy, age, line therapy number | |||
| Separation measures | Temporary breaks for slowly evolving metastases | ||||
| Minimise hospital visits and elective Admissions |
Consider delaying surgical procedure | Al Shamsi et al. [2]. | |||
| Consider surveillance for early stage cancer |
Consider postponing adjuvant CT and RT but hold any active therapy |
||||
| For all patients on active anti-cancer therapy remain vigilant for COVID-19 symptoms |
|||||
| To manage currently infected patients Schedule outpatients based on priority criteria | In-house isolation or quarantine of suspected cases to keep hospital admissions manageable | N95 mask fitting | Clear delineation of job responsibilities | Integrate all best-practice approaches into work processes to prevent further transmission |
|
| Isolate patients with suspected infection until they are cleared | Prompt identification of suspected infection among staff and application of appropriate isolation | ||||
| Patients and visitors screening before appointments | Limitation of visitors in departments | Staff temperature screening every day Staff rotation schedules |
Separate Entrance/exit | Maintain therapy schedule according to the benefit risk and availability of means for locally advanced and high-risk, patients and those already started their therapy. | Applicability in LMICs |
| Specific plan for COVID-19 suspected or positive | Should be limited | Staff COVID-19 + out of planning and self isolation 14 days | Space with limited patients in waiting room | Deep remission (3–6 months) stopping therapy is an option | |
| Separate cancer patients from other patients | Treatment of COVID-19+ patients outside of cancer center or dedicated area |
COVID-19, coronavirus disease 2019.
Patients with cancer are more susceptible to infection than individuals without cancer because of the immunosuppressive effect induced by and anti-cancer therapy. In the context of a global public health emergency related to the emergence of COVID-19, it is essential to protect patients and staff to ensure continuity of care. Thus, a major reorganization of our departments was needed to adapt the resources for oncology care maintenance to ensure timely and proportionate implementation of contingency plans that balance risks and protect patients and healthcare workers during the infections rise period. Moreover, the departments reorganization must take into account specifics of the specialities involved in the management of patients with cancer.
3.2. Surgical oncology departments
For surgery, two situations are to be distinguished: patients having undergone surgery just before the COVID-19 outbreak and patients whose surgery is already or must be planned during this period. In the first case, it is reported that COVID-19–positive patients are likely to be at higher risk of clinically severe events than those who did not have surgery. Thus, protective measures must be reinforced and their visits to the department limited. The emergency for adjuvant treatments delivery should be discussed on a case-by-case basis. For scheduled surgery, the discussion will focus on the delayed interventions, taking into account the benefit/risk ratio for each patient.
In Table 2 some recommendations are presented, from our group (AROME) and others, regarding the prioritization of urgent surgeries and the need to work with the hospital to ensure that adequate supplies (PPE, staffing, and bed capacity) will be available for non-elective, time-sensitive surgeries. Delayed oncologic surgeries may lead to disease progressions and result in tumours that are no longer resectable, leading to worse survival outcomes. Thus, all decisions for delayed surgery ± shift to neoadjuvant therapies should be taken in the frame of multidisciplinary board meetings.
3.3. Radiation oncology departments
In RT departments, staff must also be protected against COVID-19 at all times. Weekly or daily team rotation and dedicated routes for positive COVID-19 patients taken in dedicated time slots with full protection of staff is a prerequisite to reduce the risk to staff and non-infected patients. Table 2, Table 3 show the main reported global and specific recommendations.
Table 3.
Radiation Oncology departments organization during COVID-19 pandemic period.
| Recommendations for radiotherapy departments organization | |||
|---|---|---|---|
| Societies/groups/teams | Frenche RO society [39] |
Simcock et al. [22] |
Applicability in LMICs |
| March 19th | March 20th | ||
| Workers protection | |||
| WHO guidelines for preventive measures and use of Personal Protection Equipment (PPE) | X | X | Applicable |
| Reduction of the number of health professionals in radiotherapy departments to the minimum required, promoting teleworking | X | X | Teleworking probably not |
| Inviting the local Infection Control department | X | ||
| Temperature monitoring for all patients | X | Applicable | |
| Special monitoring for ‘contact’ patients (those who had close contact with confirmed COVID cases) | X | Probably not | |
| Symptomatic health professional: PCR, isolation, adapted care | X | Probably not | |
| Department Organization | |||
| Delay of follow-up medical examinations | X | Applicable | |
| Remote/telephone consultation when possible | X | X | Probably not |
| Minimize number of additional visitors, family members or careers | X | Applicable | |
| Reorganization of waiting rooms (separating fragile vs potentially infected patients, increased distances, removal of infection vectors) | X | X | |
| Optimize department areas for decontamination | X | ||
| Model for estimation of the harms of COVID infection for cancer patients | X | ||
| Creating capacity by reducing fraction numbers | X | ||
| Separation of fragile/immunocompromised vs infected/contact patients | X | ||
| Special protocol for infected/contact patients (treatment pause or dedicated treatment timeslot) | X | X | |
| When Insufficient Number Of Health Professionals | |||
| Priority to: primary radiation treatments (vs operable or adjuvant), curative (vs palliative) | X | X | Applicable |
| Delay treatment for hormone-sensitive cancersa | X | ||
| Record all changes in treatments | X | ||
| Only one therapist per treatment (standard) | X | ||
| Two therapist per treatment (complex treatments) | X | ||
| Turnover for radiation oncologists and medical physicist | X | ||
| Brachytherapy | |||
| Delay of all brachytherapy treatments | X | Applicable | |
| Prefer local/spine anaesthesia to general anaesthesia | X | ||
| Delay of treatments where surveillance is an alternative option | X | ||
| Priority to: primary treatments (vs adjuvant), single treatment (vs fractionated) | X | ||
| FFP2 masks for head and neck treatments | X | ||
| Special cases dealt with | |||
| Insufficient number of medical physicists | X | Applicable | |
| Insufficient number of radiation oncologists | X | ||
| Increase of quality control hours and prioritization of checks | X | ||
| Specific indications for omitting/delaying/hypofractionating/pausing radiotherapy treatment by cancer type and curative vs palliative treatment | X | ||
COVID-19, coronavirus disease 2019; LMICs, low- and middle-income countries.
With attention on a post-crisis unmanageable surge in activity. RO: radiation oncology.
