Abstract
Coronavirus disease 2019 (COVID-19) was initially characterized due to its impacts on the respiratory system; however, many recent studies have indicated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) significantly affects the brain. COVID-19 can cause neurological complications, probably caused by the induction of a cytokine storm, since there is no evidence of neurotropism by SARS-CoV-2. In line with this, the COVID-19 outbreak could accelerate the progression or affect the clinical outcomes of neuropsychiatric conditions. Thus, we analyzed differential gene expression datasets for clinical samples of COVID-19 patients and identified 171 genes that are associated with the pathophysiology of the following neuropsychiatric disorders: alcohol dependence, autism, bipolar disorder, depression, panic disorder, schizophrenia, and sleep disorder. Several of the genes identified are associated with causing some of these conditions (classified as elite genes). Among these elite genes, 9 were found for schizophrenia, 6 for autism, 3 for depression/major depressive disorder, and 2 for alcohol dependence. The patients with the neuropsychiatric conditions associated with the genes identified may require special attention as COVID-19 can deteriorate or accelerate neurochemical dysfunctions, thereby aggravating clinical outcomes.
Keywords: COVID-19, Neuropsychiatric disorders, Molecular markers, Elite genes, Neuroinflammation
Highlights
-
•
COVID-19 can impact the clinical outcomes of neuropsychiatric diseases (NP).
-
•
Molecular markers of NP were differentially expressed in patients with COVID-19.
-
•
Elite genes were found for schizophrenia, autism, depression and alcohol dependence.
1. Introduction
Coronavirus disease 2019 (COVID-19) outbreak is a conundrum for physicians and researchers alike, as it affects several tissues in addition to the lungs, such as the cardiovascular system, kidneys, gut and brain (Wadman, 2020; Wang et al., 2020; Zubair et al., 2020). Patients may present ophthalmological and neurological manifestations including conjunctivitis, confusion, seizure, stroke, and Guillain-Barré syndrome, which may be associated with inflammation. Moreover, there is a close relationship between the periphery of the body and the central nervous system (CNS), where respiratory, cardiac, hepatic, renal and gastrointestinal symptoms can markedly affect the brain, via an event that has been well characterized in patients with COVID-19, the cytokine storm. The cytokine storm is an overreaction of immune system; cytokines are molecules that can mediate a healthy immune response, but their overproduction can lead to switching from having a protective role to a harmful effect, resulting in attacks on healthy tissues, including the brain. Although the CNS presents functional barriers, such as the blood-brain barrier (BBB), after the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the permeability of this barrier can be affected as a consequence of the increased production, release and migration of cytokines into the CNS. This, in turn, may trigger specific inflammatory mechanisms that induce BBB breakdown and, consequently, brain damage (Alam et al., 2020; Li et al., 2020; Yachou et al., 2020; Zubair et al., 2020). Importantly, several neuropsychiatric and psychological symptoms have been reported after SARS-CoV-2 infection (Troyer et al., 2020; Wu et al., 2020). Thus, the COVID-19 outbreak could accelerate the progression or affect the clinical outcomes of these conditions.
Some studies have characterized neurologic symptoms in patients during COVID-19 infection, with different outcomes (Paterson et al., 2020; Troyer et al., 2020; Wu et al., 2020). We, herein, identified several molecular markers associated with the pathophysiology of neuropsychiatric disorders that were found to be differentially expressed in clinical samples from patients with COVID-19. To the best of our knowledge, this is the first report presenting a molecular link between neuropsychiatric disorders and SARS-CoV-2 infection through the identification of the altered expressions of genes associated with COVID-19 and brain diseases.
2. Methods
2.1. Dataset acquisition
Clinical evidences are pointing to a correlation between COVID-19 and neurological conditions. Accordingly, we performed the search for molecular impact promoted by SARS-CoV-2 infection that might be linked to selected neuropsychiatric disorders. Thus, host differentially expressed genes (DEGs) from clinical datasets were obtained from published data (Blanco-Melo et al., 2020; Ouyang et al., 2020; Xiong et al., 2020). The selected data were made with clinical samples from COVID-19 patients. Briefly, Blanco-Melo et al. (2020) used lung-epithelium derived cells and human bronchial epithelial cells to identify the differential transcriptional response and protein signature; Ouyang et al. (2020), identified DEGs from human peripheral blood mononuclear cells (PBMCs) and the corresponding serum samples; Xiong et al. (2020) also described the differential transcriptome and transcriptional changes in bronchoalveolar lavage fluid (BALF) and PBMCs specimens of COVID-19 patients.
