Abstract
The worldwide COVID-19 pandemic outburst has caused a serious public health issue with increasing needs of accurate and rapid diagnostic and screening testing. This situation requires an optimized management of the chemical reagents, the consumables, and the human resources, in order to respond accurately and effectively, controlling the spread of the disease. Testing on pooled samples maximizes the number of tested samples, by minimizing the time and the lab supplies needed. The general conceptualization of the pooling method is based on mixing samples together in a batch. Individual testing is needed only if a specific pool exhibits a positive result. The development of alternative hybrid methods, based on “in house” protocols, utilizing commercially available consumables, in combination with a reliable pooling method would provide a solution, focusing on the better exploitation of the personnel and the lab supplies, allowing for rapid screening of a population in a reasonably short time.
Keywords: Pooling methods, SARS-CoV-2, Hierarchical algorithm, Array testing, N genes
1. Introduction
The pandemic, named Coronavirus disease 2019 (COVID-19) (Wang et al., 2020) is associated with an infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is now well established that the fast and worldwide COVID-19 pandemic outburst has caused a serious public health problem with increasing needs of accurate and rapid diagnostic and screening strategies (Kumar et al., 2020). It is self-evident that maximizing the number and availability of the tests is of paramount importance to combat the epidemic (Pikovski and Bentele, 2020).
This urgent situation requires an optimized and co-ordinated management, including a proper exploitation of the chemical reagents, the consumables as well as the human resources, in order to respond promptly and efficiently towards, controlling the spread of the disease. Testing on pooled samples offers a means of maximizing the number of tested samples and minimizing the time and the laboratory consumables needed (Ben-Ami et al., 2020). The precise identification of infected individuals within a community is critical not only for restricting the spread of the disease, but also for studying it in-depth”. It appears the SARS-CoV-2 affects fundamental biological mechanisms, such as DNA replication, triggering replication stress. Replication stress is the hallmark of the oncogene-induced DNA damage model for cancer development and other pathophysiological disorders/lesions, such as granulomatous development (Halazonetis et al., 2008; Herrtwich et al., 2016).
Sample pooling is not a new approach, as it has been successfully applied in various cases of infectious diseases in the past (Dorfman, 1943; Abdalhamid et al., 2020). Dorfman proposed and applied the pooling method, in order to screen World War II soldiers for syphilis (Dorfman, 1943). It was the best way to rapidly detect and isolate patients suffering from syphilis, to treat them, and simultaneously protect others. Various types of pooling can be applied depending on individual clinical needs.
The general principle behind the pooling method is based on mixing a pre-selected number of samples together in a batch (Chen et al., 2020). The pooled sample will be subsequently tested by applying a molecular diagnostic protocol. Individual testing is needed only if a specific pool exhibits a positive result. This approach allows the increase of the number of individuals that can be tested simultaneously, utilizing the resources which are typically needed for a single test, while maintaining the detection accuracy (Furstenau et al., 2020). Hence, the pooling technique is a clear realization of the Principle of Minimum Potential Energy (Sadd, 2014), which is of crucial importance during an emergency.
It is widely accepted that during the COVID-19 pandemic, the need for immediate testing and the decreased availability or the lack of commercial kits or the delay in the delivery of them, due to the increased global demand (Carter et al., 2020), emerges the need for the development of “in house” protocols for RNA extraction and quantitative reverse transcription polymerase chain reaction (qRT-PCR) capable of detecting and quantifying the SARS-CoV-2 viral load. During the generalized quarantine, the United States of America and Europe experienced an acute shortage of certain reagents (Modesti et al., 2020). The extended shortage of supplies forced several regions around the globe to completely stop testing, resulting in a decrease of the diagnostic capacity and a potential underestimation of the epidemiological data on the disease.
Hybrid methods, based on “in house” protocols and utilizing commercially available consumables and kits, in combination with a reliable pooling method would provide a solution, focusing on the better exploitation of the personnel and the consumables, and allowing for rapid screening of a population in a reasonably short time.
2. Sample pooling strategies
Molecular RT-PCR-based tests are widely used for public health interventions such as case detection, tracking and isolation (Udugama et al., 2020). These strategies are of significant importance, considering that infection can be asymptomatic despite high viral loads (Zou et al., 2020; Walsh et al., 2020). Cycle threshold [Ct] values and viral load are typically analyzed in nasal and throat swabs. It seems that higher viral loads are detected soon after the appearance of the first symptoms (Pan et al., 2020). Also, nasal swabs which are sampled from the mid-turbinate and nasopharynx are considered more reliable than throat swabs, showing higher viral loads for the same patients.
