Abstract
Objective
To determine the incidence, characteristics, and risk factors of pulmonary embolism (PE) among patients hospitalized for COVID-19.
Patients and Methods
We performed a prospective observational study of a randomly selected cohort of consecutive patients hospitalized for COVID-19 infection between March 8, 2020 through April 25, 2020. All eligible patients underwent a computed tomography pulmonary angiography independently of their PE clinical suspicion and were pre-screened for a baseline elevated D-dimer level.
Results
119 patients were randomly selected from the 372 admitted to one tertiary hospital in Valencia (Spain) for COVID-19 infection during the period of study. Seventy-three patients fulfilled both the inclusion criteria and none of the exclusion criteria and were finally included in the study. Despite a high level of pharmacological thromboprophylaxis (89%), the incidence of PE was 35.6% (95% confidence interval [CI], 29.6 to 41.6%), mostly with a peripheral location and low thrombotic load (Qanadli score 18.5%). Multivariate analysis showed that heart rate (Hazard Ratio [HR], 1.04), room-air oxygen saturation (spO2) (HR, 0.87), D-dimer (HR, 1.02), and C-reactive protein (CRP) levels (HR, 1.01) at the time of admission were independent predictors of incident PE during hospitalization. A risk score was constructed with these four variables showing a high predictive value of incident PE (AUC-ROC: 0.86; 95% CI: 0.80 to 0.93).
Conclusions
Our findings confirmed a high incidence of PE in hospitalized COVID-19 patients. Heart rate, spO2, D-dimer, and CRP levels at admission were associated with higher rates of PE during hospitalization.
Keywords: Pulmonary embolism, COVID-19, Thrombosis, Inflammation, Computed tomography
Abbreviations: ACE2, Angiotensin converting enzyme-2; Ao, Aortic artery; AUC-ROC, Area under curve ROC; BMI, Body mass index; CHOD, CRP concentration + Heart rate + Oxygen saturation + D-dimer levels; CI, Confidence interval; CRP, C-reactive protein; CTPA, Computed tomography pulmonary angiography; CXR, chest X-ray; HR, Hazard Ratio; ICU, Intensive care units; IL6, Interleukin-6; LDH, Lactate dehydrogenase; LV, Left ventricle; PA, Pulmonary artery trunk; PCR, polymerase chain reaction; PE, Pulmonary embolism; RR, Respiratory rate; RV, Right ventricle; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SD, Standard deviation; sPESI, Simplified Pulmonary Embolism Severity Index; spO2, Oxygen saturation; VTE, Venous thromboembolism
Introduction
Since its first outbreak in Wuhan in late December 2019,1 the coronavirus infection known as COVID-19 has spread rapidly around the globe, with more than 55 million people infected and nearly 1350,000 deceased (Centers for Disease Control and Prevention [CDC], WHO; November 18, 2020). This virus, classified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), seems to enter cells by endocytosis, using angiotensin converting enzyme-2 (ACE2) protein receptors found in the lungs, heart, kidneys, and gastrointestinal tract, as well as in the blood vessels.2 The abundant expression of ACE2 in type II alveolar cells may cause diffuse alveolar damage and hyperinflammation resulting in pneumonia and acute respiratory distress syndrome, as well as several extrapulmonary manifestations, including cardiovascular, hematologic, and thrombotic complications due to direct and indirect effects of the viral illness.3, 4, 5, 6
As regards the coagulopathy observed in association with SARS-CoV-2 infection, the mechanisms that activate coagulation have been hypothesized as being linked to immune responses, through the release of pro-inflammatory mediators (such as interleukin-1, interleukin-6 [IL6] and tumor necrosis factor-α) that interact with platelets, stimulate the expression of tissue factor (initiating extrinsic coagulation cascade), induce an upregulation of plasminogen activator inhibitor-1, suppress the fibrinolytic system, lead to endothelial dysfunction and complement pathway activation, triggering thrombogenesis.7, 8, 9, 10, 11
One of the most relevant and consistent features of this process is the increase in D-dimer levels, which has been associated in COVID-19 patients with a greater need for mechanical ventilation and admission to an intensive care unit (ICU), as well as death.12, 13, 14, 15, 16, 17, 18, 19
In the last few months, much scientific literature has been devoted to descriptions of different aspects of COVID-19 infection associated with thrombotic complications, particularly pulmonary embolism (PE). The results have been mixed, probably because most studies to date have been performed only on patients attended in an ICU and have not included any systematic or comprehensive investigation of prospective protocols.20, 21, 22, 23, 24, 25, 26, 27, 28 As a consequence, the incidence, characteristics, and risk factors of hemodynamically stable PE associated with COVID-19 infection still remain unknown.
Therefore, to address this critical gap in our knowledge, the main objective of the current study was to assess the incidence of PE in a representative series of patients randomly selected from all those hospitalized for COVID-19 infection. We also aimed to determine the risk factors and thrombotic load of PE in these patients, and to construct a predictive score for PE during hospitalization with easy-to-use variables obtained at the time of admission.
Methods
Patients and study design
We conducted a prospective observational study of consecutive hospitalized patients admitted to the respiratory and internal medicine departments with confirmed COVID-19 infection in one tertiary university hospital in Spain (Polytechnic and University La Fe Hospital. Valencia) between March 8, 2020 and April 25, 2020.
