Abstract
The coronavirus disease 2019 pandemic has resulted in many changes in how we interact in society, requiring that we protect ourselves and others from an invisible, airborne enemy called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Until a vaccine is developed, and it reaches high levels of distribution, everyone must continue to be diligent to limit the viral spread. The practice of assisted reproduction during this pandemic presents unique challenges in addition to the risks identified in general clinical care. The established good tissue practices employed in laboratories are not designed to protect gametes and embryos from an airborne virus, particularly one that may be shed by an asymptomatic staff member. Armed with theoretical risks but lacking direct evidence, assisted-reproduction teams must examine every aspect of their practice, identify areas at a risk of exposure to SARS-CoV-2, and develop a mitigation plan. Several professional fertility societies have created guidelines for the best practices in patient care during the coronavirus disease 2019 pandemic. As we learn more about SARS-CoV-2, updates have been issued to help adapt infection-control and -prevention protocols. This review discusses what is currently known about SARS-CoV-2 infection risks in assisted reproductive centers and recommends the implementation of specific mitigation strategies.
Key Words: SARS-CoV-2, gametes, embryos, in vitro fertilization, infertility care, ART laboratory
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/31824
Severe acute respiratory coronavirus 2 (SARS-CoV-2) is a highly transmissible virus that causes an acute respiratory disease called coronavirus disease 2019 (COVID-19). It accomplishes this feat primarily through respiratory droplets (>5 μm in diameter) and, possibly, aerosols (≤5 μm in diameter) released when a person exhales, coughs, sings, or talks. The virus is acquired through droplet inhalation, entry into the eyes, direct contact with another person, or by touching a surface that harbors virus-loaded droplets. After direct or indirect contact, the virus is introduced to the host after they touch their eyes, nose, or mouth (1, 2, 3, 4). Much has been learned about this novel virus during the pandemic, including its variable incubation period, ranging from 2 to 14 days, and the ability to spread from nonsymptomatic or presymptomatic individuals (5, 6, 7, 8). The variable and lengthy incubation period of SARS-CoV-2; potential for asymptomatic spread; and lack of an accessible, accurate, and rapid diagnostic tool make it significantly challenging to avoid this contagion (9).
During the early stages of the COVID-19 pandemic, assisted reproduction was one of many medical services that paused patient care to protect patients and healthcare workers, conserve personal protective equipment (PPE) and human resources, and reduce strain on the healthcare system (10, 11). As centers prepared to resume patient care, it was quickly recognized that their prepandemic infection control procedures that focused on universal precautions were insufficient to defend against the highly contagious, aerosol-mediated respiratory virus that can be shed and transmitted by asymptomatic or presymptomatic individuals (12, 13, 14). In response, professional fertility societies and working groups across the globe promptly formed taskforces to develop strategies and identify the best practices for assisted reproduction programs to provide safe and effective patient care while mitigating the risk of SARS-CoV-2 infection. Several updates to these recommendations have been made as we have developed a better understanding of how to protect patients and healthcare workers and realized the risks of SARS-CoV-2 exposure of gametes, embryos, and reproductive tissues. The aim of this study was to conduct a systematic review of SARS-CoV-2 infection risks and practice guidelines for infection control in assisted reproductive technology (ART) centers providing patient care during the COVID-19 pandemic.
Materials and methods
Literature Search
We performed a review to identify studies of SARS-CoV-2 infection risk in patients seeking infertility care along with professional fertility societies’ guidelines and individual recommendations for the mitigation of SARS-CoV-2 transmission while providing assisted reproductive care. The electronic databases OVID Medline and PubMed were searched for articles published from December 1, 2019, through October 20, 2020. We performed a search using the MeSH terms “assisted reproduction,” “infertility,” and “COVID-19” and restricted the publications to English language and human subjects. An additional manual search of results was performed using Google Scholar using the same MeSH terms. Professional fertility society guidelines that have not been published as manuscripts were accessed from their websites.