2.2. Correlation and assignment of DEGs to neuropsychiatric disorders
The described clinical COVID-19 dataset containing host DEGs was checked to potential assignment to genes associated with selected neuropsychiatric disorders found in the MalaCards Human Disease Database (https://www.malacards.org) (Rappaport et al., 2017), as previously described (Beys-da-Silva et al., 2020, 2019). The assignment checking was made manually for each DEG against all genes associated with the selected neuropsychiatric disorders in MalaCards Database.
3. Results
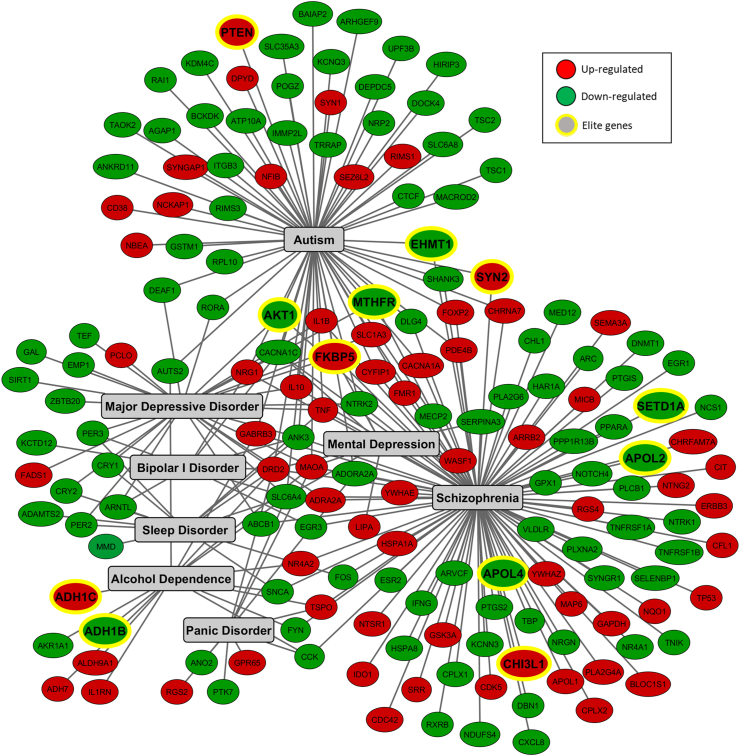
First, we constructed a database containing DEGs identified in clinical samples from COVID-19 patients, and this database was then further checked for potential associations with specific neuropsychiatric disorders. A substantial set of DEGs was found for genes are associated with alcohol dependence, autism, bipolar disorder, depression, panic disorder, schizophrenia, and sleep disorder (Fig. 1). According to several publications, these disorders have been significantly influenced by the COVID-19 outbreak (Eshraghi et al., 2020; Troyer et al., 2020; Yao et al., 2020). In addition to the identification of 171 genes linked to these neuropsychiatric disorders (Table S1), it should be highlighted that 12 genes are associated with causing some of these disorders. These genes, known as elite genes, according to the MalaCards Database (Rappaport et al., 2017), are: ADH1B (alcohol dehydrogenase 1B (class I), beta polypeptide), ADH1C (alcohol dehydrogenase 1C (class I), gamma polypeptide), AKT1 (AKT serine/threonine kinase 1), APOL2 (apolipoprotein L2), APOL4 (apolipoprotein L4), CHI3L1 (chitinase 3 like 1), EHMT1 (euchromatic histone lysine methyltransferase 1), FKBP5 (FKBP prolyl isomerase 5), MTHFR (methylenetetrahydrofolate reductase), PTEN (phosphatase and tensin homolog), SETD1A (SET domain containing 1A, histone lysine methyltransferase), and SYN2 (synapsin II).
Fig. 1.
Interaction network of genes associated with selected neuropsychiatric disorders that were found differentially expressed in clinical samples from patients with COVID-19. The interaction network was built in Cytoscape v.3.8.0, using the differentially expressed genes (DEGs) identified in these samples, and categorized with the MalaCards Human Disease Database (https://www.malacards.org). Genes are represented in circles (red, upregulated and green, downregulated). Nodes with yellow edges represent elite genes, which are those likely associated with causing the disease, since their gene-disease associations are supported by manually curated and trustworthy sources, according to MalaCards Database.