In Table 3, we present the main recommendations for workers in RT departments and staff organization, in accordance with available human resources. In addition, scenarios taking into account the reduction in the number of workers in case of COVID-19 infection have been proposed to ensure treatment with the required quality and safety.
For patients, a distinction must be made between patients who have started treatment and those who have not yet started irradiation. For the first, finishing treatment is a priority, either following initial planned fractionation schedules or after plan recalculation to shorten treatment duration. For the weekly visits, telemedicine and phone calls could be advocated to limit the time spent in the hospital every day. For COVID-19–positive patients, dedicated areas, separate exit/entrance and dedicated time slots have to be planned.
For patients who have not started RT yet, priorities should be also fixed as per tumour sites, adjuvant or neoadjuvant settings, the possibility of delaying irradiation in settings where primary chemotherapy or endocrine therapy could be administrated. These situations will be discussed in the next section.
3.4. Medical oncology departments
As for other departments, dedicated areas, specific exits/entrance and a limited number of patients in the waiting rooms are recommended to limit contacts between infected and non-infected patients. To ensure minimal presence in the hospital, some regimens can be altered to minimize infusion visits or a switch to oral drugs when possible. During treatments, systematic screening before appointments is recommended by the majority of experts.
For patients already in deep remission (stable for more than 6–12 months), stopping or delaying treatment may be an option. A temporary break could be an option for patients with slowly evolving metastases. For all patients on active anti-cancer therapy, we need to remain vigilant for COVID-19 symptoms. Their therapy should be planned in a dedicated area.
Changes in management strategy and therapy in accordance with type of cancer (Table 4 ).
Table 4.
Approach to curative intent therapy by tumour sites: summary of the published recommendations during COVID-19 crisis.
| Disease | Criteria for delay | Fractionation | Boost | Systemic therapy | References | LMIC applicability | |||
|---|---|---|---|---|---|---|---|---|---|
| Head and neck | |||||||||
| Head and neck cancer: all tumour sites | Head and neck cancer treatment break or deferral may lead to reduced tumour control | Consider mitigatinsbg with additional radiation dose after treatment or addition of chemotherapy. | [[30], [31]] | Applicable | |||||
| Patients < 70 y, with non resectable tumours: Standard time for treatment ≤ 4 weeks since diagnosis should be respected |
SIB should be considered:
|
Concomitant chemotherapy for locally advanced forms should be offered according to the usual indications | Applicable only if IMRT is available. Beside small volumes (such as larynx T1 N0), hypofractionation using 3D RT = risk of high toxicity To favour chemotherapy during the first 2 months before combined chemoradiation |
||||||
| Patients <70 y, eligible for adjuvant RT: A period of 6–8 weeks between surgery and RT should be respected. |
|
Concomitant chemotherapy for high risk tumours should be offered according to the usual indications | |||||||
| Patients >70 years old or unfit (≥PS 2 and/or with significant comorbidities) | Non resectable tumours:
|
Applicable for palliative care | |||||||
| HPV | no de-escalation for HPV + tumours | Not applicable | |||||||
| Lung cancer | |||||||||
| Early NSCLC | No delay of post-op RT No immediate RT for N2 NSCLC |
Standard RT | Standard therapy
|
[[32], [33]] | Applicable | ||||
| Locally advanced NSCLC | No delay of CRT | Standard RT | Standard therapy
|
Not applicable | |||||
| Metastatic NSCLC | PS 1, Fit patients PS 2, elderly patients |
Palliative strategy | Oncogenic alteration: standard therapy No oncogenic alteration:
|
According to availability of immunotherapy Applicable |
|||||
| SCLC | No delay of CRT | Standard RT |
|
Applicable If G-CSF available |
|||||
| Breast | |||||||||
| DCIS | Delete RT 3–6 months | 40Gy in 15f | TAM “standby” therapy possible | [3,35] | Applicable | ||||
| Invasive BC | |||||||||
| HR + post M stages I II | Delete RT 3–6 months | Preferred scheme 40Gy in 15f | Not systematic boost in low risk | ET standby therapy systematic | |||||
| Other BC subtypes and patients profiles including young and high- risk patients | No delay of RT | Standard or hypofractionation | Hypofractionation boost: 10–15Gy OR Integrated boost |
Standard therapy | |||||
| GU – Prostate | |||||||||
| Low risk | Active surveillance or delay treatment | – | – | [12,38] | Applicable | ||||
| Intermediate risk | Delay RT 3 months | In case of RT indication use hypofractionation (60Gy in 20fr) | 3–6 months of ADT before RT | [39] | Hypofractionation only if IMRT is available and no indication of nodal RT | ||||
| Delay surgery by 3–6 months | No ADT | [12, 39] | Applicable Using standard fractionation No dose escalation if IMRT-IGRT no available |
||||||
| High risk | Delay RT by 3–6 months | 3–6 months of ADT before RT | [38, 39] | ||||||
| Surgery should be switched to RT | |||||||||
| Post-operative or “rising PSA” RT indication | Delay RT by 3 months | 3 months of ADT before RT | |||||||
| Metastatic setting hormone sensitive | Delay RT for oligo-metastatic disease | ADT + New generation of ET | |||||||
| Castration-resistant patients | Delay/avoid CT and prednisone | Enzalutamide is to be preferred | [12] | If Enzalutamide is available | |||||
| GU – Bladder | |||||||||
| Muscle infiltrating (MI) | Surgery, no delay | NA Chemotherapy possible | [38] | Applicable | |||||
| MI when surgery is contraindicated | RT with or without 5Fu/myto | In case of RT indication hypofractionation should be preferred (55Gy in 20fr) | [38, 39] | Hypofractionation only if IMRT is available a | |||||
| Metastatic 1st line | cisplatin-gemcitabine + G-CSF (No MVAC) | [38] | Applicable If G-CSF is available |
||||||
| Metastatic 2nd line | Delay treatment | Unknown impact of checkpoint inhibitors on covid19 | – | ||||||
| GI – oesophagus | |||||||||
| Localized cancer | RTCT with Carboplatin-Taxol | Standard | [[40], [41], [42], [43], [44], [45], [46], [47]] | Applicable | |||||
| Inoperable or advanced | Standard | Or FOLFOX | |||||||
| Complete response to CRT | Follow-up or delay surgery | ||||||||
| Incomplete response to CRT | Delay salvage surgery up to 3 months | ||||||||
| GI-Pancreas | |||||||||
