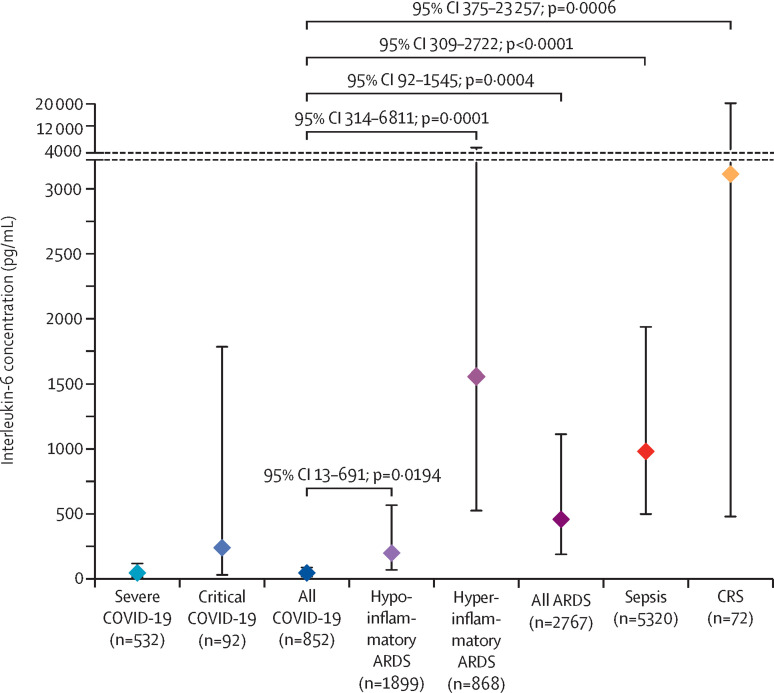
We thank Luke Chen and colleagues for their interest in our study.1 With respect to potential double counting of participants—this is a valid criticism. Indeed some participants could have been double counted if they were treated at the same hospital in overlapping time periods. Among the identified studies, only small fractions of the study periods overlap. Nevertheless, we recalculated our primary analysis using only one study—whichever was largest—from each group with potentially overlapping cohorts (figure ). The results do not change.
Figure.
Interleukin-6 concentrations in patients with COVID-19 versus comparison disorders
Markers indicate the weighted pooled mean concentration for each disease group. Errors bars indicate 95% CIs. p values and 95% CI were computed for the difference in means between the indicated group versus COVID-19. ARDS=acute respiratory distress syndrome. CAR=chimeric antigen receptor. CRS=chimeric antigen receptor T cell-induced cytokine release syndrome.
Our dataset is publicly available.1 We welcome Chen and colleagues or other researchers to explore additional analyses.
Regarding our study representing predominantly Chinese cohorts—North American and European studies were generally published after our search had been concluded. Findings from these regions are consistent with our results. For example, in 237 critically-ill patients with COVID-19 in New York City, NY, USA, median interleukin (IL)-6 concentration was 26 pg/mL (IQR 11–69 pg/mL).2 Patients enrolled in the BACC Bay trial done in Boston, MA, USA, (n=243) had median IL-6 concentration of 24·4 pg/mL (IQR 14·1–45·5 pg/mL).3
Chen and colleagues argue that elevated IL-6 is not a prerequisite for clinical response to IL-6 blockade. Although theoretically true, most randomised trials of anti-IL-6 have shown no benefit so far, as their correspondence notes.3, 4, 5 The authors invoke a prediction rather than causal framework, stating that IL-6 is “the best available biomarker for severity of COVID-19”. IL-6 is associated with disease severity but there are no data to support this superlative designation. Furthermore, unlike C-reactive protein and D-dimer, IL-6 is not a widely available test and often takes days to obtain results, limiting clinical use.
As has been proven for sepsis and acute respiratory distress syndrome before, we maintain that a cytokine storm model is overly reductive and at best unhelpful in COVID-19.
Acknowledgments
MDT is supported in part by the US National Institute of General Medical Sciences, US National Institutes of Health (NIH) (grant K08 GM 132794). CSC is supported in part by the US National Heart, Lung, and Blood Institute (R35 HL140026); she has received additional grant funding from Roche/Genentech (current) and Bayer (former), and consulting fees from Vasomune, Gen1e Life Sciences, and Quark Pharmaceuticals. CJT's institution, the Fred Hutchinson Cancer Research Center, has held equity in Juno Therapeutics; CJT has received research grants and personal fees for advisory board participation from Juno Therapeutics/Bristol Myers Squibb, during the conduct of the study. He declares fees and stock options in relation to a role on the scientific advisory boards of Precision Biosciences, Caribou Biosciences, Eureka Therapeutics, Myeloid Therapeutics, Century Therapeutics, and ArsenalBio; travel expenses and fees for participation on the advisory boards of Amgen, Novartis, Kite/Gilead, Humanigen, Aptevo, PACT Pharma, AstraZeneca, and Nektar Therapeutics; travel expenses from T-CURX; and research funding from AstraZeneca and Nektar Therapeutics, outside of the submitted work. CJT reports a patent (US patent number 10 653 756; issued May 19, 2020) for the “Identification of CD8+ T cells that are CD161hi and/or IL18Rαhi and have rapid drug efflux capacity for toxicity of CAR-T cells”, for which he receives royalties from the licensee, Juno Therapeutics; a patent pending for “Methods and compositions related to toxicity associated with cell therapy”; a patent pending for “Methods for the treatment of B cell malignancies using adoptive cell therapy”; and a patent pending for “Biomarkers and uses thereof for selecting pancreas immunotherapy intervention”. MOH is supported in part by the US National Heart Lung and Blood Institute (R00 HL 141678). ML discloses research funds from the French Ministry of Health, research support from Shingotec, lecture fees from Baxter and Fresenius, and consulting fees from Novartis. CSD is supported in part by the US National Institute of General Medical Sciences, US NIH (R01 GM 121102). He has stock options with Enlivex Therapeutics, outside of the submitted work. CSD reports honoraria from Lippincott Williams & Wilkins (Scientific Editor Critical Care Medicine) and the New York University Department of Anesthesiology (visiting professor); non-financial support for participation in the Bernard-Wiggers Task Force (accommodation) and from the Society of Critical Care Medicine (meeting registration and accommodation); an honorarium, travel expenses, and accommodation from the International Society of Hematology and Thrombosis; and royalties from Elsevier for the textbook Evidence-Based Practice of Critical Care, outside of the submitted work. All other authors declare no competing interests.
References
- 1.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]