The pathophysiologies of alcohol dependence, autism, bipolar disorder, depression, panic disorder, schizophrenia and sleep disorder remain unclear, but they share a common event: neuroinflammation. In this regard, we showed that inflammatory markers, such as TNF (tumor necrosis factor), TNFRSF1A and TNFRSF1B (TNF receptor superfamily member 1A and 1B), IFNG (interferon gamma), IL1B (interleukin 1 beta), IL1RN (interleukin 1 receptor antagonist), IL10 (interleukin 10), CXCL8 (C-X-C motif chemokine ligand 8), CD38 (CD38 molecule) and PTGS2/COX2 (prostaglandin-endoperoxide synthase 2/cyclooxygenase 2), among others, were found to be differentially expressed after SARS-CoV-2 infection (Fig. 1).
With regard to the elite genes for autism, we identified six genes, including PTEN, which is part of a signaling pathway implicated in neuronal survival, synaptic plasticity, learning, and memory (Busch et al., 2019). In addition, alterations in the expression of other genes associated with autism were observed, including AKT1, EHMT1, FKBP5, MTHFR and SYN2. Nine elite genes were identified for schizophrenia, and some of these are shared with autism, such as AKT1, EHMT1, FKBP5, MTHFR and SYN2. We identified other DEGs correlated to schizophrenia, such as APOL2, APOL4, SETD1A, and CHI3L1. With regard to depression three elite genes have been identified: AKT1, FKBP5 and MTHFR, which were previously identified for autism and schizophrenia. These genes regulate key points in the pathogenesis of depression (Park et al., 2019; Wan et al., 2018). Moreover, we identified other molecular markers that are significantly associated with depressive disorder (Fig. 1 and Table S2).
The molecular markers found also indicate significant changes linked to bipolar disorder, such as the downregulation of ABCB1 (ATP binding cassette subfamily B member 1), involved in multidrug resistance, and SLC6A4 (solute carrier family 6 member 4), which controls the transport of serotonin (Zhu et al., 2017). Moreover, we found two elite genes associated with alcohol dependence, ADH1B and ADH1C, which play a major role in ethanol metabolism. IL1RN was also found upregulated and may be linked with this condition, as it is associated with inflammation/genetic predisposition to high alcohol consumption (Blednov et al., 2015; Tilg et al., 2016).
Several genes that promote significant effects on neurons and glial cells were also identified, as up and downregulated, such as NFIB (nuclear factor I B), the transcriptional activator of the astrocytic marker GFAP (glial fibrillary acidic protein), and SLC1A3/EAAT1 (solute carrier family 1 member 3/excitatory amino acid transporter). Other DEGs were associated with signal transduction, transcription factors and the cell cycle, as well as proteins that regulate the oxidative response, cytoarchitecture and plasticity of the brain; all of these genes remarkably compromise brain functionality.
4. Discussion
Recent publications indicate that SARS-CoV-2 can cause neurological complications; however, the majority of studies are limited to epidemiological data observed in the hospitals. The impact of microbial infection on host’s gene expression is well known to trigger molecular alterations linked to pathological outcomes. Therefore, considering that clinical outcomes after COVID-19 are unknown and that their consequences are potentially dramatic, we surveyed DEGs identified in clinical samples from COVID-19 patients for a potential correlation with neuropsychiatric disorders. The early identification of potential susceptible groups with increased risk of mental health deterioration after SARS-CoV-2 infection is a matter of global public health interest due to the pandemic status of COVID-19. Accordingly, the list of molecular markers and diseases presented here might be useful for this purpose (Table S2). To the best of our knowledge, this is the first study linking SARS-CoV-2 with specific molecular markers associated with potential neuropsychiatric sequelae.
Although the neurotropism of SARS-CoV-2 remains unclear, many studies have shown neurological consequences derived from excessive inflammatory responses (Li et al., 2020; Zubair et al., 2020). In line with this, we identified changes in molecular markers associated with neuroinflammation. Pro-inflammatory and anti-inflammatory cytokines were upregulated in clinical samples of COVID-19 patients and these findings may indicate that SARS-CoV-2 can trigger an excessive inflammatory response. It is important to note that central and periphery inflammatory mediators, including components of cytokine storm, interleukin 6 (IL6) and C-reactive protein, are key elements in the pathomechanisms of several neuropsychiatric disorders, such as depression and bipolar disorder (Fries et al., 2019; Köhler-Forsberg et al., 2017). Thus, COVID-19 patients may present accelerated neuroprogression and/or psychiatric sequelae associated with inflammation.