Pooling protocols can be used to expand the capacity of the laboratory consumables, the infrastructure and the personnel, in periods when high numbers of people needed to be screened. Group testing protocols using pooled samples have been successfully employed in screening infectious diseases for many years (Dwyre et al., 2011). The efficacy of those methods increased with the development of sensitive molecular techniques. In any case, group testing is always applied with the expectation to perform significantly fewer tests than the number of specimens to be tested. When a pooling strategy has been selected and the pooled test result is negative, then every component of the batch is considered also negative, as if it had been examined separately. In case of a positive or ambiguous result, all the samples, comprising the pool, should be tested individually (Centers for Disease Control and Prevention (CDC), 2020). An ambiguous result sometimes is related to a borderline patient, at the Ct value level and sometimes is associated with a possible misfunction of the PCR reaction. When RNA levels are low (Ct value≥35), inter-assay variations may occur that are not detected without repeating the experiment. In each case, all the samples of the suspicious pools should be looked in separately. In general, the prevalence of the virus justifies the documentation of the use of the pooling protocol. Hence, if the number of positive results is expected to be low, then this strategy is approved. There are several processes that are included in group testing protocols in different stages of the procedure, such as at the swab level, at the RNA level etc. In any case, it is important that the false negative rate is maintained controllably low (Pouwels et al., 2020).
The pool size is an important parameter which can affect the specificity and sensitivity of the method (Brynildsrud, 2020). Various online tools have been developed in order to select the optimal configuration, by estimating the expected number of tests, the percentage of the reduction of the expected number of tests, compared to individual testing, the sensitivity, the specificity, the positive predictive value and the negative predictive value (A Shiny App, 2020). The extent of the saved consumables and resources through the use of the appropriate grouping method depends both on the group size and the prevalence rate (Dorfman, 1943).
3. Standard pooling protocol algorithms
Sample pooling algorithms are commonly characterized as being either hierarchical or non-hierarchical, depending on their structure (Hou et al., 2017).
3.1. Hierarchical algorithms
Hierarchical algorithms involve testing samples in non-overlapping pools over a pre-defined number of stages, until each individual can be classified as positive or negative (Wang et al., 2018).
3.1.1. Two-stage hierarchical testing
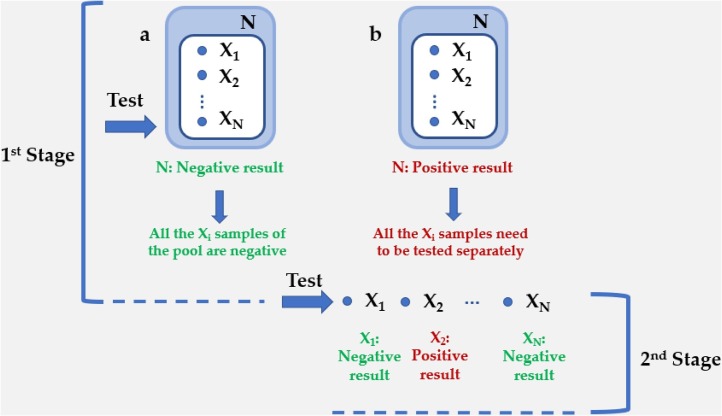
The two-stage pooling procedure is considered as the standard protocol for sample pooling. The fundamentals of this process include the definition of some critical parameters, variables and constants. Thus, the number of the samples that should be tested or the initial population of the experiment is defined as N (Centers for Disease Control and Prevention (CDC), 2020). The number of the samples in this initial pool is termed as Xi (1 < i < N). Upon a negative result of the N, all the Xi can be declared healthy (Fig. 1 a). If the result is positive, then a second stage is required. All the Xi samples of the positive pool are going to be tested separately and the final result is that of this second step (Fig. 1b).
Fig. 1.
Configuration for two-stage hierarchical testing with an initial pool N, followed by individual testing in the second stage of the algorithm. (a) Upon a negative result of the N, all the Xi can be declared healthy. (b) If the result is positive, then a second stage is required. All the Xi samples of the positive pool are going to be tested separately and the final result is that of this second step.
