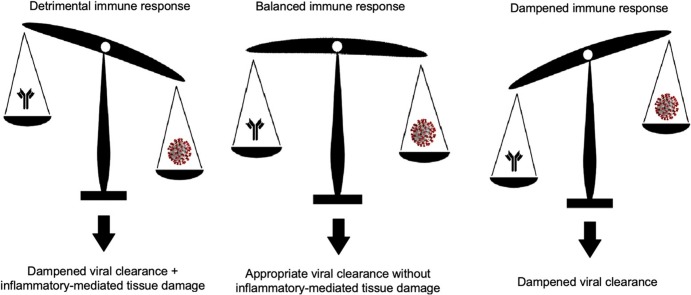
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Pharmacology, Viral clearance kinetics
Abstract
Coronavirus Disease 2019 (COVID-19) is a pandemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The clinical spectrum of COVID-19 is broad and varies from mild to severe forms complicated by acute respiratory distress and death. This heterogeneity might reflect the ability of the host immune system to interact with SARS-CoV2 or the characteristics of the virus itself in terms of loads or persistence. Information on this issue might derive from interventional studies. However, results from high-quality trials are scarce. Here we evaluate the level of evidence of available published interventional studies, with a focus on randomised controlled trials and the efficacy of therapies on clinical outcomes. Moreover, we present data on a large cohort of well-characterized patients hospitalized at a single University Hospital in Milano (Italy), correlating viral clearance with clinical and biochemical features of patients.
1. Introduction
Coronavirus Disease 2019 (COVID-19) is a major global health-emergency caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It includes mild forms, with sole upper respiratory tract involvement, and severe cases complicated by acute respiratory distress syndrome (ARDS) [[1], [2], [3], [4], [5], [6], [7]]. The cause of the clinical heterogeneity is not clear. Since the onset of the pandemic, data on the pharmacological treatment of COVID-19 has accumulated. Agents that had previously showed efficacy on other coronaviruses (i. e. SARS-CoV and MERS-CoV) were repurposed in COVID-19 patients and other approaches have been described. However, the emergency jeopardized the implementation of high-quality randomised controlled trials (RCTs). Most of the evidence regarding treatment of COVID-19 comes from lower-quality studies, e. g. case series, case control-studies and nonrandomised controlled trials. While this data might be useful, only the results of high-quality studies should be taken into consideration to evaluate the clinical efficacy of various agents and to develop reliable guidelines. Viral clearance data in treated patients may shed light on the relationship between the host immune system and SARS-CoV-2.
2. Objective and methods
We performed a literature review using PubMed (https://www.ncbi.nlm.nih.gov/pubmed) to find relevant articles published until the 22nd of May 2020. The following search terms were used: COVID-19, coronavirus and SARS-CoV-2 in combination with treatment, trial and pharmacology. Other studies were identified among the references of each of the retrieved articles. We defined the level of evidence for each study according to the guidelines of the Oxford Centre for Evidence-based Medicine [8]. We identified and thoroughly analysed the studies with a minimum level of evidence of 1c, recovering data on the clinical and biochemical characteristics of the study population and on the clinical and virological efficacy of each agent.
We also analysed the viral clearance time in a large cohort of well characterized patients (n = 1022) admitted to a single Institution (San Raffaele University Hospital, Milan, Italy). All subjects have been enrolled in the COVID-BioB protocol (ClinicalTrials.gov Identifier: NCT04318366), which conforms to the Declaration of Helsinki and has been approved by the Institutional Ethical Board. Patients diagnosed with COVID-19 based on a positive reverse transcriptase chain reaction (RT-PCR) test for SARS-CoV-2 nucleotide sequences on upper respiratory tract samples and who had performed at least two RT-PCR between February 26 and May 5, 2020 were eligible for this study. Time to negative RT-PCR test and the proportion of patients with a negative RT-PCR test within 14 and 28 days were calculated. Cox regression analysis was performed to identify viral RNA negativity predictors at 14 days, 28 days and at the last RT-PCR performed, regardless of the time it was performed. Variables that were significantly related to the primary end-point (negative conversion of viral RNA load) in univariable tests (p-value < 0.05) were included in the multivariable models. A similar test was performed to assess the presence of predictors of mortality in our study population. Microsoft Excel® 2019 and Statacorp STATA 15 were used to perform the analysis.