Each COVID-19 patient admitted into hospital was randomly assigned to one pulmonologist or one internal medicine specialist. Around 50% of the specialists involved (seven pulmonologists and three internal medicine specialists) were randomly selected to participate in the study (and all accepted to participate) and all their patients were initially included in the study, independently of their pre-test clinical probability of PE (n = 119). D-dimer levels were assessed before final inclusion on the basis that normal levels render acute PE unlikely and D-dimer is more frequently elevated in hospitalized patients, in infection or inflammatory disease, and in COVID-19 patients.29 , 30
The inclusion criteria were a) patient age≥18 years and b) elevated D-dimer levels, defined as>500 ng/mL, except in patients older than 50 years, where an age-adjusted cut-off was used (age x 10 ng/mL).31 , 32
The exclusion criteria were a) pregnancy; b) hemodynamic instability, defined as cardiac arrest or obstructive shock (systolic blood pressure < 90 mmHg or vasopressors required to achieve blood pressure ≥ 90 mmHg;29 c) contraindicated exposure to radiological contrast due to severe renal failure [creatinine clearance < 30 mL/min/1.73 m2]; d) known iodine allergy; e) supine intolerance, and/or f) any other indication of full-dose anticoagulation (i.e., atrial fibrillation, prosthetic heart valve).
Patients were treated at their attending physician's discretion, according to our internal protocol and their clinical judgement. All the eligible patients (n = 73) underwent a computed tomography pulmonary angiography (CTPA) to assess the presence of PE, in accordance with the research protocol.
The research protocol was approved by the ethics committee of the Hospital Universitari i Politècnic La Fe (Valencia, Spain), with the register number 2020–197–1. In accordance with this committee's requirements, all the patients provided informed consent to participate in the study orally, because of the biological risk involved in obtaining written consent. We followed the guidelines for observational studies of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.33
Diagnosis of COVID-19 infection and pneumonia
Diagnosis of COVID-19 was confirmed either by a positive result in a real-time polymerase chain reaction (PCR) test of a nasopharyngeal or sputum sample or by a positive result from serological testing, COVID-19 suggestive opacities on chest X-ray and clinically compatible presentation (fever, cough, dyspnea, asthenia, myalgia, or diarrhea).1 , 34, 35, 36
The extent of the effects on the lung in a chest X-ray (CXR) was assessed by an expert thoracic radiologist, following our internal protocol. Each lung was divided on CXR in upper (suprahilar), medium (hilar) and inferior field (infrahilar) with a total of 6 lung fields. The affected lung extent was graded according to the fields involved: I: no lung opacities; II: mild extent (< 1 field); III: moderate extent (1–2 fields); IV: severe extent (3–4 fields); V: very severe extent (5–6 fields).
Clinical and analytical measurements
A complete set of clinical variables, including clinical history, clinical picture, onset of symptoms, previous treatments, comorbidities, physical examination, and daily evolution, was carefully recorded from all patients at admission and during hospitalization. Peripheral blood samples were collected at admission to analyze a set of acute phase reactants, including lactate dehydrogenase (LDH), total and differential leukocyte count, platelet count, D-dimer levels, IL6 levels, high sensitivity C-reactive protein (CRP), and albumin. Treatments used for the COVID-19 infection were also carefully recorded (Supplemental Table 1). The local D-dimer laboratory test was HemosILⓇ D-dimer HS500, a quantitative D-dimer assay with a threshold of 500 ng/mL.37 , 38 The clinical risk of patients with confirmed PE was determined by the simplified Pulmonary Embolism Severity Index (sPESI). On this basis, the patients were divided into two groups: low-risk (0 points) and high-risk (1 point or more) (Supplemental Table 2).39, 40
Computed tomography pulmonary angiography
The images were acquired with a 256-slice multidetector CT (Philips Brilliance iCT) after intravenous injection of iodinated contrast agent (Iomeprol-400) at 4 ml/s, triggered on the main pulmonary artery.
Each CTPA was read by two independent experienced thoracic radiologists, blinded to patient status as well as to clinical and biological features. PE was defined by the presence of at least one intravascular contrast filling defect, and the closest pulmonary vessel affected was used to characterize the PE location as being in the main, lobar, segmental, or subsegmental pulmonary arteries. If any images proved difficult to interpret, a third independent radiologist examined them to provide a consensus.
The report also included the thrombotic load, measured via the Qanadli Score (Supplemental Figure 1),41 the pulmonary artery trunk diameter (PA) -at the level of the pulmonary artery bifurcation-, the ratio of the PA/Aortic diameter (Ao), the right ventricle (RV) diameter and the RV/left ventricle (LV) ratio. The extent of lung involvement was visually assessed as the percentage of lungs with COVID-19 suggestive ground-glass opacities or consolidations on CTPA.42 Five categories of involvement were described: I: no lung opacities; II: mild extent (≤ 25%); III: moderate extent (26–50%); IV: severe extent (51–75%); V: very severe extent (≥76%).
Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD), and qualitative or dichotomous variables as percentages. Normal distribution of the variables was assessed by the Kolmogorov-Smirnov test. To analyze the characteristics of patients according to the presence or not of PE, a Student's t-test (or non-parametric test when appropriate) and Chi-Square test (or Fisher exact when appropriate) were used to compare the groups.
A sample size was calculated to analyze the estimated incidence of PE (main variable of the study) in the whole group of 372 patients hospitalized because of COVID-19 infection. According to the current literature, the expected incidence of PE in hemodynamically stable (mainly non-ICU) patients with elevated D-dimer and routine thrombosis prophylaxis (as similar as possible to our patients) is 10%.43 Assuming a calculation error of 6%, a confidence level of 95%, 73 patients with a valid reading of CTPA scan were needed. Agreement between radiologists on the diagnosis of PE was assessed via the Kappa index. The study was completed when the sample size of valid patients was reached.