3.1.2. Three-stage hierarchical testing
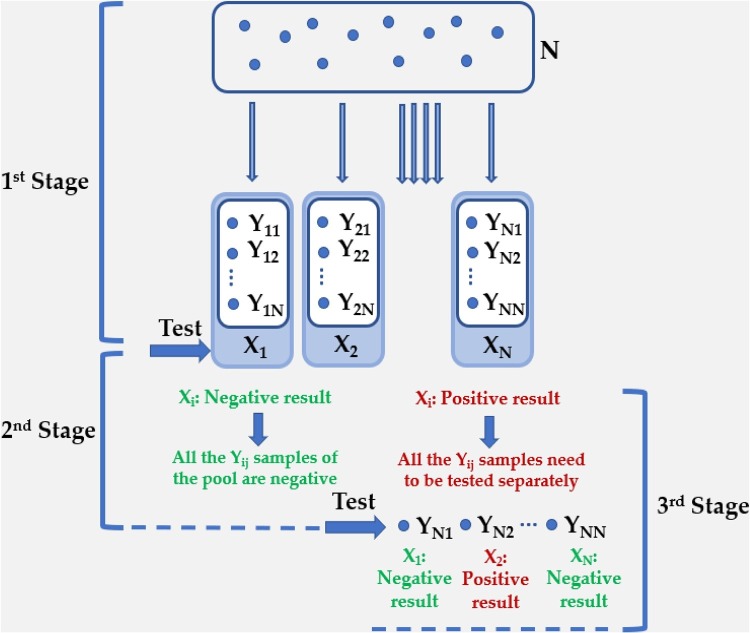
When a higher number of stages is required, due to a large initial number of samples that need to be tested, intermediate testing of specimens should occur in wider pools, comprising “sub-pools” at various levels. Thus, a three-stage algorithm also begins by testing specimens in non-overlapping pools. The number of the samples that should be tested or the initial pool of the experiment is defined as N. The number of the groups or the divisions of the population is termed as Xi (1 < i < N). Yij is the number of the samples in each pool (1 < i < N, 1 < j < N) and is the same for all the pools. Xi pools are examined individually. Upon a negative result of the Xi, all the Yij can be declared healthy. If the result is positive, then non-overlapping sub-pools of its members are formed for a second stage of testing, to isolate those that contain positive samples (Hou et al., 2017). Consequently, if a sub-pool is positive, then individual testing of all its members is going to be implemented in the third and final stage (Fig. 2 ). Based on our experience, by applying the qRT-PCR assay with primers and probes targeting the N genes (N1 and N2), which is the region encoding a nucleocapsid protein of SARS-CoV-2 (Perchetti et al., 2020a) (Supplementary Fig. 1), the detection of the positive samples is efficient, employing three-stage hierarchical testing in pools of 5, 8, 10 and 30 samples, in cases with different viral load (104, 103, 102 and 10 copies/reaction), namely from intermediate to low viral load (Supplementary Fig. 1). In these positive samples, Next Generation Sequencing (NGS) with Ion Torrent Suite™ software was also implemented. High homology (more than 99 %) with the SARS-CoV-2 sequence was assessed by employing the Basic Local Alignment Search Tool (BlastN) (Wang et al., 2018; Johnson et al., 2006). We observed that there is a striking resemblance with other SARS-CoV-2 strains, that were identified in other countries, like USA, Turkey, Sri Lanka, Germany, India and Czech Republic (Supplementary Fig. 2). This phenomenon confirms that there is a worldwide spread of the virus through populations. Furthermore, with this analysis, we can validate samples being positive, and essentially, we can verify existing or new mutations, first at the nucleotide level and foremost at the protein level. Thus, Next Generation Sequencing (NGS) allows a periodical auto-check of our method, since certifies that SARS-CoV-2 is the virus that is detected. We typically select samples obtained from all the range of the possible viral load to be examined through Next Generation Sequencing (NGS). Particularly the borderline positive samples should be tested, ensuring the outcome of the results.
Fig. 2.