De Felice et al. emphasized the importance of determining SARS-CoV-2 viral load in cerebrospinal fluid, since neurological complications can be primary or secondary consequences of infection (De Felice et al., 2020). Independently, several genes that directly affect neurotransmission and synaptic plasticity have been reported as modulated in clinical samples of COVID-19 patients, suggesting that SARS-CoV-2 may have the ability to disturb key neurotransmitter systems, which represent a common point and a potential trigger for neuropsychiatric disorders. Notably, the potential neuroinvasion of SARS-CoV-2 can be associated with an inflammatory response-induced BBB breakdown (Alam et al., 2020; Yachou et al., 2020; Zubair et al., 2020). In line with this, astrocytes form (and reform) the BBB (Vainchtein and Molofsky, 2020), and we verified an upregulation of a modulator of GFAP, an astrocyte marker. Accordingly, plasma levels of GFAP have been shown to be increased in patients with severe and moderate COVID-19 (Kanberg et al., 2020).
As a peculiarity of depression, patients with the other neuropsychiatric disorders discussed in this study can also have depression. Depression should be highlighted, due to its high prevalence in the population and its association with other chronic pathological conditions. In addition, depression has been associated with social isolation and all other psychosocial aspects of the pandemic (Dubey et al., 2020). Moreover, attention should be paid to subjects with bipolar disorder that are COVID-19 positive to avoid the aggravation of clinical manifestations related to this mood disorder, since patients can become depressed and the mood swings can affect sleep (Volkert et al., 2015). Additionally, alterations in the expression of genes linked to panic and sleep disorders were observed.
In summary, we observed a remarkable number of molecular markers associated with neuropsychiatric disorders with altered expression in clinical samples from COVID-19 patients. Herein, we present a list containing 171 molecular markers that potentially need to be further surveyed in patients with COVID-19 or those recovered from COVID-19. This clinical follow-up might be particularly important in those patients previously diagnosed with alcohol dependence, autism, bipolar disorder, depression, panic disorder, schizophrenia and sleep disorder. These groups may require special attention from healthcare professionals because their pre-existing neuropsychiatric conditions might deteriorate after COVID-19, due to these molecular alterations. Four points are critical: i) all neuropsychiatric disorders addressed are associated with inflammatory processes and COVID-19 triggers a cytokine storm; ii) changes in the expression of genes associated with these disorders, especially elite genes, can accelerate neurochemical dysfunctions, aggravating neurological damage in these groups; iii) psychosocial effects associated with all aspects of the pandemic, such as social distancing and quarantine, economic burden, mass hysteria, and financial losses might also aggravate or help to trigger these disorders; and iv) after COVID-19 recovery, patients may need different management and pharmacological support to avoid or reduce associated neurological sequelae.
Funding
The researchers of this work are supported by Universidade Federal do Rio Grande do Sul, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
CRediT authorship contribution statement
André Quincozes-Santos: and, performed data collection and analysis, The manuscript was written by, and all authors commented on previous versions of the manuscript, All authors read and approved the final manuscript. Rafael Lopes Rosa: and, performed data collection and analysis, The manuscript was written by. Emanuela Fernanda Tureta: and, performed data collection and analysis, The manuscript was written by. Larissa Daniele Bobermin: performed data collection and analysis, The manuscript was written by, and all authors commented on previous versions of the manuscript, All authors read and approved the final manuscript. Markus Berger: and, performed data collection and analysis, The manuscript was written by. Jorge Almeida Guimarães: and all authors commented on previous versions of the manuscript, All authors read and approved the final manuscript. Lucélia Santi: and, performed data collection and analysis, The manuscript was written by, and all authors commented on previous versions of the manuscript, All authors read and approved the final manuscript. Walter Orlando Beys-da-Silva: contributed to the study conception and design, performed data collection and analysis, The manuscript was written by, and all authors commented on previous versions of the manuscript, All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors of this study are supported by Universidade Federal do Rio Grande do Sul, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100196.
Contributor Information
André Quincozes-Santos, Email: andrequincozes@ufrgs.br.