Multivariable logistic regression was used to determine the optimal independent variables associated with the diagnosis of PE at admission. The variables included in the logistic model were those considered clinically relevant by the authors after an assessment of the current literature.
In order to construct a predictive score for PE in our patients, those quantitative variables related to the presence of PE in the logistic regression analysis were dichotomized. The cut-off point for each variable was selected using the best area under curve ROC (AUC-ROC) for the prognosis of PE via the Youden index. After dichotomization, these variables were again included in a logistic regression model. The beta coefficient value of each variable (rounded to the closest whole value) was selected to assess the relative weight of each variable in the model. The sum of the relative weight of all the variables included in the score corresponded to its total value. The score was divided into terciles and a post-test probability of suffering a PE during hospitalization was calculated for each tercile. Finally, a ROC-curve for the prognostic value of the score was constructed.
A Spearman test was used to assess the correlation between the Qanadli score and the analytical and CTPA measurements.
A two-sided p<0.05 was considered to be statistically significant and all the calculations were made with the corresponding 95% confidence interval (CI). Statistical analysis was carried out using SPSS software, version 24 (SPSS, Inc., Chicago, IL, USA).
Results
Characteristics of patients at baseline and treatment
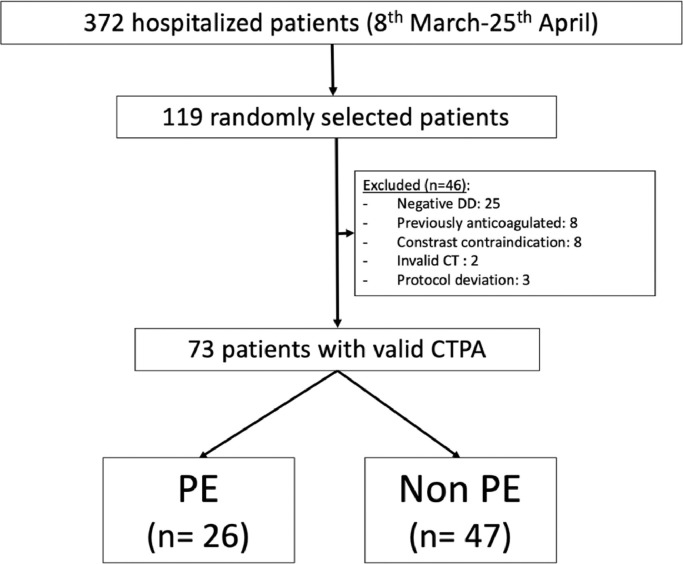
In order to achieve 73 valid patients, we randomly screened a total cohort of 119 (32% patients from the total of 372 hospitalized from March 8, 2020 to April 25, 2020) with a confirmed diagnosis of COVID-19 infection. Although our initial protocol included the possibility of a serological test for inclusion in the study, in the end all the patients were included after a positive PCR test. Forty-six patients were excluded, mainly for a D-dimer value below the threshold level (25 patients) (Fig. 1 ).
Fig. 1.
Flow chart of the study.
Abbreviations: CT, computed tomography; CTPA, computed tomography pulmonary angiography; DD, D-dimer; PE, pulmonary embolism.
Supplemental Table 3 shows that there were no differences between the randomly selected (n = 119) and non-selected (n = 253) groups of patients.
Table 1 shows the main characteristics of the 73 patients finally included in the study. Their mean age was 65.4 (SD 16) years (range: 28 to 92) and 52 (71%) were men. The mean body mass index (BMI) was 29.3 kg/m2 (SD 5.8). The most common comorbidities were arterial hypertension (50%), diabetes (18%), asthma (10%), and obstructive sleep apnea syndrome (10%). The most common symptoms at the time of admission were cough (76%) and dyspnea (45%). Most patients presented multi-lobe pneumonia (77%) and one third were moved to ICU (34%). During the hospitalization, patients received hydroxychloroquine or chloroquine (93%), azithromycin (92%), corticosteroids (46%), lopinavir/ritonavir (34%), tocilizumab (22%), and baricitinib (13%). Furthermore, 86.6% received hydroxychloroquine or chloroquine plus azithromycin, and 43% triple combination plus systemic corticosteroids. Significantly, most of the patients received pharmacological thromboprophylaxis (93%), mainly with enoxaparin 40 mg per day (70%) or 60 mg per day (19%). The mean hospitalization period was 27.3 days (SD 16.1).
Table 1.
Baseline comparative characteristics of patients with and without pulmonary embolism. Data are expressed as mean (SD) or n (%).