3. Results
3.1. Pharmacological treatment of COVID-19
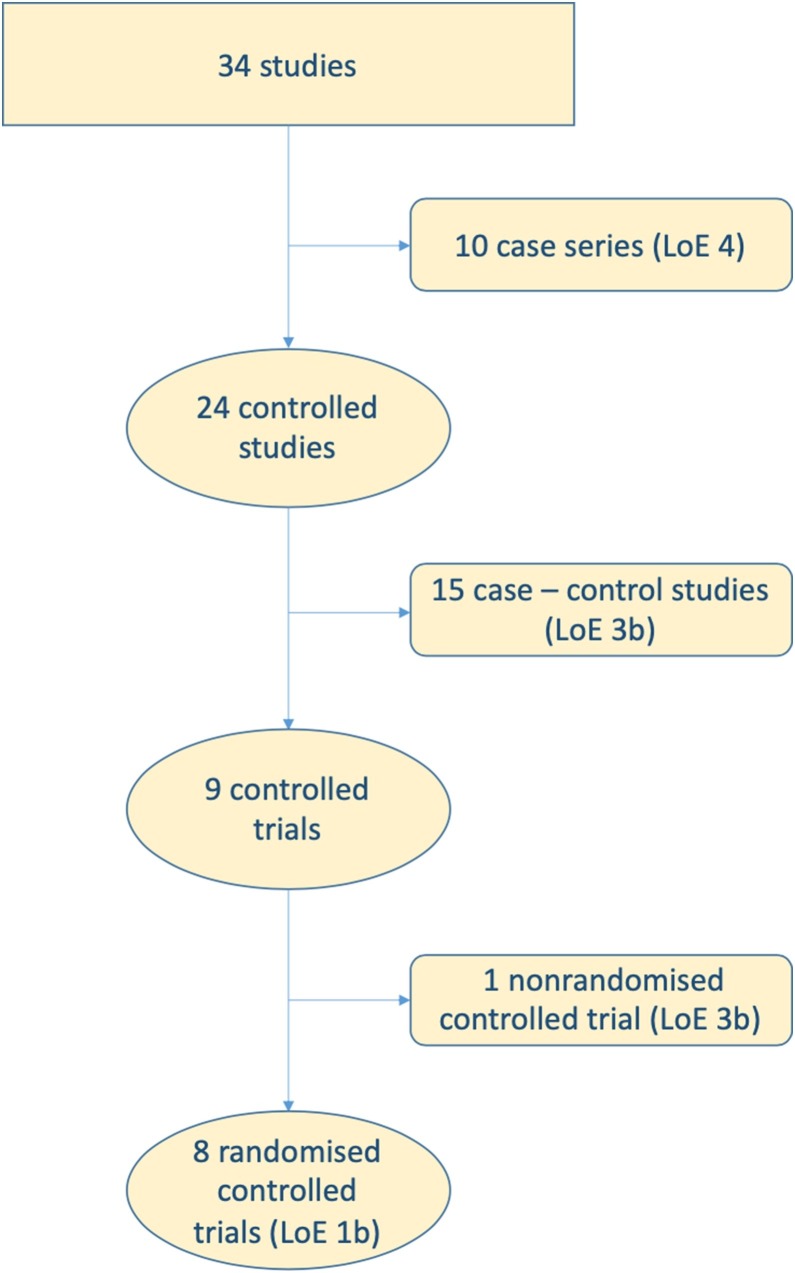
Several antiviral agents have been studied in patients with COVID-19 for their ability to accelerate viral clearance and prevent cytopathic damage. Excessive or dysregulated immune responses might contribute to tissue damage [1,9]. Thus, immunomodulatory drugs, including glucocorticoids and anti-cytokine agents, have been tested to reduce the burden of lung inflammation. A third group of agents that has been tested in COVID-19 patients includes compounds with broad spectrum pharmacodynamic properties, such as antimalarial agents. We identified 34 studies that evaluated the use of experimental drugs in COVID-19 patients (Fig. 1 ). We retrieved data from eight RCTs (level of evidence of 1b). The results of RCTs will be discussed in detail in the following paragraphs and are summarized in Table 1 . Moreover, we have identified one nonrandomised controlled trial (level of evidence 3b), 15 case-control studies (level of evidence 3b) and 10 case series (level of evidence 4). Studies with level of evidence 3b and 4 reported mixed clinical and virological results on remdesivir, lopinavir/ritonavir, favipiravir, umifenavir, interferon α-2b, glucocorticoids, tocilizumab, anakinra, hydroxychloroquine, heparin and convalescent plasma [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]] (Table 2 ).
Fig. 1.
Process of identification of interventional studies in COVID-19 patients and attribution of levels of evidence (LoE) according to the guidelines of the Oxford Centre for Evidence-based Medicine.
Table 1.
Randomised controlled trials evaluating the efficacy of diverse pharmacological therapies in COVID-19 patients.