| Operable/bordrline | Patients who does not fit for neo-adjuvant chemotherapy should be considered as high priority for surgery | NA FOLFOX to delay surgery | [40, 47] | Applicable according to drugs availability | |||||
| Locally advanced | Avoid CRT during COVID-19 outbreack Completion of NA chemotherapy when already started or patients included in clinical trials should be also considered as a high priority |
CT with schemes using Capecitabine | |||||||
| Post-operative setting | Delay adjuvant treatments according to the benefit risk | FOLFIRINOX is recommended (depending on benefit in OS) | [40] | ||||||
| GI – Rectum | |||||||||
| CRT completed or ongoing | Delay surgery up to 3 months | [40, [42], [43], [44]] | Applicable | ||||||
| All new patients | Pre operative RT | 25Gy in 5fr and surgery after 3 months | |||||||
| T4 rectal cancer | Chemoradiation | CAP 50 and surgery after 11weeks | |||||||
| Low rectum with complete response to chemoradiation | Tumour excision or watch and wait (GRECCAR 2 criteria) | ||||||||
| GI – Anal canal | |||||||||
| Localized | Standard chemo-radiation based on capecitabine or mytomicin C | [40, 47] | Applicable | ||||||
| Recurrence or metastatic setting | Chemotherapy with capecitabine/oxaliplatin or carboplatin/capecitabine | ||||||||
| Gynaecological – Cervical cancers | |||||||||
| Cervical cancer |
|
Standard RT or RCT | [36, 37] | Applicable | |||||
| Gynaecological – Endometrium | |||||||||
| Low and intermediate risk or stage IA | Delay surgery up to 1–2 months | Total hysterectomy with bilateral annexectomy associated with sentinel node procedure | Applicable | ||||||
| High-risk or stage II |
|
Consider if brachytherapy alone is a reasonable substitute for these patients after weighing risks and benefits | PET-CT availability | ||||||
| Advanced stage III IV |
|
6 cycles of Carboplatin - Taxol up-front and then delay the pelvic RT until after chemotherapy completion. | Applicable | ||||||
| Gynaecological – others | |||||||||
| Vulvar cancer | Early-stage: surgery could be delayed up to 1–2 months No surgery indication: RTCT without delay |
Applicable | |||||||
| Vaginal cancer | To favour imaging for staging in order to omit LN surgery RTCT if no surgery indication without delay |
||||||||
| Ovarian | Early-stage: delay surgery up to 1–2 months Advanced stage: to favour primary systemic therapy No intraperitoneal hyperthermia chemotherapy (CHIP). |
Not available mainly | |||||||
RTCT, radiochemotherapy RT, radiotherapy; NA, neoadjuvant; LN, lymph nodes; COVID-19, coronavirus disease 2019; LMICs, low- and middle-income countries; MSKCC: Memorial Sloan Kettering Cancer Center; PET-CT: positron emission tomography-computes tomography; G-CSF: Granulocyte colony-stimulating factor
3.4.1. Head and neck cancer
The medical complexity of head and neck cancer management may lead to prolonged delays that worsen treatment outcomes. Therefore, those caring for patients with head and neck cancer must take action to reduce these negative impacts as the country rallies to overcome the challenges posed by this pandemic [24]. Thus, therapeutic adaptation possibilities reported by the French groups are based mainly on expert opinion [30,31]. Thus, in daily practice, any adaptation must be discussed with the patient and in the frame of multidisciplinary board meetings. For surgery community, the aim is to minimize the risk of care opportunity loss for patients and to anticipate the increased number of cancer patients to be treated at the end of the pandemic, taking into account the degree of urgency, the difficulty of the surgery, the risk of contaminating the caregivers (tracheotomy) and the local situation (whether or not the hospital and intensive care departments are overstretched) [30,31].
All indications for combined chemoradiotherapy must be maintained, as well as the usual delays between diagnosis and RT (≤4 weeks) or between surgery and RT (6–8 weeks). Fractionation must be optimized: favour hypofractionation (early-stage larynx, elderly or comorbid patients) and simultaneous integrated boost [30].
3.4.2. Lung cancer
Following the ESTRO (European society of therapeutic radiation oncology)-ASTRO (American society of therapeutic radiaion oncology) consensus statement [32] and the French recommendations [33], we need to distinguish recommendations according to pathology and stage of the disease.
For early non–small-cell lung cancer, surgery could be delayed up to 6 weeks for stage I II, N0 disease. Another alternative approach is to deliver stereotactic RT with a limited number of fractions (1 to 3).
In the post-operative setting, patients undergoing treatment have to complete their program. For adjuvant RT, given the uncertainty about its impact in this context, it is not recommended to initiate RT for patients with N2 disease.
In locally advanced non–small-cell lung cancer, patients are more at risk of developing severe respiratory acute complications requiring intensive care. Thus, only patients who are already undergoing therapy should complete their program while initiation of RT in new patients is discussed in the frame of multidisciplinary bord meetiings. For patients undergoing chemotherapy, carboplatin is preferred over cisplatin for its rapidity of administration and its lower toxicity.
The administration of durvalumab must be carried out if the safety conditions are reasonable, by adapting the treatment regimen [33].
3.4.3. Breast cancer
The American Society of Breast Surgeons (ASBS) [34], the French Saint Paul-de-Vence (SPDV) group [35] and the international RT network [3] reported their recommendations in March. The ASBS recommended breaking into three priority categories based on patient conditions, ranging from immediately life-threatening conditions to patients stable enough that services can be delayed for the duration of the COVID-19 pandemic. In the SPDV conception, different scenarios were detailed.
In summary, for post-operative patients and those on follow-up telemedicine is recommended. Interventions for biopsies in abnormal mammograms, neoadjuvant patients finishing treatment, hormone therapy during 6–12 months for luminal hormone receptor (HR)-positive patients, adjuvant antibody treatment reasonably be curtailed after 7 months instead of 12 months of treatment for HER2-positive and limit reconstructive surgery to expander only are the main recommendations. In addition, more specifically in patients with breast luminal cancer (early or locally advanced) primary endocrine treatment could safely delay surgery up to 3 to 6 months, in a ccoradnce with several published trials.