| Variable | All patients (n = 73) | With PE (n = 26) | Without PE (n = 47) | p |
|---|---|---|---|---|
| Age, yr | 65.4 (16) | 65.3 (14.5) | 65.5 (16.9) | 0.93 |
| Gender, male/female% | 71%/29% | 69%/31% | 72%/28% | 0.78 |
| BMI, Kg/m2 | 29.3 (5.8) | 29.9 (6.2) | 28.6 (5.5) | 0.49 |
| Symptoms,% -Fever (> 37 °C) -Dyspnea -Cough -Chest pain -Hemoptysis -Abdominal pain -Neurological disorder -Diarrhea -Limbs edema -DVT signs |
77% 45% 76% 17% 4% 6% 1% 30% 3% 3% |
77% 54% 83% 19% 8% 8% 0% 27% 8% 6% |
78% 40% 72% 16% 2% 13% 2% 31% 0% 0% |
0.93 0.26 0.36 0.69 0.29 0.47 0.45 0.74 0.06 0.19 |
| Treatment during hospitalization,% -Systemic steroids -Baricitinib -Lopinavir/ritonavir -Tocilizumab -Azithromycin -Chloroquine (or hydroxychloroquine) -Thromboprophylaxis |
46% 13% 34% 22% 92% 93% 94% |
48% 14% 42% 39% 88% 93% 88% |
44% 11% 29% 10% 93% 93% 98% |
0.87 0.37 0.26 0.001 0.48 0.48 0.14 |
| Cardiovascular disease,% -Arterial hypertension -Diabetes -Dyslipidemia -Stroke -Ischemic heart disease -Heart failure -Atrial fibrillation -Valvular disease |
50% 18% 34% 6% 10% 3% 4% 3% |
50% 8% 35% 4% 8% 4% 4% 0% |
51% 24% 33% 7% 11% 2% 4% 4% |
0.92 0.08 0.91 0.62 0.64 0.69 0.90 0.29 |
| Respiratory disease,% -Asthma -COPD -OSA -Smoke habit (current or former) |
10% 1% 10% 30% |
15% 0% 12% 38% |
7% 2% 9% 19% |
0.24 0.45 0.72 0.13 |
| Other risk factors,% -NSAID -Ongoing cancer -Thrombophilia -Previous VTE -Chronic venous disease -Previous surgery -Antiaggregant therapy |
3% 5% 1% 1% 8% 3% 15% |
3% 8% 0% 0% 15% 4% 16% |
3% 2% 2% 2% 4% 2% 13% |
0.89 0.39 0.45 0.45 0.11 0.69 0.71 |
| At admission -Temperature, °C -Heart rate, bpm -Respiratory rate, rpm -Systolic BP, mmHg -Diastolic BP, mmHg -spO2/FiO2 ratio -room-air sp02,% |
37.3 (0.9) 94.7 (17) 21.5 (5.5) 133 (19.3) 79.7 (9.8) 429 (58) 91.5 (8.6) |
37.4 (0.8) 102 (19) 26 (15) 133.6 (18.7) 81 (10.5) 411 (79) 89.1 (9.8) |
37.2 (1) 88.4 (12.3) 20 (5) 132.3 (19.3) 78.3 (9.6) 440 (38) 92.5 (7.9) |
0.61 0.001 0.01 0.82 0.40 0.043 0.001 |
| ICU,% | 34% | 42% | 28% | 0.25 |
| Orotracheal intubation,% | 27% | 38% | 19% | 0.09 |
| Onset of symptoms, days | 7.3 (6.2) | 6.3 (4.9) | 7.8 (6.8) | 0.31 |
| Length of stay, days | 27.6 (16.1) | 27.8 (17.9) | 27.4 (14.3) | 0.92 |
| CXR pneumonia score -Mild -Moderate -Severe -Very severe |
23.3% 27.4% 20.5 28.7% |
23.1% 23.1% 23.1% 34.6% |
23.4% 29.8% 19.1% 27.7% |
0.39 |
| Analytical at admission -D-dimer, ng/mL -GPT, IU/mL -GOT, IU, mL -Ferritin, pg/mL -IL-6, pg/mL -LDH, IU/mL -CRP, mg/L -Leukocytes, cell/uL -Hemoglobin,% -Neutrophils, cell/uL -Lymphocytes, cell/uL -Platelets, (x103) -proBNP, mg/L -HS troponin T, mg/mL -INR -Quick Index,% -Fibrinogen, mg/dL -aPTT, secs |
4327 (8321) 40.9 (72) 58.9 (77.6) 704.5 (533) 185 (379) 328 (124) 105 (107) 8130 (3.3) 14.7 (13.4) 6245 (3.01) 1289 (1.1) 224 (87) 672 (966) 58.9 (142) 1.1 (0.1) 84.7 (14.1) 651 (91) 31.6 (15) |
6270 (13,814) 57 (110) 79 (115) 915 (618) 226 (456) 379 (147) 154 (128) 8991 (3.6) 12.7 (1.8) 6913 (3.2) 1330 (1.6) 236 (99) 605 (1155) 62 (23) 1.1 (0.2) 83.8 (13.7) 690 (18) 28.9 (3.7) |
2384 (6134)31 (35)46 (44)572 (442)161 (326)297 (127)97 (96)7758 (3.1)15.8 (16.7)5923 (2.9)1120 (0.6)217 (80)686 (259)117 (53)
|
0.006 0.16 0.27 0.11 0.04 0.04 0.001 0.14 0.34 0.21 0.45 0.37 0.74 0.58 0.73 0.76 0.02 0.31 |
Abbreviations: aPTT, activated partial thromboplastin time; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRP, c-reactive protein; CXR, chest X-ray; DVT, deep venous thrombosis; fiO2, inspiratory oxygen concentration; GOT, glutamate-oxaloacetate transaminase; GPT, glutamate pyruvate transaminase; HS, high-sensivity; ICU, intensive care unit; IL6, interleukin-6; INR, international normalized ratio; LDH, lactate dehydrogenase; NSAID, nonsteroidal anti-inflammatory drug; O2, oxygen; OSA, obstructive sleep apnoea; PE, pulmonary embolism; SpO2, oxygen saturation; VTE, venous thromboembolism.