Walter Orlando Beys-da-Silva, Email: walter.beys@ufrgs.br.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alam S.B., Willows S., Kulka M., Sandhu J.K. Severe acute respiratory syndrome coronavirus-2 may be an underappreciated pathogen of the central nervous system. Eur J Neurol ene. 2020;14442 doi: 10.1111/ene.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beys-da-Silva W.O., da Rosa R.L., Santi L., Tureta E.F., Terraciano P.B., Guimarães J.A., Passos E.P., Berger M. The risk of COVID-19 for pregnant women: evidences of molecular alterations associated with preeclampsia in SARS-CoV-2 infection. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;165999 doi: 10.1016/j.bbadis.2020.165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beys-da-Silva W.O., Rosa R.L., Santi L., Berger M., Park S.K., Campos A.R., Terraciano P., Varela A.P.M., Teixeira T.F., Roehe P.M., Quincozes-Santos A., Yates J.R., Souza D.O., Cirne-Lima E.O., Guimarães J.A. Zika virus infection of human mesenchymal stem cells promotes differential expression of proteins linked to several neurological diseases. Mol. Neurobiol. 2019;56:4708–4717. doi: 10.1007/s12035-018-1417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y.A., Benavidez J.M., Black M., Mayfield J., Harris R.A. Role of interleukin-1 receptor signaling in the behavioral effects of ethanol and benzodiazepines. Neuropharmacology. 2015;95:309–320. doi: 10.1016/j.neuropharm.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch R.M., Srivastava S., Hogue O., Frazier T.W., Klaas P., Hardan A., Martinez-Agosto J.A., Sahin M., Eng C., Developmental Synaptopathies Consortium Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Transl. Psychiatry. 2019;9:253. doi: 10.1038/s41398-019-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S., Biswas P., Ghosh R., Chatterjee Subhankar, Dubey M.J., Chatterjee Subham, Lahiri D., Lavie C.J. Psychosocial impact of COVID-19. Diabetes & metabolic syndrome. Clin. Res. Rev. 2020;14:779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi A.A., Li C., Alessandri M., Messinger D.S., Eshraghi R.S., Mittal R., Armstrong F.D. COVID-19: overcoming the challenges faced by individuals with autism and their families. The Lancet Psychiatry. 2020;7:481–483. doi: 10.1016/S2215-0366(20)30197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries G.R., Walss-Bass C., Bauer M.E., Teixeira A.L. Revisiting inflammation in bipolar disorder. Pharmacol. Biochem. Behav. 2019;177:12–19. doi: 10.1016/j.pbb.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;10 doi: 10.1212/WNL.0000000000010111. 1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O., Buttenschøn H.N., Tansey K.E., Maier W., Hauser J., Dernovsek M.Z., Henigsberg N., Souery D., Farmer A., Rietschel M., McGuffin P., Aitchison K.J., Uher R., Mors O. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 2017;62:344–350. doi: 10.1016/j.bbi.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Li H., Xue Q., Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox. Res. 2020;38:1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y., Yin J., Wang W., Shi H., Shi Y., Xu B., Qiao L., Feng Y., Pang L., Wei F., Guo X., Jin R., Chen D. Downregulated gene expression spectrum and immune responses changed during the disease progression in patients with COVID-19. Clinical Infectious Diseases ciaa. 2020:462. doi: 10.1093/cid/ciaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Rosenblat J.D., Brietzke E., Pan Z., Lee Y., Cao B., Zuckerman H., Kalantarova A., McIntyre R.S. Stress, epigenetics and depression: a systematic review. Neurosci. Biobehav. Rev. 2019;102:139–152. doi: 10.1016/j.neubiorev.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S., the UCL Queen Square National Hospital for Neurology and Neurosurgery COVID-19 Study Group The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain awaa. 2020:240. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport N., Twik M., Plaschkes I., Nudel R., Iny Stein T., Levitt J., Gershoni M., Morrey C.P., Safran M., Lancet D. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45:D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Moschen A.R., Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64:955–965. doi: 10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein I.D., Molofsky A.V. Astrocytes and microglia: in sickness and in health. Trends Neurosci. 2020;43:144–154. doi: 10.1016/j.tins.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert J., Kopf J., Kazmaier J., Glaser F., Zierhut K.C., Schiele M.A., Kittel-Schneider S., Reif A. Evidence for cognitive subgroups in bipolar disorder and the influence of subclinical depression and sleep disturbances. Eur. Neuropsychopharmacol. 2015;25:192–202. doi: 10.1016/j.euroneuro.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Wadman M. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science. 2020 doi: 10.1126/science.abc3208. https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes [DOI] [Google Scholar]
- Wan L., Li Y., Zhang Z., Sun Z., He Y., Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl. Psychiatry. 2018;8:242. doi: 10.1038/s41398-018-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Yuan, Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachou Y., El Idrissi A., Belapasov V., Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020;41:2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Chen J.-H., Xu Y.-F. Patients with mental health disorders in the COVID-19 epidemic. The Lancet Psychiatry. 2020;7:e21. doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Klein-Fedyshin M., Stevenson J.M. Serotonin transporter gene polymorphisms and selective serotonin reuptake inhibitor tolerability: review of pharmacogenetic evidence. Pharmacotherapy. 2017;37:1089–1104. doi: 10.1002/phar.1978. [DOI] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.