For adjuvant RT, up to 16 weeks of last surgery or chemotherapy with high-risk indications for radiation, such as inflammatory disease, node positive disease, triple negative breast cancer, post-neoadjuvant chemotherapy with residual disease at surgery, young age (<40) with additional high-risk features, are recommended.
Patients older than 65–70 y with lower risk stage I HR-positive/HER2- negative cancers and ductal carcinoma in situ (DCIS), adjuvant endocrine therapy can be encouraged to defer/omit radiation without affecting overall survival. Hypofractionated regimens are recommended, including in cases of DCIS, post-mastectomy or nodal RT. The boost is reserved for high-risk patients, and SIB or hypofractionation is to be preferred [3,22,29,35].
3.4.4. Gynaecological cancers
Since the beginning of March, numerous scientific societies and research groups provided recommendations for cervical, endometrial and ovarian cancer [36,37].
In summary, for cervical cancer, it has been suggested that the value of lymph node staging surgeries must be reviewed on a case-by-case basis in accordance with comorbidities, imaging results and stage of disease. For therapy, the recommendation is to omit any changes in radiochemotherapy regimens or interrupt or postpone RT that could lead to tumour response reduction. After concomitant radiochemotherapy, surgery should not be systematic in cases of complete response.
In endometrial cancer, recommendations are in accordance with the stage of disease [36]. Surgery remains the standard of care for early-stage disease. The minimally invasive laparoscopic approach, robot-assisted (or not) is the preferred approach. For low and intermediate pre-operative European society of medical oncology (ESMO) risk, total hysterectomy with bilateral ovariectomy associated with a sentinel node procedure should be preferred. It is lawful to consider postponing surgery by 1–2 months in low-risk endometrial cancers. For high ESMO risks involving staging by pelvic and lumbo-aortic nodal dissection, it seems recommended to favour the Memorial Sloan Kettering Cancer Center (MSKCC) algorithm, associating positrons emission tomography - computed tomography (PET-CT) and sentinel node biopsy procedure to omit nodal dissection which increases the risk of pre- and post-operative complications.
For ovarian cancers, recommendations are as per the stage of disease. For early-stage ovarian cancer, surgery may be postponed by 1–2 months, whereas for advanced disease, neoadjuvant chemotherapy or primary ‘debulking’ surgery should be considered. In cases of neoadjuvant chemotherapy, additional cycles up to 6 could be considered before surgery [36].
For vulvo-vaginal cancers, the main messages consist of delaying surgery by 1–2 months and indicate chemoradiation in locally advanced cases, whereas PET-CT for staging should be favoured to delay lymph node dissection.
3.4.5. Genito-urinary cancers
Recommendations concerned mainly prostate, bladder and kidney cancers. In summary, the French recommendations [12,38] for prostate cancer are oriented towards delaying all RT planning by 3–6 months. During this period, ADT administration is recommended in intermediate and high-risk patients. For RT, schedules should be hypofractionated if RT is started during the COVID-19 period [39,40]. For low-risk patients, surgery could be delayed in favour of active surveillance. Moreover, surgery should not be delayed more than two months in high-risk and locally advanced patients [12]. This will be considered in accordance with the availability of operating rooms and the post-operative complications risk.
In patients with bladder cancer, surgery should not be delayed more than three months in the majority of cases. When RT is indicated, it should be hypofractionated. Primary chemotherapy and number of cycles should be discussed on a case-by-case basis. If surgery is preferred, a maximum of three months after diagnosis is proposed [12].
For the kidneys, beside locally advanced and thrombosis situations, surgery has to be delayed after confirmation in the tumour board [12].
3.4.6. Gastrointestinal cancer
In summary, surgery could be postponed by three months for gastrointestinal (GI) cancers when other therapies are indicated. All decisions should be taken in accordance with the stage of the disease and the risk of spread in case of delaying optimal therapy [25].
Oesophagus cancer should be treated whenever possible with RT and concomitant carboplatin and taxol. In the neoadjuvant setting or salvage therapy, surgery could be delayed up to three months [40,41]. Detailed RT recommendations, including definitive combined radiochemotherapy and fractionation have been reported by Jones et al. [26]. They also suggested that as the impact of RT on disease severity in patients with a diagnosis of COVID-19 is unknown and it may be appropriate to avoid RT in such patients.
Surgery for rectal cancer should be delayed up to three months [40] and neoadjuvant treatment for new patients should be carried out with the short-course scheme [40,42], reserving the classical CAP50 therapy. The watch and wait attitude is possible for patients with complete response after standard chemo-RT [40,43,44]. In anal canal carcinomas, chemo-RT capecitabine should replace 5-FU+/- mitomycin C[40,45]. Brachytherapy, if indicated, could be delayed [39].
For pancreatic and other GI cancers, ESMO guidelines defined high priorities for surgery and systemic therapy [46,47]. All resectable cancers and borderline patients who are not fit for neoadjuvant chemotherapy should be considered as high priority. In locally advanced disease, initiation or completion of neoadjuvant chemotherapy when already started or patients included in clinical trials should be also considered as a high priority [47].
4. Discussion
This review aims to summarize some national/international guidelines and literature regarding the organization of patient management and specific recommendations by tumour sites. Indeed, outbreaks of COVID-19 disease may result in the interruption of medical care provided to patients with cancer and induce undertreatment in addition to the risk of infection and death from COVID-19. Early data from China and Italy suggest that patients with cancer may be at higher risk of contracting COVID-19, particularly when multiple visits are needed, and also developing more severe forms of the disease [[48], [49]]. One prospective study on 1590 patients pointed out the higher incidence of COVID-19 in patients with cancer [49]. In addition, patients with cancer seem to be at a greater risk of ventilation need and death [50,51]. Thus, preventing patients with cancer from being exposed to COVID-19 is a critical public health priority that needs an important effort in terms of staff and department organization and patient screening to adapt therapy delay and management despite COVID-19 infection risk. The benefit risk/ratio is a key point for all newly diagnosed cancers, as delaying treatment is not recommended if, at all, avoidable [28,52].
In LMICs the problem of delay in diagnosis already exists. So the fear with the COVID-19 pandemic is to see an increase in the number of patients with locally advanced cancers who do not receive treatment in time. In certain countries, with the screening program being stopped during the pandemic, locally advanced forms may resurface in local epidemiology if screening remains suspended for a long time. For head and neck cancers for example, a panel from ASTRO and ESTRO have published statement with two pandemic scenarios: early (risk mitigation) and late (severely reduced RT resources) and treatment recommendations for five head and neck cases. There was agreement (or strong agreement) across a number of practice areas including: treatment prioritization, whether to delay initiation or interrupt RT [27].