Radiological findings and PE location
Twenty-six out of 73 patients included in the study had a confirmed diagnosis of PE with an incidence of 35.6% (95% CI, 29.6 to 41.6); 51.4% of PE had a sPESI=0 points and 48.6% had a sPESI>0 points. Supplemental Figure 2 shows the distribution of the observed PE. Most of the intraluminal filling defects were detected in peripheral (segmental and subsegmental) branches (45.1% of the right PE and 42.4% of the left PE of the total lumen contrast filling defects seen). No patient had a main artery trunk PE, 5 (19.2%) presented PE in the right or left artery trunk, and 18 (69.2%) presented at least one lobar PE. An example of patient with a lobar and subsegmental PE can be seen in Fig. 2 . The Kappa index between radiologists for the diagnosis of PE was 81% (100% for main, lobar and segmental pulmonary arteries and 69% for subsegmental pulmonary arteries). The mean time lapse between the onset of symptoms and the application of CTPA was 18.2 (SD 13.2) days.
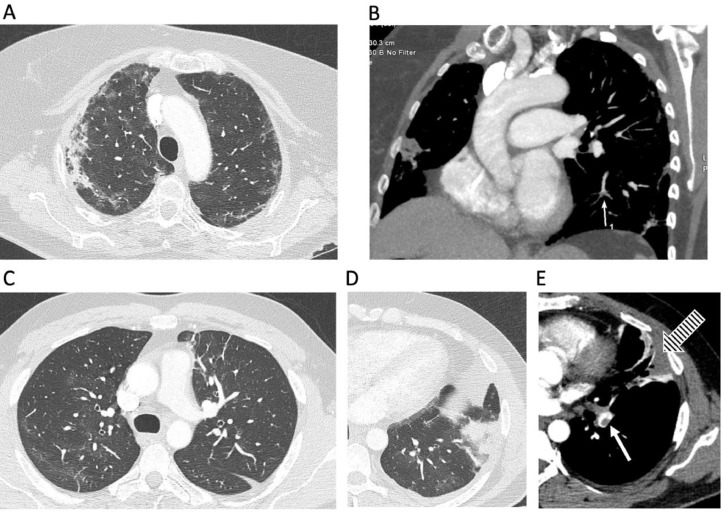
Fig. 2.
Computed tomography (CT) pulmonary angiography: A and B: a 66-year old female patient hospitalized for COVID-19 pneumonia; axial lung tissue setting (A) and soft tissue setting coronal (B) CT image reconstructions showing characteristic COVID-19 pulmonary lesions with ground-glass opacities and consolidations involving both the lung parenchyma in predominantly peripheral distribution (A) and a subsegmental contrast filling defect (white arrow, B). C, D and E: axial CT image reconstructions of a 38-year old male patient who had fever, cough, dyspnea, and left-side chest pain and was admitted with COVID-19 pneumonia; upper lung levels showing patchy ground-glass opacities in the left lung (C), and left lower lung with a triangular subpleural consolidation (D) corresponding to a pulmonary infarction (red arrow) related to the presence of pulmonary thrombus in the left lower lobe, visible in a soft tissue setting (white arrow, E).
Risk factors for PE
The comparative characteristics of patients with PE versus those without PE are shown in Table 1. At the time of admission, patients with PE presented lower room-air oxygen saturation (spO2), higher respiratory (RR) and heart rates, and higher values of systemic inflammation (D-dimer, LDH, CRP, and IL6 concentrations). We failed to find any differences in age, gender, body mass index, blood pressure, or extent of affected parenchyma lung involvement extent at admission. Furthermore, patients with PE were more frequently critically ill, transferred to ICU (42% vs 28%; p = 0.06), and in need of orotracheal intubation (38% vs 19%; p = 0.07).
Supplemental Table 4 shows the comparative CTPA characteristics of those patients with and without PE. There were no differences between groups as regards vascular and heart dimensions, or as regards the extent of pneumonic infiltration.
The following variables assessed at admission were included in a logistic regression model examining their potential clinical relevance: heart rate, RR, LDH, D-dimer and CRP concentrations, BMI, age, and room-air spO2. Of these, only room-air spO2, heart rate, and D-dimer and CRP concentrations at admission were independently associated with the occurrence of PE ( Table 2 ).
Table 2.
Logistic regression. Baseline (hospital admission) quantitative variables independently associated with pulmonary embolism.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Heart rate, bpm | 1.04 | 1.01–1.09 | 0.036 |
| Baseline room-air sp02,% | 0.87 | 0.76–0.98 | 0.041 |
| D-dimer, ng/mL | 1.02 | 1.01–1.04 | 0.022 |
| CRP, mg/L | 1.01 | 1.01–1.05 | 0.037 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; spO2, oxygen saturation.
The chod score
The AUC-ROC method was used to determine the diagnostic value of each selected quantitative variable. The Youden index was used to dichotomize the selected variables and assess the cut-off point with the best prognostic value, and the corresponding AUC-ROC were calculated (Supplemental Table 5).
The dichotomized variables were included in another multivariable logistic regression in order to construct the score. The beta coefficients were rounded to establish the relative weight of each variable in the score (Supplemental Table 6).
Table 3 shows the final score that was constructed. It was named CHOD, an acronym of (CRP concentration + Heart rate + Oxygen saturation + D-Dimer levels).
Table 3.