Study Selection
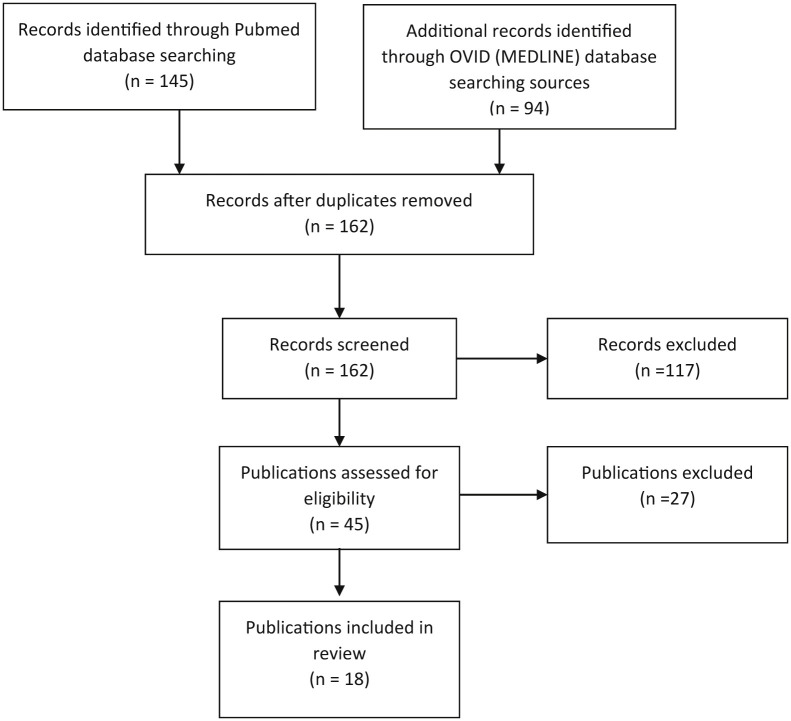
The initial search of the OVID Medline and PubMed databases helped identify 271 publications relevant to assisted reproduction or infertility and COVID-19. An overview of the study inclusion is presented in Figure 1 . This review included studies of risk assessment for SARS-CoV-2 infection in ART centers; recommendations from regional, national, and international fertility working groups and professional fertility societies for infection control; and SARS-CoV-2 infection in gametes, embryos, and gonads. Studies focusing on the impact of COVID-19 on reproductive health and pregnancy and psychological effects of delay in infertility care because of the pandemic and letters to the editor commenting on national or international responses to the SARS-CoV-2 infection prevention guidelines were excluded.
Figure 1.
PRISMA flowchart showing the study’s inclusion and exclusion. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
The articles and dates of acceptance describing SARS-CoV-2 infection risks specific to different ART clinics and laboratories are shown in Table 1 (15, 16, 17, 18, 19, 20, 21, 22). Guidelines accessed from professional fertility societies’ websites are listed in Table 2 (22, 23, 24, 25, 26, 27, 28). Readers must be mindful of the dates of manuscript acceptance or guideline publications because they reflect the knowledge of SARS-CoV-2 threats to the ART centers at the time.
Table 1.
Publications providing guidance for SARS-CoV-2 infection precautions in assisted reproduction practices.
| Author | Organization/location | Acceptance date | Comments |
|---|---|---|---|
| Hickman et al. (15) | International IVF laboratory managers (China, Italy, Spain, France, United Kingdom, Brazil, Australia presented. Additional participants: Austria, Belgium, Denmark, Germany, Greece, Japan, Malaysia, Mexico, New Zealand, Saudi Arabia, Sweden, United States participated) | May 12, 2020 | Proceedings of an international symposium held April 3, 2020 |
| Zivkovic et al. (16) | Croatian Society of Clinical Embryologists | May 2020 | |
| Andrabi et al. (17) | India, Milann-The Fertility Centre | May 31, 2020 | |
| Maggiulli et al. (18) | Italy, GENERA Group | June 19, 2020 | Failures modes and effective analysis of SARS-CoV-2 infection |
| Hallak and Esteves (19) | Brazil | July 5, 2020 | Specific to andrology services |
| Pomeroy and Schiewe (20) | United States | July 24, 2020 | Good tissue practices, Cryopreservation |
| Choucair et al. (21) | Middle East Fertility Society Embryology Special Interest Group | September 22, 2020 | |
| Alaluf et al. (22) | SAMeR (Argentina Society for Reproductive Medicine) | September 25, 2020 |
Note: IVF = in vitro fertilization; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Table 2.