In the last three months, a number of scientific societies, oncology groups and experts’ networks have suggested that all efforts should be made to prevent patients with cancer from being exposed to COVID-19. They also proposed several recommendations for urgent new organization in oncology departments with possible patient selection, treatments and schedules tailored in accordance with patients and tumour criteria, so as to continue to provide adequate strategy for the majority of patients [1,2,7,11,16].
In accordance with the local health system organization and tools availability, different approaches have been undertaken by cancer centres and oncologists in countries with early epidemics, in response to the risk of infection, as well as strain on health systems. The main proposals are summarized in Table 2, Table 3. They are as follows:
-
(i)
Minimizing the risk of exposure for patients (with a clear policy of screening before appointments) and staff (with daily temperature screening) to protect the immune compromised patients.
-
(ii)
Department reorganization with minimizing patient turnover times (‘fast track’ area) and number of visits to the hospital. Use of telemedicine and phone calls for postponed appointments and non-urgent surgeries or other treatments [53,54].
-
(iii)
Delaying locoregional therapy data to reduce risk of COVID-19 in patients with cancer are limited. The American College of Surgeons recommends balancing the risk of delay of an elective surgery with the potential likelihood for a post-operative need for respirator utilization [54]. In addition, patients with a history of surgical resection may have continued immunosuppression or other prolonged effects of surgery, which can contribute to COVID-19 risk [55].
-
(iv)
Shortening RT duration and plan recalculations for hypofractionated schedules have been proposed in accordance with the type of cancer and curative or palliative intent situations. In some cases (e.g., low-risk breast and prostate cancers), endocrine therapy is advocated to delay RT by 2–4 months [3,38,39].
-
(v)
Switching patients from intravenous to oral therapies to limit the number of inpatient visits has been recommended in France [1] and Italy [52]. In all cases, delaying systemic therapy is not recommended if at all avoidable [52]. For patients already in deep remission (stable for more than 6–12 months), stopping treatment may be an option [[1], [2], [35]].
The vast majority of the published recommendations for department organization, staff and patients' appointments/visits are quite feasible in the majority of LMICS. At times, there may be complex geographic situations, unavailability of equipment or means of communication (telemedicine) and staff which can negatively impact the situation. The selection of patients for whom care can be deferred becomes a major issue in the organizational management of the weeks in which the contagiousness to COVID-19 is high. However, the definition of priorities can be complicated to do in certain LMICs. For example, the NICE recommendation says that during COVID-19 pandemic ‘use RT only id unavoidable’ [28]. This could be in agreement with the availability of resources and means in LMICs unlike others [[28], [29], [30], [31], [32]] are more precise in terms of patients’ selection that takes into account cancer type and disease stage to not compromise the prognosis.
Table 4 summarizes the main recommendations by tumour sites. There is mainly a deal between avoiding COVID-19 contamination for patients without making them lose chances of cure due to deferring or suspending standard effective treatment. Even if limited evidence exists on the modification of treatment plans to reduce the risk of COVID-19 in patients with cancer, these recommendations are an important support for many oncologists to help with the decision. Adapted to the local situation, they can also serve as a basis for decisions in LMICs. However, we must note the fact that, in addition to the limited means in some countries, the local epidemiological context can limit these recommendations applicability. One example is combined radio chemotherapy for oesophageal cancers. In case of unavailable modern techniques in these countries that could ovoid significantly organ at risk exposure, ovoiding RT has been suggested as an option as the disease severity expected in patients with a diagnosis of COVID-19 is unknown [26].
For surgery scheduling, the American College of Surgeons recommends balancing the risk of delay of an elective surgery with the potential likelihood for post-operative ICU or respirator use [54]. Presumably, in certain LMICs, oncological surgery continues to be carried out in general surgery departments which are very widely transformed into UCI during the COVID-19 crisis. Thus, this transformation necessary to take care of patients with COVID-19 with respiratory distress directly impacts the cancellation of oncological surgery.
Whatever the type of cancer, the consensus is to postpone surgery. The most obvious cases concern those who benefit from screening, such as breast and prostate cancer. The recommended delay can go up to 2–3 months. However, patients’ selection for delaying surgery is a crucial point, as delayed oncologic surgeries may lead to disease progression and result in tumours that are no longer resectable, leading to worse survival outcomes [52]. For locally advanced and high-risk patients, neoadjuvant therapies represent a solution before undergoing surgery in breast and some GI cancers [12,38]. The dilemma in this case is to weigh the risk/benefit ratio of treating patients optimally during the COVID-19 period with the consequences of immunosuppression [[55], [56]] and repeated visits to the hospital to receive RT when it is indicated for combined therapy. The delay between the end of pre-operative treatment and surgery can also be lengthened in many cases, such as rectal [43], endometrial [36] and bladder cancer [12,38].
In addition, prior RT, the primary treatment, can constitute a “waiting” solution during the COVID-19 period. The recommendations are fairly unanimous for high-risk and locally advanced prostate cancers [12,38,39]. Indeed, as the radiohormonotherapy trials of prostate cancer have all used schemes with a primary hormone therapy between 2 and 6 months before RT, it is easier to defer the RT (up to 6 months) without any prejudice for the patient [38,39]. Recently, Zaorsky et al. [23] have to establish recommendations and a framework by which to evaluate prostate RT management decisions. They concluded that the Remote visits, and Avoidance, Deferment, and Shortening of RT framework can be applied to prostate cancers and other disease sites to help with decision-making in the COVID-19 pandemic. This concept could applicable in LMICs with however some specificities regarding local situations of health care and means.
For breast cancer, the data are less robust for primary endocrine therapy while ‘waiting’ for RT. However, the consensus recommends in many situations to postpone or even omit RT in elderly patients with low risk, as no evidence of impact on survival has been demonstrated in this subset of patients [35].
RT hypofractionation, already largely used in LMICs, is systematically recommended in the adjuvant setting without chemotherapy. A recent report from USA has pointed out the evidence-based guidelines for omitting or abbreviating breast cancer RT, where appropriate, in an effort to mitigate risk to patients and optimize resource use [29].