The CHOD score.
| Variables (at hospital admission) | Values |
|---|---|
|
C-Reactive protein <50 mg/L ≥ 50 mg/L |
0 1 |
|
Heart Rate < 90 bpm ≥ 90 bpm |
0 2 |
|
Oxygen Saturation (room air) > 92% ≤92% or less |
0 2 |
|
D-dimer < 956 ng/mL ≥ 956 ng/mL |
0 2 |
| TOTAL | Range (0–7) points |
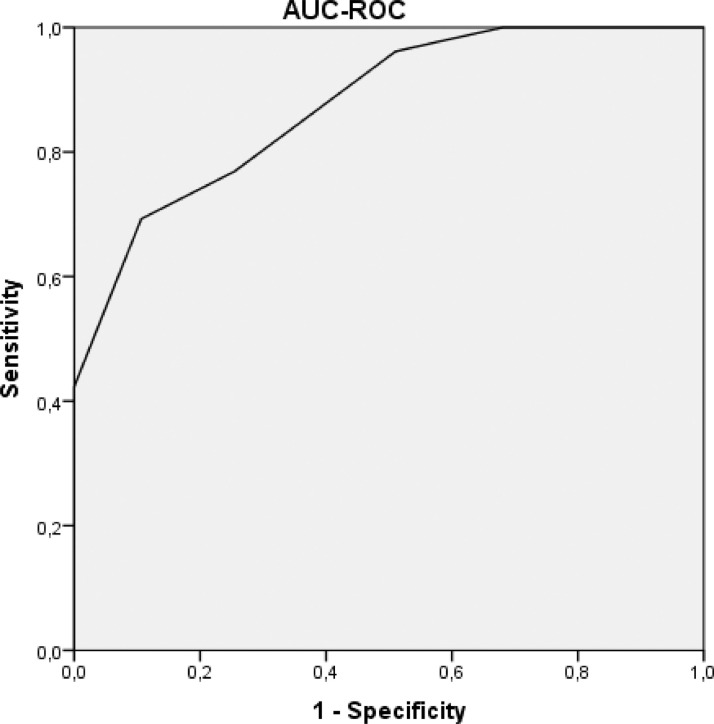
The AUC-ROC of the CHOD score was 0.86 (95% CI: 0.80 to 0.93) ( Fig. 3 ). The score ranged from 0 to 7 points. The probability of incident PE during the hospitalization was low (4.5%) at 0–2 points, moderate (36.8%) at 3–5 points, and high (100%) at 6–7 points.
Fig. 3.
Area under curve ROC of the CHOD score.
Factors associated with the thrombotic load
The mean value of the thrombotic load assessed with the Qanadli score was 18.5% (SD 15.9). Table 4 shows the significant correlations between the Qanadli index and various biomarkers and CTPA findings. A greater thrombotic load was seen in those patients with higher numbers of platelets and higher D-dimer values. The higher the thrombotic load, the larger the PA diameter, PA/Ao ratio, and RV/LV ratio.
Table 4.
Correlations between thrombotic load and peripheral biomarkers and computed tomography pulmonary angiography (CTPA) findings.
| Variables | Spearman correlation |
|---|---|
| At admission | |
| Platelets | r = 0.40; p = 0.045 |
| D-dimer | r = 0.47; p = 0.016 |
| CT findings | |
| PA/Ao ratio | r = 0.40; p = 0.046 |
| PA diameter | r = 0.42, p = 0.034 |
| RV/LV ratio | r = 0.35; p = 0.04 |
Abbreviations: Ao: Aortic diameter; LV; Left Ventricle; PA, Pulmonary artery; RV, Right ventricle.
Discussion
Our data, obtained from a randomly selected cohort of hospitalized patients with confirmed COVID-19 infection and D-dimer value above the threshold level, reveal that the cumulative incidence of PE was higher than expected (35.6%), thus suggesting that SARS-CoV-2 infection has a thrombogenic element. At admission, the risk factors for developing PE during hospitalization were a high heart rate, high D-dimer and CRP levels, and lower room-air oxygen saturation. These variables were used to construct an easy-to-use score that showed an excellent predictive value. The overall thrombotic load of PE was low, predominantly peripheral, and associated with various inflammatory biomarkers and CTPA findings. In our cohort, however, we failed to find any relationship between the extension of radiological pneumonia and the PE diagnosis. We suggest that physicians attending hospitalized patients with COVID-19 infection should be aware of their increased risk of PE, particularly in a severe form with systemic inflammation and hypoxemia.
The data currently available on thromboembolic risk in COVID-19 patients are still scarce and mainly refer to patients admitted to an ICU.43 . 44 In ICU patients, a high cumulative incidence of 18–50% of venous thromboembolism (VTE) has been reported, depending on the research methodology used.21 , 27 , 45, 46, 47, 48, 49, 50 As for non-ICU patients, Middeldorp et al. have observed that the cumulative incidence of VTE in those patients attended in an ICU (48%, 95% CI, 33 to 61%) was significantly greater than that seen in non-ICU patients (10%; 95% CI, 3 to 24%).45 Similarly, Bompard et al. retrospectively analyzed the CTPA performed on those COVID-19 patients with clinical suspicion of PE or elevated D-dimer levels. Again, PE incidence was greater in the ICU patients (50%, 95% CI, 30 to 70%), compared to non-ICU patients (18%, 95% CI, 12 to 27%).23 More recently, a retrospective single-center study found that the incidence of symptomatic PE in patients with COVID-19 was lower (5.4%), but CTPA was only performed on 14% (214/1477) of the subjects and the diagnostic yield of CTPA in these patients was similar to our findings (37%).22 However, these studies had several limitations, such as a lack of sample size calculation to estimate the incidence in the whole population of hospitalized patients, as well as a lack of analysis of the risk factors or thrombotic burden of PE in patients infected by COVID-19.