Configuration for three-stage hierarchical testing with an initial pool N. The number of the groups or the divisions of the N population is termed as Xi (1 < i < N). Yij is the number of the samples in each pool (1 < i < N, 1 < j < N) and is the same for all the pools. Xi pools are examined individually. Upon a negative result of the Xi, all the Yij can be declared healthy. If the result is positive, then sub-pools of its members are formed for a second stage of testing, to isolate those that contain positive samples. Consequently, if a sub-pool is positive, then individual testing of all its members is going to be implemented in the third and final stage.
3.2. Non-hierarchical algorithms
Non-hierarchical algorithms involve testing over stages. The difference is that in this approach, individuals may be tested more than once per stage through overlapping pools (Verdun et al., 2020).
3.2.1. Array testing
Array testing, also known as matrix pooling, is the most common among the non-hierarchical processes. Individual specimens with an equal disease probability are arranged in a two-dimensional (2-D) grid and pooled by row and by column in the first stage of the algorithm. The number of rows and columns is defined as N and M, respectively (Wang et al., 2018). The second stage of the 2-D array testing involves separately retesting specimens not classified as negative in the first stage. These are the samples that lie at intersections of positive rows and columns (see Fig. 3 ). Three-dimensional or higher-dimensional procedures can also be used, first testing a master pool, depending on the need of the experiment (Kim and Hudgens, 2009).
Fig. 3.
Configuration for array testing with a N x M matrix development. Individuals are pooled by row and by column in the first stage of testing. The second stage of the 2-D array testing involves separately retesting specimens not classified as negative in the first stage. These are the samples that lie at intersections of positive rows and columns (in red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Furthermore, there are alternative methods for employing array testing, in case of samples with unequal disease probability. If higher-risk individuals are expected in the initial pool, then gradient or spiral clustering should be used during the implementation of the array test (McMahan et al., 2012). In a gradient construction clustering, higher-risk individuals are put in the leftmost columns of the matrix. Particularly, this process starts by placing the highest-risk individual in the (1, 1) cell, the second higher-risk individual in the (2, 1) cell and so on. When the first column is filled, the cell (1, 2) is subsequently filled and this task is repeated, moving across the array, from left to right. The lowest-risk individual is placed in the cell (N, M) (Fig. 4 a). This procedure should be selected in low prevalence settings, in cases with a low number of samples which are expected to be positive (McMahan et al., 2012). The main advantage of this method compared to the standard uninformative array testing is that it isolates the highest-risk individuals on one side of the array, and it reduces the number of columns which test positive.
Fig. 4.
Configuration for array testing with a N x M matrix development in gradient (a) and spiral (b) mode. These approaches should be selected in case of samples with unequal disease probability. In a gradient array test, higher-risk individuals are put in the leftmost columns of the matrix. When the first column is filled, the second one is filled form the top to the bottom and so on. The lower-risk individual is placed in the cell (N, M). In the spiral methods the higher-risk individuals are put in a square sub-matrix within the master one. Typically, the highest-risk individual is assigned in the upper left-hand corner of the array. The common motif starts with the highest-risk individual at the (1, 1) cell, the next three highest-risk individuals are allocated to the (2, 1), (2, 2), and (1, 2) cells, respectively. Then, the samples are put in the cells of the hypothetical array in the same motif, in descending order of disease probability.
In the spiral methods the higher-risk individuals are assigned to a square sub-matrix within the master one. Typically, the highest-risk individual is put in the upper left-hand corner of the array. The common motif starts with the highest-risk individual at the (1, 1) cell, the next three higher-risk individuals are put at the (2, 1), (2, 2), and (1, 2) cells, respectively. Then, the samples are put in the cells of the hypothetical array (3, 1), (3, 2), (3, 3), (2, 3), (1, 3), in descending order of disease probability (Fig. 4b). The aim of this approach is to isolate the positive individuals in a small square, expecting as negative the majority of the other rows and columns of the matrix.
4. Conclusion and future perspectives
The rapid screening of a large number of individuals and the accurate detection of the infected ones, enables the study of the infection-mediated molecular mechanisms and thus a deep understanding of the COVID19 biology. Performing test using pooled samples can maximize the number of tested samples in a short time.