In breast and prostate cancer, high evidence of equivalence between standard and hypofractionation has been demonstrated in the literature. However, in prostate and breast cancer, this is generally true only for limited irradiation volume in patients without extended nodal RT. Large volume of lung RT could increase the risk of lung damage if the patient became infected by COVID-19 during RT and developed severe respiratory acute syndrome [57].
For systemic therapies, there is currently no evidence to support changing chemotherapy and immunotherapy regimens. In addition, delaying treatment is not recommended if at all avoidable [52]. However, some regimens can be altered to minimize infusion visits. fFor all cytotoxic regimens, dose intensity is important and multiple studies report poorer survival in cases of dose intensity reduction. In the surgical consensuses, the option of an increased number of cycles allowing a surgery delay of 2–6 weeks is advocated [12,33]. However, this should be carried out with caution regarding treatment efficacy and its potential toxicity. In cases of systemic therapy maintenance, switching IV to oral therapies largely available in LMICs, is mostly recommended to limit the number in-patient visits to the hospital. For patients already in complete remission, stopping or delaying treatment may be an option. The use of systemic treatments is not contraindicated during the COVID-19 pandemic period. However, given the risk of possible immunosuppression, it is important to discuss the indications and to prioritize the treatment strategies in accoradance with the benefit-risk ratio as this has been underlined in the various reference systems cited previously [46,47,52].
5. Conclusion
The COVID-19 pandemic has and will have a major impact on the organization of healthcare systems. While novel vaccines and drugs that target SARS-CoV-2 are under development, the challenge for oncology community is to continue to provide the best therapy to all patients. It will be crucial to consider the benefit risk ratio for optimal anti-cancer therapy (to minimize the risk of care opportunity loss for patients) and minimize COVID-19 contaminations during therapy that may interrupt or delay therapy, and thus, compromise patients’ outcome. Several recommendations published from the early period of the crisis have helped for urgent new organization in oncology departments with possible patient selection, treatments and schedules tailored according to patients and tumour criteria.
In LMICs, the challenge is to define strategies to try mitigating the deleterious effect of COVID-19 pandemic on cancer care. These negative effects will be probably amplified due to (i) epidemiology of cancer diagnosed in more advanced stages in these countries, (ii) limited means for diagnostic and treatment that will delay cancers management; (iii) the need to manage COVID-19 patients in limited number of centres that will impose delay for cancer therapy and (iv) the economic impact of the COVID-19 pandemic on health system priorities and investment in oncology.
Of note, the vast majority of these recommendations for department organization, are quite feasible in the majority of LMICs where the context for cancers patients is related multiple parameters such as: late diagnosis, long waiting lists for therapy and the lack of therapeutic innovations due to the economic situation which may be worsened by the COVI-19 pandemic.
Financial support
None.
Conflict of interest statement
A.I. declares the following relevant financial activities outside the submitted work: has received Grants from Transgene, Sanofi, Air Liquide, Nutritheragene; has received travel funding from Leo Pharma; Grant research support and travel funding from Carthera. J.G. declare the following financial personnal fees for activities outside the submitted work or served as consultant or advisory board/ has received symposium and travel funding from: Roche-Genentech, Novartis, Onxeo, Dachii Sankyo, MSD, Isai, Genomic Health, Ipsen, Macrogenics, Pfizer, Mylan, Lilly, Immunomedics, Sandoz. J.-P.S. declares the following financial personnal fees for activities outside the submitted work or served as consultant or advisory board/ has received Symposium and travel funding from: MSD, Lilly, Roche, Mylan, Pfizer, PF Oncology, LeoPharma, Novartis, Biogaran, Astra Zeneca, Gilead, BMS. All the other authors have no conflict of interest to declare.
Acknowledgements
The authors would like to thank Ms Myrna Perlmutter for her help in editing English language for this manuscript.
References
- 1.You B., Ravaud A., Canivet A., Ganem G., Giraud P., Guimbaud R., et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020 Mar 25;(20):30204–30207. doi: 10.1016/S1470-2045(20)30204-7. pii: S1470–2045, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shamsi H.O., Alhazzani W., Alhuraiji A., Coomes E.A., Chemaly R.F., Almuhanna M., et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncol. 2020 Apr 3 doi: 10.1634/theoncologist.2020-0213. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S., et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020 May;32(5):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez P.T., Chiva L., Eriksson A.G.Z., Frumovitz M., Fagotti A., Martin A.G., et al. COVID-19 global pandemic: options for management of gynecologic cancers. Int J Gynecol Canc. 2020 Mar 27 doi: 10.1136/ijgc-2020-001419. pii: ijgc-2020–001419, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Lung Cancer Study Group Chinese Thoracic Society, Chinese Medical Association; Chinese Respiratory Oncology Collaboration. Expert recommendations on the management of patients with advanced non-small cell lung cancer during epidemic of COVID-19 (Trial version)] Zhonghua Jiehe He Huxi Zazhi. 2020 Mar 3;43:E031. doi: 10.3760/cma.j.cn112147-20200221-00138. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Simonato A., Giannarini G., Abrate A., Bartoletti R., Crestani A., De Nunzio C., et al. Members of the research urology network (RUN). Pathways for urology patients during the COVID-19 pandemic. Minerva Urol Nefrol. 2020 Mar 30 doi: 10.23736/S0393-2249.20.03861-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Jazieh A.R., Al Hadab A., Al Olayan A., Al Hejazi A., Al Safi F., Al Qarni A., et al. Managing oncology services during a major coronavirus outbreak: lessons from the Saudi arabia experience. JCO Glob Oncol. 2020 Mar;6:518–524. doi: 10.1200/GO.20.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ficarra V., Novara G., Abrate A., Bartoletti R., Crestani A., De Nunzio C., et al. Members of the research urology network (RUN). Urology practice during COVID-19 pandemic. Minerva Urol Nefrol. 