| Lopinavir Ritonavir | Favipiravir | Interferon β1b Ribavarin | Remdesivir | Chloroquine | Hydroxychloroquine | ||||
|---|---|---|---|---|---|---|---|---|---|
| Study arms | L/R vs Standard care | Favipiravir vs Umifenavir | IFN + RBV + L/R vs L/R | Remdesivir vs Standard care | CQ high-dose vs CQ low-dose | HCQ vs Standard care | |||
| Number of patients | 199 | 236 | 127 | 237 | 1059 | 81 | 62 | 150 | |
| Median age (years);gender | 58;M 68 % | NA;M 49 % | 52;M 54 % | 65;M 59 % | 59;M 64 % | 51;M 75 % | 45;M 47 % | 46;M 55 % | |
| Baseline PaO2:FiO2ratio | < 300 | NA | NA | < 300 | NA | NA | > 300 | > 300 | |
| Median baseline CRP (mg/dL) | NA | 1.10 | 3.00 | NA | NA | 8.48 | NA | 0.86 | |
| Median delay from symptom onset (days) | 13 | NA | 5 | 11 | 9 | NA | NA | 17 | |
| Clinical efficacy | None | Faster amelioration | Faster recovery | None | Faster recovery | NA | Faster amelioration | NA | |
| Viral clearance rate at 28 days (%) | 59 vs 58 | NA | NA | 76 vs 83 | NA | NA | NA | 75 vs 71 | |
| Time to viral clearance (days) | NA | NA | 7 vs 12 * | NA | NA | NA | NA | 8 vs 7 | |
| References | Cao et al | Chen et al | Hung et al | Wang et al | Beigel et al | Borba et al | Chen et al | Tang et al | |
NA = not available; L/R = lopinavir/ritonavir; IFN = interferon β−1b; RBV = Ribavarin; CQ = chloroquine; HCQ = hydroxychloroquine; M = male. * = statistically significant.
Table 2.
Studies other than randomised controlled trials evaluating the efficacy of diverse pharmacological therapies in COVID-19 patients.
| Drug | Level of evidence | References |
|---|---|---|
| Remdesivir | 4 | Grein et al |
| Lopinavir/Ritonavir | 3b | Yan et al |
| Umifenavir | 3b | Wang et al |
| 3b | Zhu et al | |
| 3b | Deng et al | |
| 3b | Xu et al | |
| Favipiravir | 3b | Cai et al |
| Glucocorticoids | 3b | Wang et al |
| 3b | Whu et al | |
| 3b | Gong et al | |
| Tocilizumab | 4 | Xu et al |
| 4 | Morena et al | |
| 4 | Sciascia et al | |
| 3b | Campochiaro et al | |
| Anakinra | 3b | Cavalli et al |
| 4 | Aouba et al | |
| 4 | Dimopoulos et al | |
| 4 | Pontali et al | |
| Chloroquine & Hydroxychloroquine | 3b | Gautret et al |
| 3b | Geleris et al | |
| Heparin | 3b | Tang et al |
| Convalescent Plasma | 4 | Shen et al |
| 4 | Duan et al | |
| 4 | Mingxiang et al | |
| 4 | Ahn et al |
3.1.1. Lopinavir/Ritonavir
Lopinavir is a HIV aspartate protease inhibitor that is administered in combination with ritonavir, an inhibitor of cytochrome P450 that increases the half-life of the former. Previous data suggested a possible efficacy of this combination in SARS-CoV and MERS-CoV infected patients, especially when agents were used early in the natural history of the infection [33,34]. Cao and colleagues published the results of a randomised, open-label, controlled trial of 199 COVID-19 patients (60 % male, mean age 58 years) in whom treatment with lopinavir/ritonavir was started with an average delay of 13 days from the onset of symptoms. All patients had an oxygen saturation (SatO2) of 94 % or less while they were breathing ambient air or a ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) at or below 300. About 16 % of patients needed high-flow oxygen or mechanical ventilation at baseline. No information about inflammatory markers has been reported. Antiviral treatment has not been associated with either faster clinical improvement or lower mortality than standard care. However, a sub-analysis revealed that the time to clinical improvement tended to be shorter in patients in whom lopinavir/ritonavir was started in the first 12 days. However, the difference was not statistically significant and an increase in the 28-day viral clearance rate was not observed [35].
3.1.2. Favipiravir
Favipiravir is an RNA polymerase inhibitor with in vitro antiviral activity against SARS-CoV-2. Chen et al. conducted a multicenter, open-label, randomised, superiority study comparing favipiravir and umifenavir. Both of these influenza drugs had previously proven effective in COVID-19 patients compared to lopinavir/ritonavir and standard care in one nonrandomised controlled trial and three retrospective studies [22,26,27,29]. In this RCT the efficacy of the drugs was compared in 236 COVID-19 patients (49 % male, 30 % above 65 years of age). The median value of erythrocyte sedimentation rate was 17 mm/hour while median C-reactive protein concentration was 1.10 mg/dL. No data about baseline SatO2, PaO2:FiO2 ratio or need for supplemental oxygen of patients is reported. Favipiravir was not associated with significant differences in terms of recovery rate at one week when compared to umifenavir, despite a significantly faster improvement in symptom relief [36].
3.1.3. Interferon β-1b and Ribavirin
Ribavirin is a guanosine analogue displaying broad antiviral activity against RNA and DNA viruses. A combined treatment with this antiviral agent and interferon β -1b, a cytokine with antiviral properties, has shown efficacy against other coronaviruses [37]. Interferon β -1b might enhance the antiviral activity of ribavirin in an early phase of SARS-CoV-2 infection, whilst a delayed administration might worsen the inflammatory features that are thought to characterize the late stages of COVID-19 [9]. Hung et al. led a multicentre, prospective, open-label, randomised trial comparing in 127 COVID-19 patients the combination therapy with interferon β−1b, ribavirin and lopinavir/ritonavir with the sole antiretroviral therapy. The median delay from symptom onset to treatment initiation was five days and only patients who were enrolled before seven days after disease onset received interferon β−1b as part of the combination therapy, while the others were treated only with lopinavir/ritonavir and ribavirin. Precise clinical information as far respiratory function of enrolled patients is concerned are missing (beside them having a median national early warning score 2 of 2 at baseline). The median age was 52 years and 54 % of the study population was male. The median values were 3 mg/dL for C-reactive protein, 19 for erythrocyte sedimentation rate and 1.6 pg/mL for interleukin 6. Combination treatment was associated with a shorter time to both negative viral load and clinical improvement. These favorable results however lost their significance when comparing the control group with patients who did not receive interferon (i. e. those who started treatment at least seven days after symptom onset) [38].