A main question about the pooling method is the possible impact on the accuracy of the results. qRT-PCR has an inherent bias itself, like most other testing procedures. Therefore, it can misclassify some negative specimens as positive and vice versa, during the quantification process. These inaccuracies may derive from the low viral load in some patients, the bad quality of swab specimen collection, the insufficient sample loading and RNA degradation during the sample handling process. These effects are supposed to be amplified in a grouped test; therefore, it is important to acknowledge them as potential technical limitations. Generally, the larger the pool size, the higher the probability of obtaining false-negative results. Ben-Ami et al. support that a mixture of 8–10 samples is considered to yield a reliable outcome (Ben-Ami et al., 2020). Lohse et al. suggest that pooling of up to the maximum number of 30 samples per pool can increase test capacity with the existing supplies and equipment, detecting positive samples with satisfactory accuracy (Lohse et al., 2020). A recent report by Mutesa et al. introduced a strategy for detecting SARS-CoV-2 at low prevalence based on a two-stage algorithm which involves a first round of group tests, with a larger group size (up to 100) (Mutesa et al., 2020). At the second stage, groups identified as positive proceed to a round of “slice tests,” circumventing any need for individual testing. Moreover, our experimental data, employing the qRT-PCR assay with primers and probes targeting the N genes (N1 and N2) indicate that the detection of the positive samples is efficient, in pools of 5, 8, 10 and 30 samples, in cases with incremental viral load (104, 103, 102 and 10 copies/reaction), from mean to low viral load. Therefore, the detection if the positive sample was feasible either in samples with intermediate viral load (104 copies / reaction), or in cases with low viral load (∼10 copies /reaction) in pools of 5, 8, 10 and 30 samples. These data came from a series of test repetitions. Actually, the experiments have been repeated three times and the samples were tested in triplicates. Thus, the majority of the studies focusing on testing infection-suspected samples in pools, demonstrate that cases with a mean viral load (∼25 Ct Value / 104-105 copies/mL) are detected in pools of 5 or 10 samples. Hirotsu et al. showed that the primer/probe targeting the N1 site succeeded to detect samples with high and moderate viral load, but not those with low viral load when the samples were examined in pools of 20. The N2 site was proven to be more effective target for analyzing the pooled samples, since it was detected even if in cases with low viral load (Hirotsu et al., 2020). Perchetti et al. showed that pooling correctly identified SARS-CoV-2 in 94 % of the samples which were classified as samples with median viral load. The detection of the positive samples with low viral load was not feasible by pooling. On the other hand, all the samples which individually determined as negative, were also negative when tested in pools (Perchetti et al., 2020b).
Generally, employing pooled tests generates a large worry about the probability of increasing the false negative rate. This concern is based on the fact that during group testing a few positive samples are mixed with many negative samples which could push the concentration of viral genetic material below the detection limit (Arnaout et al., 2020). Hence, borderline positive patients, namely patients with very low viral load, may not be identified as positive, since these levels of viral genetic material may not be quantified. This is a common phenomenon in samples obtained by convalescent patients 14–21 days post symptomatic infection (Lohse et al., 2020). Since borderline positive samples might escape detection in large-pool testing, this might be a crucial point for further discussion, regarding the clinical significance of performing tests on those patients. It is not totally clarified if patients with low viral concentration or those who are asymptomatic carriers of the disease can rapidly transmit it. Zou et al. suggest that the viral load was detectable in both asymptomatic and symptomatic patients. Hence, asymptomatic or slightly symptomatic patients may have the potential to transmit the disease (Zou et al., 2020). Since it is unclear whether COVID19 transmission can occur early in the course of the infection, and the detection and isolation of COVID19 may require different strategies from those required for the general control of SARS-CoV-2, it is important to promptly establish a comprehensive plan of action for sufficient SARS-CoV-2 detection based on the already existing protocols and algorithms.
Depending on the situation and taking into consideration the probability of a possibly high-risk area in each case, it is important to balance the pros and cons between the need of a quick screen of the asymptomatic population and the accurate detection of the borderline positive patients, who may not be detected in a pooled test.
Funding
This research was funded by the research and diagnostic project “Immediate diagnosis of individuals with suspected infection by the new coronavirus SARS-CoV-2 and taking of measures to prevent secondary transmission of the virus”, Research Code: 17068, Special Account for Research Grants, National and Kapodistrian University of Athens.
It was also supported by the National Public Investment Program of the Ministry of Development and Investment / General Secretariat for Research and Technology, in the framework of the of the Flagship Initiative to address SARS-CoV-2 (2020ΣE01300001) and H. Pappas donation.