To the best of our knowledge, this is the first study to use a representative and random selection of patients to calculate the cumulative incidence of PE in the entire population of patients hospitalized with COVID-19 infection. The resulting incidence of 35.6% (95% CI, 29.6 to 41.6) is greater than any previously reported in non-ICU patients. Interestingly, this reported rate is similar to those previously reported in ICU patients with influenza A (H1N1) virus infection.51 Moreover, this is also the first study to analyze the main risk factors for suffering a PE during hospitalization, using clinical and analytical variables easily measured at the point of admission into hospital: increased heart rate, CRP, and D-dimer levels, and decreased room-air oxygen saturation. Significantly, all these variables could be altered by either PE of any origin or COVID-19 infection, but it seems that any alterations are more pronounced when both diseases appear simultaneously, or when COVID-19 infection produces a state of hypercoagulability that could lead to PE. In fact, current evidence suggests that D-dimer values are increased in COVID-19 patients, and this elevation correlates with the severity and prognosis of the disease, independently of the presence of PE.13, 14, 15 , 17 , 19 Furthermore, autopsy specimen studies have revealed that most lung samples present thrombotic features, especially in peripheral vessels.52, 53, 54, 55
Another novel finding of our study is the construction of a score to predict PE during hospitalization using four easy-to-measure variables (CRP, heart rate, oxygen saturation, and D-dimer) at admission. This CHOD score was constructed after a dichotomization of the variables, choosing the cut-off points with the best prognostic value (>50 UI/mL, >90 bpm, <92% and >956 mg/dl, respectively), and it has a range of values from 0 to 7 points. The prognostic value of the CHOD score was excellent (AUC-ROC: 0.86 [CI 95%, 0.80 to 0.93]). A CHOD score of 0 to 2 points at admission showed a low probability of PE during hospitalization (4.5%), compared with a very high probability (100%) in those patients with 6 or 7 points (and a moderate probability, 36.8%, in those with 3 to 5 points).
Thus, this score could be a useful tool for identifying those COVID-19 patients who, independently of the clinical suspicion of the attending physician, could benefit from a CTPA scan at admission because of a pro-thrombotic state caused by SARS-CoV-2. The predictive value of the CHOD score remained consistent in different series of patients with even very high cut-off points of D-Dimer levels (>2000 ng/ml) and different baseline characteristics.
It is worth noting that, despite the high incidence of PE, the vast majority of patients had no identifiable clinical symptoms suggestive of PE. This is probably because there was an overlap of symptoms of COVID-19 infection and PE, or because most of the PE observed were segmentary and subsegmentary asymptomatic thrombus. It is also striking that there is no association between the extension of radiological pneumonia (measured by both CXR and CTPA) and the confirmation of PE, even though a possible correlation could have been expected as both pneumonia extension and PE are related to a hyper-inflammatory state.5 These two situations could, however, correspond to different pathophysiological pathways: PE to a state of hypercoagulability with systemic inflammation and endothelial dysfunction, and the extension of pneumonia to a more local pulmonary inflammation. This possible divergence needs to be corroborated by further studies. Finally, it is also remarkable that PE had such a high incidence of PE in spite of the massive use of pharmacological thromboprophylaxis at correct doses (at least 40 mg of enoxaparin) in more than 80% of patients, reinforcing the theory of high pro-thrombotic risk from COVID-19 infection.
Finally, we observed that the thrombotic load, as assessed by the Qanadli index, was low, due to the high percentage of patients who presented effects on only segmentary and subsegmentary branches. Our results concur with those found by van Dam et al., who reported that PE patients with COVID-19 have a more peripheral and less extensive diagnosis than those with no COVID-19 infection, suggesting that PE in COVID-19 patients could be a combination of thromboembolic disease and in situ thrombosis.56 Not surprisingly, as in the case of PE patients with no COVID-19 infection, the thrombotic load of those with both diseases was associated with both more biomarkers of coagulation (number of platelets and D-dimer levels) and CTPA evidence of vascular repercussions, such as increased rates of PA/Ao and RV/LV.
The main strength of our study is that is the first to analyze the incidence and risk factors of PE in a consecutive hospitalized cohort of patients with COVID-19 infection, independently of the clinical probability of PE. However, some limitations also need to be considered: Our study is observational in nature, and therefore no causal association could be established. Our results are only applicable to patients with elevated D-dimer levels (although in the overall group of hospitalized patients in our study less than 20% presented D-dimer levels below the threshold).
Moreover, we did not screen for the presence of deep venous thrombosis, so the incidence of VTE could be even higher. On the other hand, by excluding patients with shock and cardiac arrest a number of PEs might have been lost and anticoagulation is not 100% protective against PE, so this could be a selection bias. Antifactor Xa levels were not assessed to monitor enoxaparin thromboprophylaxis dosing. Finally, a larger-scale external validation of our findings is still needed before our results can be applied to clinical practice.
In summary, our study confirmed the presence of a high cumulative incidence of PE in hemodynamically stable hospitalized patients with both a confirmed diagnosis of COVID-19 infection and increased D-dimer levels, despite appropriate thromboprophylaxis and independently of any clinical suspicion of PE. At admission to hospital, increased heart rate, CRP, and D-dimer levels and low oxygen saturation while breathing room air were the main risk factors for developing PE during hospitalization.