3.1.4. Remdesivir
Remdesivir is an RNA polymerase inhibitor with broad-spectrum in vitro antiviral activity that has been previously used to treat patients with Ebolavirus infection. After an initial report of efficacy in an uncontrolled study-cohort of patients with severe COVID-19 [10], the results of a randomised, double-blind, placebo-controlled, multicentre trial have been published by Wang et al. The study population comprised 237 COVID-19 patients with a baseline SatO2 of 94 % or lower on room air or PaO2:FiO2 ratio of 300 or less. Less than 20 % of patients received high flow oxygen or mechanical ventilation. The average age was 65 years and 59 % were male. The median interval from symptom onset and treatment start was 11 days. No information about inflammatory markers is reported. No differences were found as far viral clearance rate is regarded and remdesivir was not associated with a significant faster clinical improvement, despite a positive trend [39]. An association between treatment with remdesivir and a decrease in time to clinical recovery was confirmed and defined as statistically significant in a more recent double-blind, randomised, placebo-controlled trial led on 1059 COVID-19 patients with a median delay from symptom onset to treatment start of 9 days. The median age was 59 years and 64 % were male. At baseline only 12 % of patients did not need supplemental oxygen and 44 % needed high-flow oxygen or mechanical ventilation [40].
3.1.5. Chloroquine and hydroxychloroquine
Immunomodulatory activity and the potential antiviral properties of the antimalarial agents chloroquine and hydroxychloroquine might be useful in the treatment of patients with COVID-19 [41,42]. Shortly after the beginning of SARS-CoV-2 pandemic, two observational studies suggested that both agents were effective in treating COVID-19 patients [17,43]. A more recent observational study has not confirmed the efficacy of antimalarials in this disease [16], while a recently published multinational registry analysis raising concerns on the safety of these agents has been retracted.
Results of three RCTs evaluating these two drugs have been published to date. Borba et al. compared two different chloroquine regimens (high-dose vs low-dose) in 81 patients with severe COVID-19, defined as presenting with a respiratory rate higher than 24 rpm and/or heart rate higher than 125 bpm (in the absence of fever) and/or SatO2 lower than 90 % in ambient air and/or shock. Supplemental oxygen was required in 89 % of patients at baseline and median C-reactive protein level was 8.48 mg/dL. The median age was 51 years and 75 % were male. The lethality at 13 days in the whole study population was 27 %, which the authors claimed was consistent with that of patients who did not receive chloroquine. A placebo-group was not part of the study design. Lethality was higher in the high-dosage group, but the difference was no longer significant when performing a multivariate analysis and controlling by age. Reported data about virological outcomes are incomplete [44]. Chen and colleagues led a placebo-controlled randomised controlled trial to evaluate the efficacy of hydroxychloroquine in 62 non-severe COVID-19 patients, with SatO2 > 93 % or a PaO2:FIO2 ratio > 300. The median age was 44.7 years and 47 % were male. No data are available on the biochemical characteristics, the delay from the onset of symptoms and the viral clearance rate. Patients treated with hydroxychloroquine had faster clinical recovery and greater radiological improvement [45]. Tang et al. led a multicentre, open label, randomised controlled trial comparing 75 COVID-19 patients receiving hydroxychloroquine with 75 patients receiving standard of care. The median delay from symptom onset to treatment initiation was 16.6 days, the median age was 46 years and 55 % were male. Only two patients had a baseline PaO2:FIO2 ratio < 300; the median erythrocyte sedimentation rate was 28 mm/h, mean C-reactive protein was 0.86 mg/dL. No differences were observed in viral clearance rate between the two groups at 28 days. Clinical outcomes were not reported [46].
3.1.6. Summary of the evidence of efficacy for current treatments
Results on potential clinical efficacy of various pharmacologic agents in COVID-19 patients have been only partially confirmed by available RCTs. As far as these trials are considered, no treatment reduces mortality in COVID-19 patients. Favipiravir, interferon β−1b, ribavirin, remdesivir and hydroxychloroquine have variable degrees of efficacy in shortening time to clinical amelioration and recovery.