CRediT authorship contribution statement
Nefeli Lagopati: Writing - original draft, Writing - review & editing, Visualization. Panagiota Tsioli: Writing - original draft, Visualization. Ioanna Mourkioti: Writing - review & editing, Visualization. Aikaterini Polyzou: Writing - original draft. Angelos Papaspyropoulos: Writing - original draft. Alexandros Zafiropoulos: Writing - review & editing. Konstantinos Evangelou: Methodology, Writing - review & editing. George Sourvinos: Supervision. Vassilis G. Gorgoulis: Conceptualization, Methodology, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the contribution of Dr. Athanassios Kotsinas, Assistant Professor at the Faculty of Medicine, NKUA, for providing representative NGS results.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2020.114044.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- A Shiny App for Pooled Testing. Available online: https://bilder.shinyapps.io (accessed on 15/11/2020).
- Abdalhamid B., Bilder C.R., McCutchen E.L., Hinrichs S.H., Koepsell S.A., Iwen P.C. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am. J. Clin. Pathol. 2020;153(6):715–718. doi: 10.1093/ajcp/aqaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout R., Lee R.A., Lee G.R., Callahan C., Yen C.F., Smith K.P., Arora R., Kirby J.E. SARS-CoV2 testing: the limit of detection matters. bioRxiv. 2020 doi: 10.1093/cid/ciaa1382. 2020.06.02.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R., Klochendler A., Seidel M., Sido T., Gurel-Gurevich O., Yassour M., Meshorer G., Benedek E., Fogel I., Oiknine-Djian E., Gertler A., Rotstein Z., Lavi B., Dor Y., Wolf D.G., Salton M., Drier Y. The hebrew university-hadassah COVID-19 diagnosis team. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin. Microbiol. Infect. 2020;26(9):1248–1253. doi: 10.1016/j.cmi.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynildsrud O. COVID-19 prevalence estimation by random sampling in population - optimal sample pooling under varying assumptions about true prevalence. BMC Med. Res. Methodol. 2020;20(1):196. doi: 10.1186/s12874-020-01081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., Sasso J.M., Gregg A.C., Soares D.J., Beskid T.R., Jervey S.R., Liu C. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020;6(5):591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2020. Interim Guidance for Use of Pooling Procedures in SARS-CoV-2 Diagnostic, Screening, and Surveillance Testing.https://www.cdc.gov/coronavirus/2019-ncov/lab/pooling-procedures.html [Google Scholar]
- Chen W., Zhang S., Williams J., Ju B., Shaner B., Easton J., Wu G., Chen X. A comparison of methods accounting for batch effects in differential expression analysis of UMI count based single cell RNA sequencing. Comput. Struct. Biotechnol. J. 2020;18:861–873. doi: 10.1016/j.csbj.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman R. The detection of defective members of large populations. Ann Math Statist. 1943 Corpus ID: 120890326. [Google Scholar]
- Dwyre D.M., Fernando L.P., Holland P.V. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92–98. doi: 10.1111/j.1423-0410.2010.01426.x. [DOI] [PubMed] [Google Scholar]
- Furstenau T.N., Cocking J.H., Hepp C.M., Fofanov V.Y. Sample pooling methods for efficient pathogen screening: practical implications. bioRxiv. 2020 doi: 10.1371/journal.pone.0236849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halazonetis T.D., Gorgoulis V.G., Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319(5868):1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- Herrtwich L., Nanda I., Evangelou K., Nikolova T., Horn V., Sagar Erny D., Stefanowski J., Rogell L., Klein C., Gharun K., Follo M., Seidl M., Kremer B., Münke N., Senges J., Fliegauf M., Aschman T., Pfeifer D., Sarrazin S., Sieweke M.H., Wagner D., Dierks C., Haaf T., Ness T., Zaiss M.M., Voll R.E., Deshmukh S.D., Prinz M., Goldmann T., Hölscher C., Hauser A.E., Lopez-Contreras A.J., Grün D., Gorgoulis V., Diefenbach A., Henneke P., Triantafyllopoulou A. DNA damage signaling instructs polyploid macrophage fate in Granulomas. Cell. 2016;167(5):1264–1280. doi: 10.1016/j.cell.2016.09.054. [DOI] [PubMed] [Google Scholar]
- Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., Sueki H., Hayakawa M., Mochizuki H., Tsutsui T., Kakizaki Y., Miyashita Y., Omata M. Pooling RT-qPCR testing for SARS-CoV-2 in 1000 individuals of healthy and infection-suspected patients. Sci. Rep. 2020;10(1):18899. doi: 10.1038/s41598-020-76043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P., Tebbs J.M., Bilder C.R., McMahan C.S. Hierarchical group testing for multiple infections. Biometrics. 2017;73(2):656–665. doi: 10.1111/biom.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Zaretskaya I., Raytselis Y., Merezhuk Y., McGinnis S. Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2006;36(Web Server issue (2008)):W5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Hudgens M.G. Three-dimensional array-based group testing algorithms. Biometrics. 2009;65(3):903–910. doi: 10.1111/j.1541-0420.2008.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Gupta P.K., Srivastava A. A review of modern technologies for tackling COVID-19 pandemic. Diabetes Metab. Syndr. 2020;14(4):569–573. doi: 10.1016/j.dsx.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse S., Pfuhl T., Berkó-Göttel B., Rissland J., Geißler T., Gärtner B., Becker S.L., Schneitler S., Smola S. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan C.S., Tebbs J.M., Bilder C.R. Two-dimensional informative array testing. Biometrics. 2012;68(3):793–804. doi: 10.1111/j.1541-0420.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti P.A., Wang J., Damasceno A., Agyemang C., Van Bortel L., Persu A., Zhao D., Jarraya F., Marzotti I., Bamoshmoosh M., Parati G., Schutte A.E. Indirect implications of COVID-19 prevention strategies on non-communicable diseases: an opinion paper of the european society of hypertension working group on hypertension and cardiovascular risk assessment in subjects living in or emigrating from low resource settings. BMC Med. 2020;18(1):256. doi: 10.1186/s12916-020-01723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutesa L., Ndishimye P., Butera Y., Souopgui J., Uwineza A., Rutayisire R., Ndoricimpaye E.L., Musoni E., Rujeni N., Nyatanyi T., Ntagwabira E., Semakula M., Musanabaganwa C., Nyamwasa D., Ndashimye M., Mwikarago I.E., Muvunyi C.M., Mazarati J.B., Nsanzimana S. A pooled testing strategy for identifying SARS-CoV-2 at low prevalence. Nature. 2020 doi: 10.1038/s41586-020-2885-5. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchetti G.A., Nalla A.K., Huang M.L., Jerome K.R., Greninger A.L. Multiplexing primer/probe sets for detection of SARS-CoV-2 by qRT-PCR. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchetti G.A., Sullivan K.W., Pepper G., Huang M.L., Breit N., Mathias P., Jerome K.R., Greninger A.L. Pooling of SARS-CoV-2 samples to increase molecular testing throughput. J. Clin. Virol. 2020;131 doi: 10.1016/j.jcv.2020.104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikovski A., Bentele K. Pooling of coronavirus tests under unknown prevalence. Epidemiol. Infect. 2020;148:e183. doi: 10.1017/S0950268820001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels K.B., Roope L., Barnett A., Hunter D.J., Nolan T.M., Clarke P.M. Group Testing for SARS-CoV-2: Forward to the Past? PharmacoEconomics. 2020;4(2):207–210. doi: 10.1007/s41669-020-00217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd M.H. Elasticity. third edition. 2014. Chapter 6 - strain energy and related principles in theory, applications, and numerics; pp. 119–139. [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Verdun C.M., Fuchs T., Harar P., Elbrachter D., Fischer D.S., Berner J., Grohs P., Theis F.J., Krahme F. Group testing for SARS-CoV-2 allows for up to 10-fold efficiency increase across realistic scenarios and testing strategies. medRxiv. 2020 doi: 10.3389/fpubh.2021.583377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K.A., Jordan K., Clyne B., Rohde D., Drummond L., Byrne P., Ahern S., Carty P.G., O’Brien K.K., O’Murchu E., O’Neill M., Smith S.M., Ryan M., Harrington P. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020;81(3):357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., McMahan C.S., Tebbs J.M., Bilder C.R. Group testing case identification with biomarker information. Comput. Stat. Data Anal. 2018;122:156–166. doi: 10.1016/j.csda.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., He Y., Tong J., Qin Y., Xie T., Li J., Li J., Xiang J., Cui Y., Higgs E.S., Xiang J. Characterization of an asymptomatic cohort of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected individuals outside of Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.