Declaration of Competing Interest
The authors declare no conflict of interest related to this study.
Acknowledgments
Authors' contributions
Study design: AGO, GO and MAMG.
Acquisition, analysis, and interpretation of data: all authors.
Data collecting: JDGO, AB, GO, ABG, RM, GA, LF, PG, SR, EZ, AF, CM, LT, CF, AC, RG and CM.
Statistical analysis: AGO, GO and MAMG.
Authors of the manuscript: AGO, GO and MAMG.
Critical review of the manuscript: PC, RM and RM.
All the authors provided intellectual input and approved the final draft of the manuscript.
Funding
This study has not received any funding or grant.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.01.003.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C., Chen X., Cai Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly B.S., Siguret V., Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1603–1606. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30404-5. S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T., Levy J.H. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16:231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 8.Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of Coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippi G., Favaloro E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: a Pooled Analysis. Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P. COVID-19 Coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidali S., Morosetti D., Cossu E., Luisi M.L.E., Pancani S., Semeraro V. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res. 2020;6:260. doi: 10.1183/23120541.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price L.C., McCabe C., Garfield B., Wort S.J. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longchamp A., Longchamp J., Manzocchi-Besson S., Whiting L., Haller C., Jeanneret S. Venous thromboembolism in critically Ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4:842–847. doi: 10.1002/rth2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyte M.B., Kelly P.A., Gonzalez E., Arya R., Roberts L.N. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020;195:95–99. doi: 10.1016/j.thromres.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macías M., Toledo-Samaniego N. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K. Acute Pulmonary Embolism and COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J., Wang X., Zhang S., Lin B., Wu X., Wang Y. Characteristics of acute pulmonary embolism in patients with COVID-19 associated pneumonia from the City of Wuhan. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstantinides S.V., Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2019;40:3453–3455. doi: 10.1093/eurheartj/ehz726. [DOI] [PubMed] [Google Scholar]
- 30.Miron M.J., Perrier A., Bounameaux H., de Moerloose P., Slosman D.O., Didier D. Contribution of noninvasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients. Eur Respir J. 1999;13(6):1365–1370. doi: 10.1183/09031936.99.13613719. [DOI] [PubMed] [Google Scholar]
- 31.Roncon L., Zuin M., Zonzin P. Age-adjusted D-dimer cut-off levels to rule out venous thromboembolism in COVID-19 patients. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Righini M., Van Es J., Den Exter P.L., Roy P., Verschuren F., Ghuysen A. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 33.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 34.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 35.Ahn D., Shin H., Kim M., Lee S., Kim H., Myoung J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for Novel Coronavirus Disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 37.Legnani C., Cini M., Scarvelis D., Toulon P., Wu J.R., Palareti G. Multicenter evaluation of a new quantitative highly sensitive D-dimer assay, the Hemosil D-dimer HS 500, in patients with clinically suspected venous thromboembolism. Thromb Res. 2010;125:398–401. doi: 10.1016/j.thromres.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Elferink O., Rob F.M., Loot A.E., Van De K., Chantal G.J., Hulsebos-Huygen M. Clinical evaluation of eight different D-dimer tests for the exclusion of deep venous thrombosis in primary care patients. Scand J Clin Lab Invest. 2015;75:230–238. doi: 10.3109/00365513.2014.993697. [DOI] [PubMed] [Google Scholar]
- 39.Sam A., Sánchez D., Gómez V., Wagner C., Kopecna D., Zamarro C. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J. 2011;37:762–766. doi: 10.1183/09031936.00070110. [DOI] [PubMed] [Google Scholar]
- 40.Righini M., Roy P.-., Meyer G., Verschuren F., Aujesky D., Le Gal G. The Simplified Pulmonary Embolism Severity Index (PESI): validation of a clinical prognostic model for pulmonary embolism. J Thromb Haemost. 2011;9:2115–2117. doi: 10.1111/j.1538-7836.2011.04469.x. [DOI] [PubMed] [Google Scholar]
- 41.Qanadli S.D., El Hajjam M., Vieillard-Baron A., Joseph T., Mesurolle B., Oliva V.L. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415–1420. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 42.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiménez D., García-Sánchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P. Incidence of venous thromboembolism and bleeding among hospitalized patients with COVID-19: a systematic review and meta-analysis. Chest. 2020 doi: 10.1016/j.chest.2020.11.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi L., Xu J., Duan G., Yang H., Wang Y. The pooled prevalence of pulmonary embolism in patients with COVID-19. Intensive Care Med. 2020;46(11):2089–2091. doi: 10.1007/s00134-020-06235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middeldorp S., Coppens M., Haaps TFv, Foppen M., Vlaar A.P., Muller M.C.A. Incidence of Venous Thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llitjos J., Leclerc M., Chochois C., Monsallier J., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal P.P., Cinti S., Kazerooni E.A. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009;193(6):1488–1493. doi: 10.2214/AJR.09.3599. [DOI] [PubMed] [Google Scholar]
- 52.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo W.R., Yu H., Gou J.Z., Li X.X., Sun Y., Li J.X. Histopatological findings in the explant lungs of a patient with COVID-19 treated with bilateral orthotopic lung transplant. Transplantation. 2020;104(11):e329–e331. doi: 10.1097/TP.0000000000003412. [DOI] [PubMed] [Google Scholar]
- 54.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Dam L.F., Kroft L.J.M., van der Wal L.I., Cannegieter S.C., Eikenboom J., de Jonge E. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.