Considerable heterogeneity in the characteristics of the study samples between different RCTs was observed. First, the size of study samples ranges from 62 to 1059 patients. This may have influenced the results of the studies, as suggested by the case of remdesivir. The first RCT did not reveal statistically significant results [39]. However, when the agent was tested on a larger study population with similar characteristics, significantly faster clinical recovery was found [40]. Although other factors may have contributed (e.g. shorter delay to the start of treatment), the size of the larger sample may have been crucial in explaining the discrepancy. The severity of the disease also differs in the eight study populations. Critical parameters comprise respiratory function and inflammatory load, which vary widely between different RCTs. For example, hydroxychloroquine was evaluated in mild patients with a PaO2:FIO2 ratio > 300, while RCTs evaluating remdesivir and lopinavir/ritonavir involved patients with PaO2:FIO2 ratio < 300. A single RCT on the efficacy of chloroquine included patients with a marked elevation in C-reactive protein levels (> 5 mg/dL). The host response to SARS-CoV-2 might lead to an exaggerated inflammatory response and to more severe forms of COVID-19. The possibility to analyze levels of inflammatory markers is of paramount importance to identify groups of patients with similar characteristics, which might respond differently to the agents tested in the RCTs.
The delay from symptom onset to therapy initiation might also reasonably influence the clinical and virological response of the patients. Except for the RCT evaluating the triple antiviral therapy [38], in which the median delay was five days, the median interval was at least nine days in the other trials, with a peak of 17 days [46]. Clinical efficacy has been suggested to be based on early administration of treatment [33]. Thus, these differences could represent a confounder when evaluating the efficacy of trialed drugs.
3.1.7. Therapeutic role of immunomodulatory agents
The response elicited by SARS-CoV-2 might be directly responsible for tissue damage in a subset of COVID-19 patients [1,9,47]. Increased levels of interleukin 1beta and interleukin 6 have been detected in sera of patients with severe COVID-19 [30]. Post-mortem pathological analysis revealed massive lung inflammatory infiltrates [48,49]. Observational data suggest that agents targeting inflammatory cytokines (anakinra and tocilizumab) limit COVID-19 severity in patients with marked elevation in inflammatory markers [[12], [13], [14], [15], [16],32,50]. No results of ongoing RCTs evaluating anti-cytokine agents have been published yet. So far, little evidence is available on strategies to accelerate the active termination of immune responses, such as those based on the cholinergic anti-inflammatory pathway [51,52] or the direct targeting of regulatory lymphocytes or of pattern recognition receptors [[53], [54], [55], [56]].
3.2. SARS-CoV-2: time to clearance
To date, we know little on the interaction between the host immune system and SARS-CoV-2. Factors determining elimination have not been identified. Timing of clearance and disease severity might be related [48].
3.2.1. Existing evidence regarding SARS-CoV-2 clearance
A virological outcome, defined as the time to negative SARS-CoV-2 RNA load in upper respiratory tract specimens and/or the proportion of patients with undetectable SARS-CoV-2 RNA 28 days after enrollment, has been considered in four RCTs in addition to clinical efficacy (Table 1). Viral clearance rates at 28 days ranged from 58 % to 83 % among the RCTs and they were never influenced by the tested agents. Likewise, the median time to negative viral load was variable, ranging from 7 to 12 days. The combined therapy with interferon β-1b, ribavirin and lopinavir/ritonavir was the only approach associated with a shorter time to viral clearance. Lopinavir/ritonavir monotherapy, remdesivir and hydroxychloroquine did not influence the time to negative viral load. However, patients enrolled in the triple therapy RCT had the shortest delay from symptom onset to enrollment [38] and this feature could have prompted the viral clearance. Other studies have focused merely on virological aspects of the disease. Retrospective studies reported that viral RNA was detectable for a median period of up to 23 days, with several factors proposed as predictors of delayed viral clearance, including age and disease severity [[57], [58], [59], [60]]. However, these studies involved a relatively small number of patients. The largest retrospective cohort included 191 patients while the largest RCT study population evaluating a virological outcome comprised 237 patients. Hence, definitive conclusions about SARS-CoV-2 clearance cannot be drawn yet.
3.2.2. Timing of viral clearance at our Institution
We identified 1022 patients who were diagnosed with SARS-CoV-2 infection and who had a positive RT-PCR test performed on upper respiratory tract specimens during the study period. At least two serial RT-PCR tests for SARS-CoV-2 nucleotide sequences on upper respiratory tract specimens were available for 508 patients. Baseline clinical and biochemical characteristics of our study population are showed in Table 3 . All patients received combination therapy with hydroxychloroquine and lopinavir/ritonavir since the diagnosis. Anti-cytokine agents were administered to 106 (21 %) patients; more specifically 29 (6%) were treated with tocilizumab, 63 (12 %) with high doses of intravenously administered anakinra, 14 (3%) with sarilumab and 5 (1%) mavrilimumab. Glucocorticoids were prescribed according to clinicians’ judgement in order to improve respiratory function in critical patients. However, the great variability in dosage and length of glucocorticoid therapy among the study population prevents a homogenous analysis of its impact on the study end-point.
Table 3.