2020 Mar 23 doi: 10.23736/S0393-2249.20.03846-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Filippi A.R., Russi E., Magrini S.M., Corvò R. CVID-19 outbreack in northern Italy : first practical indications fro radiotherapy. Int J Radiat Oncol Biol Phys. 2020 Mar 18;(20):30930–30935. doi: 10.1016/j.ijrobp.2020.03.007. pii: S0360–3016, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Givi B., Schiff B.A., Chinn S.B., Clayburgh D., Gopalakrishna Iyer N., Jalisi S., et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 Pandemic. JAMA Otolaryngol Head Neck Surg. 2020 Mar 31 doi: 10.1001/jamaoto.2020.0780. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Rahimi F., Talebi Bezmin Abadi A. Practical strategies against the novel coronavirus and COVID-19-the imminent global threat. Arch Med Res. 2020 Apr;51(3):280–281. doi: 10.1016/j.arcmed.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mejean A., Rouprêt M., Rozet F., Bensalah K., Murez T., Game X., et al. Le Comité de Cancérologie de l’Association Française d’Urologie (CCAFU). Recommendations CCAFU on the management of cancers of the urogenital system during an epidemic with Coronavirus COVID-19. Prog Urol. 2020 Apr;30(5):221–231. doi: 10.1016/j.purol.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aklaidos C., Azias H., Ballester M., Bendifallah S., Bolze P.-A., Bourdel N., et al. Guidelines for surgical management of gynaecological cancer during pandemic COVID-19 period - FRANCOGYN group for the CNGOF. Gynecol Obstet Fertil Senol. 2020 Mar 25;(20):30130–30136. doi: 10.1016/j.gofs.2020.03.017. pii: S2468–7189, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z., Bai H., Duan J.C., Wang J. Individualized treatment recommendations for lung cancer patients at different stages of treatment during the outbreak of 2019 novel coronavirus disease epidemic] Zhonghua Zhongliu Zazhi. 2020 Mar 3;42:E007. doi: 10.3760/cma.j.cn112152-20200228-00146. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Li X., Liu M., Zhao Q., Liu R., Zhang H., Dong M., et al. Preliminary recommendations for lung surgery during 2019 novel coronavirus disease (COVID-19) epidemic period] Zhongguo Fei Ai Za Zhi. 2020 Mar 20;23(3):133–135. doi: 10.3779/j.issn.1009-3419.2020.03.01. Epub 2020 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngoi N., Lim J., Ow S., Jen W.Y., Lee M., Teo W., et al. National University Cancer Institute Singapore (NCIS). A segregated-team model to maintain cancer care during the COVID-19 outbreak at an academic center in Singapore. Ann Oncol. 2020 Mar 31;(20):36410–36413. doi: 10.1016/j.annonc.2020.03.306. pii: S0923–7534, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020 Apr 2 doi: 10.1038/s41571-020-0362-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Felice F., Petrucciani N. Treatment approach in locally advanced rectal cancer during Coronavirus (COVID-19) pandemic: long course or short course? Colorectal Dis. 2020 Apr 1 doi: 10.1111/codi.15058. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Di Saverio S., Pata F., Gallo G., Carrano F., Scorza A., Sileri P., et al. Coronavirus pandemic and Colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020 Mar 31 doi: 10.1111/codi.15056. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Grellety T., Ravaud A., Canivet A., Ganel G., Giraud P., Guimbaud R., et al. SARS-CoV-2/COVID 19 Infection and solid cancers: synthesis of recommendations for health professionals. Bull Cancer. 2020 Apr;107(4):400–402. doi: 10.1016/j.bulcan.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Wang S., Yu K. COVID-19 infection epidemic: the medical management strategies in Heilongjiang Province, China. Crit Care. 2020 Mar 18;24(1):107. doi: 10.1186/s13054-020-2832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simcock R., Thomas T.V., Estes C., Filippi A.R., Katz M.A., Pereira I.J., et al. COVID-19 global radiation oncology’s targeted response for pandemic preparedness. Clin Trans Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaorsky N.G., Yu J.B., McBride S.M., Dess R.T., Jackson W.C., Mahal B.A., et al. Prostate cancer radiotherapy recommendations in response to COVID-19. Adv Radiat Oncol. 2020 Apr 1 doi: 10.1016/j.adro.2020.03.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner M.T., Carey R.M., Albergotti W.G., Lukens J.N., Brody R.M. Impact of the COVID-19 pandemic on the management of head and neck malignancies. Otolaryngol Head Neck Surg. 2020 Apr 21 doi: 10.1177/0194599820921413. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Tuech J.J., Gangloff A., Di Fiore F., Michel P., Brigand C., Slim K. Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Vis Surg. 2020 Mar 31;(20):30070–30079. doi: 10.1016/j.jviscsurg.2020.03.008. pii: S1878–7886, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones C.M., Hawkins M., Mukherjee S., Radhakrishna S., Crosby T. Considerations for the treatment of oesophageal cancer with radiotherapy during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 2020 Jun;32(6):354–357. doi: 10.1016/j.clon.2020.04.001. pii: S0936–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson D.J., Palma D., Guckenberger M., Balermpas P., Beitler J.J., Blanchard P., et al. Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020 Apr 14;(20):31034–31038. doi: 10.1016/j.ijrobp.2020.04.016. pii: S0360–3016, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahase E. Covid-19: use radiotherapy only if "unavoidable," says NICE. BMJ. 2020 Apr 1;369:m1338. doi: 10.1136/bmj.m1338. [DOI] [PubMed] [Google Scholar]
- 29.Braunstein L.Z., Gillespie E.F., Hong L., Xu A., Bakhoum S.F., Cuaron J., et al. Breast radiotherapy under COVID-19 pandemic resource constraints - approaches to defer or shorten treatment from a Comprehensive Cancer Center in the United States. Adv Radiat Oncol. 2020 Apr 1 doi: 10.1016/j.adro.2020.03.013. [Epub ahead of print] Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://www.gortec.net/images/covid19/Radiotherapie_ORL_Recommandations_GORTEC_COVID.pdf.
- 31.Fakhry N., Schultz P., Morinière S., Breuskin I., Bozec A., Vergez S., et al. French Society of Otorhinolaryngology, Head and Neck Surgery (SFORL), French Society of Head and Neck Carcinology (SFCCF). French consensus on management of head and neck cancer surgery during COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020 May;137(3):159–160. doi: 10.1016/j.anorl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guckenberger M., Belka C., Bezjak A., Bradley J., Daly M.E., DeRuysscher D., et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. April 6, 2020. [DOI] [PMC free article] [PubMed]
- 33.Girard N., Greillier L., Zalcman G., Cadranel J., Moro-Sibilot D., On behalf of the French-Language Society of Pulmonology (SPLF) / French-language Oncology Group Proposals for managing patients with thoracic malignancies during COVID-19 pandemic. Respir Med Res. 2020 May 24:100769. doi: 10.1016/j.resmer.2020.100769. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.https://www.breastsurgeons.org/docs/news/2020-03-26-ASBrS-ACR-Joint-Statement.pdf.