Baseline characteristics of study population.
| Characteristic | Study population (n = 508) |
|---|---|
| Age (years) | 61 (52–71) |
| Male sex | 359 (69) |
| Coronary artery disease | 48 (9.4) |
| Chronic kindey disease | 40 (8) |
| Chronic obstructive pulmunary disease | 20 (34) |
| Malignant neoplasia | 35 (6.9) |
| Arterial hypertension | 210 (41.3) |
| Diabetes mellitus | 78 (15) |
| C-reactive protein (mg/dL) | 6.6 (2.6−13.3) |
| Lactate dehydrogenase (U/L) | 338 (262−443) |
| Aspartate aminotransferase | 42 (29−63) |
| Alanine aminotransferase | 36 (24−55) |
| Glucose (mg/dL) | 107 (97−128) |
| Creatinine (mg/dL) | 0.9 (0.8−1.2) |
| Hemoglobin (g/dL) | 13.7 (12.3−15) |
| Lymphocyte count (cell/mm3) | 1000 (700−1300) |
| Body temperature (°C) | 37.9 (37−38.5) |
| PaO2:FiO2 ratio | 295 (226−338) |
Continuous variables are expressed as median (interquartile range); categorical variables are expressed as absolute number (%).
Viral clearance rates within 14 and 28 days were 32 % and 54 %, respectively. The median time to negative conversion of viral RNA load was 25 days (IQR 17–31 days). COVID-19 patients with concomitant neoplasia were characterized by a lower probability of negative conversion at 28 days (Hazard Ratio [HR] 0.127; Confidence Interval [CI] 95 % 0.023−0.702; p = 0.018). Conversely, neither baseline demographic and disease features nor biologic treatment were associated with significant modifications in viral clearance rate at 14 days.
There were no significant differences in terms of viral clearance rate when comparing patients with COVID-19 with moderate to severe ARDS (i.e. PaO2:FIO2 ratio < 200 [61]) to those with milder disease (HR 0.939; CI95 % 0.704–1.253; p = 0.671). Age was not found to influence conversion rate neither in the whole study population (HR 0.998; CI95 % 0.990–1.005; p = 0.534) nor in patients with severe ARDS (HR 1.001; CI95 % 0.978–1.04; p = 0.922). Higher baseline levels of C-reactive protein were associated with a reduced probability of viral clearance, regardless of the time of RT-PCR test (HR 0.998; CI95 % 0.995–1.000; p = 0.042).
As shown in Table 4 , neither viral clearance rates at 14 and 28 days nor time to negative viral RNA load were predictors of mortality rate in COVID-19 patients, whereas a lower baseline PaO2:FiO2 ratio and a personal history of coronary artery disease were associated with a greater mortality rate.
Table 4.
Multivariate Cox-regression analysis to evaluate predictors of death in COVID-19 patients.
| Hazard Ratio | Confidence Interval 95 % | p-value | |
|---|---|---|---|
| Absence of negative conversion at 14 days | 0.979 | 0.413 – 2.324 | 0.962 |
| Absence of negative conversion at 28 days | 1.634 | 0.567 – 4.712 | 0.364 |
| Time to negative conversion | 1.001 | 0.943 – 1.084 | 0.776 |
| Baseline PaO2:FiO2 ratio | 0.990 | 0.984 – 0.997 | 0.005 |
| Coronary artery disease | 6.584 | 1.180 – 36.728 | 0.032 |
Viral clearance rate at 28 days in our population was in line with that reported by Cao et al. [35], despite being inferior to those found in both the placebo- and interventional arms of the RCTs evaluating remdesivir and hydroxychloroquine [39,46]. Even if it has been proposed that older age and disease severity are predictors of a reduced viral clearance, we did not find any association between baseline demographic and clinical features and viral clearance rate. Neither baseline PaO2:FIO2 ratio nor body temperature, which we considered to be reliable surrogates of disease severity, were associated with a worse virological outcome. In contrast, a higher baseline C-reactive protein level was a predictor of a reduced probability of negative viral conversion. This is in line with the proposed hypothesis of a reduced efficacy of endogenous antiviral interferon-mediated mechanisms and higher viral load in patients with elevated inflammatory mediators [62]. Moreover, we found concomitant neoplasia to represent a predictor of reduced viral clearance. This result could reflect acquired immunosuppression linked to the neoplastic process, especially in case of haematologic neoplasms [63].
Viral elimination did not predict mortality. An increased interval between diagnosis and negative viral RNA load and a prolonged persistence of detectability of SARS-CoV-2 in respiratory specimens did not represent a negative prognostic factor in our study population. This observation bears relevance for the daily clinical practice, since clinicians could be influenced in the evaluation of patients by an alleged hypothesis that the absence of negative conversion of RT-PCR test has a negative impact on the patients’ prognosis. The inability to eliminate the virus, unlike compromised respiratory function and the presence of cardiovascular comorbidities [64], does not identify patients at higher mortality risk.