- 35.Gligorov J., Bachelot T., Pierga J.Y., Antoine E.C., Balleyguier C., Barranger E., et al. COVID-19 and people followed for breast cancer: French guidelines for clinical practice of Nice-St Paul de Vence, in collaboration with the Collège Nationale des Gynécologues et Obstétriciens Français (CNGOF), the Société d’Imagerie de la FEMme (SIFEM), the Société Française de Chirurgie Oncologique (SFCO), the Société Française de Sénologie et Pathologie Mammaire (SFSPM) and the French Breast Cancer Intergroup-UNICANCER (UCBG) Bull Cancer. 2020 May;107(5):528–537. doi: 10.1016/j.bulcan.2020.03.008. pii: S0007–4551, French. Publication en ligne 2020 avr. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akladios C., Azais H., Ballester M., Bendifallah S., Bolze P.A., Bourdel N., et al. Recommendations for the surgical management of gynecological cancers during the COVID-19 pandemic - FRANCOGYN group for the CNGOF. J Gynecol Obstet Hum Reprod. 2020 Jun;49(6):101729. doi: 10.1016/j.jogoh.2020.101729. Published online 2020 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yale radiation oncology Flowchart for COVID-19 – Version. March 20, 2020. https://www.medscape.com/viewarticle/927258 [Google Scholar]
- 38.French GETUG recommendations Therapeutic options for genitourinary cancers during the epidemic period of COVID-19. Bull Cancer. 2020 Mar 27;20:30154–30155. doi: 10.1016/j.bulcan.2020.03.003. pii: S0007–4551, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giraud P., Monpetit E., Lisbona A., Chargari C., Marchesi V., Dieudonné A., et al. Covid-19 epidemic: guidelines issued by the French society of oncology radiotherapy (SFRO) for oncology radiotherapy professionals] Canc Radiother. 2020 Mar 31;20:30077–30079. doi: 10.1016/j.canrad.2020.03.007. pii: S1278–3218, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuech J.J., Gangloff A., Di Fiore F., Michel P., Brigand C., Slim K., et al. Strategy for the practice of digestive and oncological surgery during the COVID-19 epidemic. J Visc Surg. 2020 Mar 31 doi: 10.1016/j.jviscsurg.2020.03.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Fiore F., Bouché O., Lepage C., Sefrioui D., Gangloff A., Schwarz L., et al. COVID-19 epidemic: Proposed alternatives in the management of digestive cancers: A French intergroup clinical point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver Dis. 2020 Jun;52(6):597–603. doi: 10.1016/j.dld.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erlandsson J., Holm T., Pettersson D., Berglund A., Cedermark B., Radu C., et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017 Mar;18(3):336–346. doi: 10.1016/S1470-2045(17)30086-4. [PMID:28190762] [DOI] [PubMed] [Google Scholar]
- 43.Renehan A.G., Malcomson L., Emsley R., Gollins S., Maw A., Myint A.S., et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016 Feb;17(2):174–183. doi: 10.1016/S1470-2045(15)00467-2. [PMID:26705854] [DOI] [PubMed] [Google Scholar]
- 44.Rullier E., Rouanet P., Tuech J.J., Valverde A., Lelong B., Rivoire M., et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicenter, phase 3 trial. Lancet. 2017 Jul 29;390(10093):469–479. doi: 10.1016/S0140-6736(17)31056-5. [PMID:28601342] [DOI] [PubMed] [Google Scholar]
- 45.Meulendijks D., Dewit L., Tomasoa N.B., van Tinteren H., Beijnen J.H., Schellens J.H.M., et al. Chemoradiotherapy with capecitabine for locally advanced anal carcinoma: an alternative treatment option. Br J Canc. 2014 Oct 28;111(9):1726–1733. doi: 10.1038/bjc.2014.467. [PMID:25167226] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vecchione L., Stintzing S., Pentheroudakis G., Douillard J.-Y., Lordick F. ESMO management and treatment adapted recommendations in the COVID-19 era: colorectal cancer. ESMO Open. 2020;5(Suppl 3):e000826. doi: 10.1136/esmoopen-2020-000826. Published online 2020 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catanese S., Pentheroudakis G., Douillard J.-Y., Lordick F. ESMO management and treatment adapted recommendations in the COVID-19 era: pancreatic cancer. ESMO Open. 2020;5(Suppl 3):e000804. doi: 10.1136/esmoopen-2020-000804. Published online 2020 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krengli M., Ferrara E., Mastroleo F., Brambilla M., Ricardi U. Running a radiation oncology department at the time of coronavirus: an Italian experience. Adv Radiat Oncol. 2020 Mar 20 doi: 10.1016/j.adro.2020.03.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30096-6/fulltext.
- 51.https://ourworldindata.org/coronavirus.
- 52.Cortiula F., Pettke A., Bartoletti M., Puglisi F., Helleday T., et al. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31:5. doi: 10.1016/j.annonc.2020.03.286. Available online 19 March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohannessian R., Duong T.A., Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-9 pandemic: a call to action. JMIR Publ Health Surveill. 2020 Apr-Jun;6(2):e18810. doi: 10.2196/18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American College of surgery. COVID 19: Elective case triage guidelines for surgical care. https://www.facs.org/covid-19/clinical-guidance/elective-case/cancer-surgery.
- 55.Spolverato G., Capelli G., Restivo A., Bao Q.R., Pucciarelli S., Pawlik T.M., et al. The management of the surgical patients during the coronavirus dieases 2019 (COVID-19) pandemic. Surgery. 2020 May 1;6(2) doi: 10.1016/j.surg.2020.04.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia Y., Jin R., Zhao J., Li W., Shen H. Risk of COVID-19 for cancer patients. Lancet Oncol. 2020 Apr;21(4) doi: 10.1016/S1470-2045(20)30150-9. Epub 2020 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grellier N., Hadhri A., Bendavid J., Adou M., Demory A., Bouchereau S., et al. Regional lymph node irradiation in breast cancer may worsen lung damage in COVID-19 positive patients. Adv Radiat Oncol. 2020 May 19 doi: 10.1016/j.adro.2020.04.033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]