4. Conclusions
Despite the efforts of the scientific community, there is a dearth of high-quality data deriving from RCTs. Promising results from retrospective case series have only been partially confirmed by RCTs and no agent has shown to reduce the mortality rate in COVID-19 patients. Only an early combined approach with interferon, ribavirin and lopinavir/ritonavir showed an association with an improved virological result. Nonetheless, data from the literature and from our own experience with serial COVID-19 swab testing suggests that viral clearance might be uncoupled from inflammation and tissue damage if COVID-19 patients are considered as a homogeneous group. Conversely, distinct patterns of interaction between the immune system and SARS-CoV-2 among different individuals might sustain heterogeneous disease phenotypes, which, in turn, could possibly be susceptible to different pharmacological interventions. A precise characterisation of the variability of immune response dynamics during SARS-CoV-2 infection is therefore urgently needed to better understand the reasons behind the failure of trialed drugs in reducing COVID-19 mortality and to provide a reliable basis for the rational design of future studies. As our review indicates, these trials should be led on a large number of patients and on relatively homogenous groups of patients in order to avoid possible confounders in the assessment of treatment efficacy.
Even if a link between the high inflammatory burden that develops in a subset of patients and a failure in the clearance of SARs-CoV-2 might be intuitive, there is paucity of published data to support this connection. We analysed virological data of a large COVID-19 population and found that both neoplasms and a higher inflammatory burden can lead to a delayed negative conversion of viral RNA load, suggesting that an exaggerated and misdirected inflammatory response might not be sufficient to eliminate the virus, despite possibly contributing to tissue lung damage. Moreover, even if in daily clinical practice RT-PCR tests are often serially repeated to check for viral elimination, this information does not represent a significant prognostic factor since viral clearance does not seemingly affect the mortality rate.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work of the authors has been supported by a COVID-19 program project grant from the IRCCS San Raffaele Hospital and by the grant COVID-2020-12371617 from the Italian Ministry of Health.
References
- 1.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D’Angelo A., De Cobelli F., Rovere-Querini P., Tresoldi M., Dagna L., Zangrillo A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA - J. Am. Med. Assoc. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Satlin M.J., Campion T.R., Nahid M., Ringel J.B., Hoffman K.L., Alshak M.N., Li H.A., Wehmeyer G.T., Rajan M., Reshetnyak E., Hupert N., Horn E.M., Martinez F.J., Gulick R.M., Safford M.M. Clinical characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020;382(24):2372–2374. doi: 10.1056/nejmc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangrillo A., Beretta L., Silvani P., Colombo S., Scandroglio A.M., Dell’Acqua A., Fominskiy E., Landoni G., Monti G., Azzolini M.L., Monaco F., Oriani A., Belleti A., Sartorelli M., Pallanch O., Saleh O., Sartini C., Nardelli P., Lombardi G., Morselli F., Scquizzato T., Frontera A., Ruggeri A., Scotti R., Assanelli A., Dagna L., Rovere-Querini P., Castagna A., Scarpellini P., Di Napoli D., Ambrosio A., Ciceri F., Tresoldi M. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit. Care Resusc. 2020;22(2):91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciceri F., Castagna A., Rovere Querini P., De Cobelli F., Ruggeri A., Galli L., Conte C., De Lorenzo R., Poli A., Ambrosio A., SIgnorelli C., Bossi E., Fazio M., Tresoldi C., Colombo S., Monri G., Fominskiy E., Martinenghi C., Franchini S., Spessot M., Carlucci M., Beretta L., Scanoglio A.M., Clementi M., Locatelli M., Tresoldi M., Scarpellini P., Martino G., Bosi E., Dagna L., Lazzarin A., Landoni G., Zangrillo A. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009) - CEBM, (n.d.). https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed May 30, 2020).
- 9.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan D., Liu X.-Y., Zhu Y.-N., Huang L., Dan B.-T., Zhang G.-J., Gao Y.-H. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur. Respir. J. 2020;56(1):2000799. doi: 10.1183/13993003.00799-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A., Monti G., De Cobelli F., Zangrillo A., Tresoldi M., Castagna A., Dagna L. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., Tomelleri A., Farina N., Ruggeri A., Rovere-Querini P., Di Lucca G., Martinenghi S., Scotti R., Tresoldi M., Ciceri F., Landoni G., Zangrillo A., Scarpellini P., Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., Netea M.G., Spyridopoulos T., Verheggen R.J., Hoogerwerf J., Lachana A., van de Veerdonk F.L., Giamarellos-Bourboulis E.J. Favorable anakinra responses in severe COVID-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28(1):117–123. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., Angelelli A., Caorsi R., Feasi M., Calautti F., Castagnola E., Rollandi G.A., Ravelli A., Cassola G., Gattorno M. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J. Allergy Clin. Immunol. 2020;146(1):213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of Hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet (London, England). 2020;S0140-6736(20):31180–31186. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA - J. Am. Med. Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan. China, Clin. Infect. Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., Xia X., Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Kim Y.S., Lee H., Yong D., Kim H.O., Kim S., Choi J.Y. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020;35(14):e149. doi: 10.3346/JKMS.2020.35.E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J. Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu P., Huang J., Fan Z., Huang W., Qi M., Lin X., Song W., Yi li. Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microbes Infect. 2020;22(4–5):200–205. doi: 10.1016/j.micinf.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Y., Guan L., Jin Z., Chen S., Xiang G., Gao B. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID‐19 patients under 50 years old. J. Med. Virol. 2020 doi: 10.1002/jmv.26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., Torre A., Cossu M.V., Minari C., Ballone E., Perotti A., Mileto D., Niero F., Merli S., Foschi A., Vimercati S., Rizzardini G., Sollima S., Bradanini L., Galimberti L., Colombo R., Micheli V., Negri C., Ridolfo A.L., Meroni L., Galli M., Antinori S., Corbellino M. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020;92(6):556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M.L., Tse M.W., Que T.L., Peiris J.S.M., Sung J., Wong V.C.W., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 35.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J., Yin P., Wang X. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA - J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 38.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/s0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. ACTT-1 Study Group Members, Remdesivir for the Treatment of Covid-19 - Preliminary Report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- 44.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.-T., Monteiro W.M., Lacerda M.V.G. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Netw. Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 46.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., Wu Y., Xiao W., Liu S., Chen E., Chen W., Wang X., Yang J., Lin J., Zhao Q., Yan Y., Xie Z., Li D., Yang Y., Liu L., Qu J., Ning G., Shi G., Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Luca G., Cavalli G., Campochiaro C., Della-Torre E., Angelillo P., Tomelleri A., Boffini N., Tentori S., Mette F., Farina N., Rovere-Querini P., Ruggeri A., D’Aliberti T., Scarpellini P., Landoni G., De Cobelli F., Paolini J.F., Zangrillo A., Tresoldi M., Trapnell B.C., Ciceri F., Dagna L. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/s2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo W., Yu H., Gou J., Li X., Sun Y., Li J., Liu L. Preprints; 2020. Clinical Pathology of Critical Patient With Novel Coronavirus Pneumonia (COVID-19) [Google Scholar]
- 50.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mannon E.C., Sun J., Wilson K., Brands M., Martinez-Quinones P., Baban B., O’Connor P.M. A basic solution to activate the cholinergic anti-inflammatory pathway via the mesothelium? Pharmacol. Res. 2019;141:236–248. doi: 10.1016/j.phrs.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersson U. The cholinergic anti-inflammatory pathway alleviates acute lung injury. Mol. Med. 2020;26(1):64. doi: 10.1186/s10020-020-00184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gladstone D.E., Kim B.S., Mooney K., Karaba A.H., D’Alessio F.R. Regulatory t cells for treating patients with COVID-19 and acute respiratory distress syndrome: two case reports. Ann. Intern. Med. 2020;(L20-681) doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahbaz S.K., Sadeghi M., Koushki K., Penson P.E., Sahebkar A. Regulatory T cells: possible mediators for the anti-inflammatory action of statins. Pharmacol. Res. 2019;149:104469. doi: 10.1016/j.phrs.2019.104469. [DOI] [PubMed] [Google Scholar]
- 55.Tao L., Wang Y., Xu J., Su J., Yang Q., Deng W., Zou B., Tan Y., Ding Z., Li X. IL-10-producing regulatory B cells exhibit functional defects and play a protective role in severe endotoxic shock. Pharmacol. Res. 2019;148:104457. doi: 10.1016/j.phrs.2019.104457. [DOI] [PubMed] [Google Scholar]
- 56.Shafabakhsh R., Pourhanifeh M.H., Mirzaei H.R., Sahebkar A., Asemi Z., Mirzaei H. Targeting regulatory T cells by curcumin: a potential for cancer immunotherapy. Pharmacol. Res. 2019;147:104353. doi: 10.1016/j.phrs.2019.104353. [DOI] [PubMed] [Google Scholar]
- 57.Hu X., Xing Y., Jia J., Ni W., Liang J., Zhao D., Song X., Gao R., Jiang F. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci. Total Environ. 2020;728:138812. doi: 10.1016/j.scitotenv.2020.138812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi L., Yang Y., Jiang D., Tu C., Wan L., Chen X., Li Z. Factors associated with duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int. J. Infect. Dis. 2020;96:531–537. doi: 10.1016/j.ijid.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W., Li Y., Ni Q., Zou R., Li X., Xu M., Zhang Y., Zhao H., Zhang X., Yu L., Su J., Lang G., Liu J., Wu X., Guo Y., Tao J., Shi D., Yu L., Cao Q., Ruan B., Liu L., Wang Z., Xu Y., Liu Y., Sheng J., Li L. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin. Infect. Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome advances in diagnosis and treatment. JAMA - J. Am. Med. Assoc. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 62.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pene F., Marin N., Roche N., Szwebel T.-A., Smith N., Merkling S., Treluyer J.-M., Veyer D., Mouthon L., Blanc C., Tharaux P.-L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kerneis S., Terrier B. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. Science. 2020 doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yıldız A., Albayrak M., Pala Ç., Şahin O., Öztürk H.B.A., Güneş G., Maral S., Okutan H. Infections in patients with lymphoma: an analysis of incidence, relationship and risk factors. J. Infect. Dev. 2018;12(9):741–747. doi: 10.3855/jidc.10399. [DOI] [PubMed] [Google Scholar]
- 64.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N. Eng. J. Med. 2020;382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]