Professional fertility societies’ practice guidelines for providing ART treatment during the COVID-19 pandemic.
| Organization | Initial publication | Last updated |
|---|---|---|
| American Society for Reproductive Medicine (ASRM) (23) | March 17, 2020 | October 6, 2020 |
| European Society of Human Reproduction and Embryology (24) | April 2, 2020 | October 14, 2020 |
| Canadian Fertility and Andrology Society (25) | March 13, 2020 | September 30, 2020 |
| Association of Reproductive and Clinical Scientists and the British Fertility Society (26) | March 16, 2020 | September 30, 2020 |
| Society for Assisted Reproduction, Society for Reproductive Biologists and College of Reproductive Biologists (ASRM affiliates, United States; specific to laboratory services) (27) | May 1, 2020 | October 5, 2020 |
| Indian Fertility Society, Indian Society for Assisted Reproduction and the Academy of Clinical Embryologists (28) | May 26, 2020 |
Note: ASRM = American Society for Reproductive Medicine; ART = assisted reproductive technology; COVID-19 = coronavirus disease 2019.
Risk assessment
Centers must assume that everyone is potentially infectious and implement additional infection prevention and control practices to their standard policies and procedures, thereby reducing the risk of SARs-CoV-2 transmission (9, 12). To achieve this goal, the program needs to identify all levels of risk associated with the continuation of patient care delivery. A failure mode and effect analysis conducted by a multidisciplinary ART team at the Gynaecology, Endocrinology, Embryology, Assisted Reproduction (GENERA) centers in Italy (18) helped identify the following 4 areas of potential risk factors for bidirectional infection in an ART clinic: patient-staff, staff-staff, staff-cell, and cell-cell. Their assessment revealed the risks associated with all phases of ART laboratory procedures, failures that can result in SARS-CoV-2 exposure, and corrective measures to avoid such failures. Professional fertility societies’ guidelines advised programs to conduct their own risk assessments for providing patient care during the pandemic because the risks vary based on staffing, physical space, PPE supplies, local infection rates, and other factors unique to their program (23, 24, 26). The risk categories identified by the GENERA team were used to structure the findings of our review of infection precautions for ART centers.
Mitigation of patient-staff transmission
The following strategies were identified by all professional fertility societies to reduce the risk of patient-to-staff and staff-to-patient transmission in ART centers.
Code of Conduct
Patients and staff must be educated and must follow the best practices for infection control to reduce the risk of exposure to SARS-CoV-2 at home, in the community, and at the workplace (Table 3 ). All fertility societies recommended including these practices in a written document for staff and patients, with regular reminders and repeated confirmation of compliance (23, 24, 25, 26, 27, 28).
Table 3.
Universal methods to reduce the risk of exposure to SARS-CoV-2.
|
Symptom Assessment Triage
COVID-19 symptom assessment should be conducted for all staff members, patients requiring an in-person visit, or any other individuals who must gain entry to the ART center. The components of question-based assessments were consistent across all fertility societies’ guidelines, with some guidelines recommending temperature checks and one recommending pulse oximeter checks for everyone entering the center (Supplemental Table 1). Patient screening may be conducted over the phone before their visit or through a mobile application. A recent review of screening recommendations from 4 national and international reproductive medicine societies has suggested that the adoption of a COVID-19 self-assessment application might relieve the staff of the additional phone call burden and assure that triage questions align with currently identified symptoms and geographic viral prevalence (29). Recommendations for patient testing as an adjuvant to triage with or without a positive screen varied with the professional fertility societies, often deferring to institutional or other authoritative sources to guide testing and quarantine measures.
Limiting Patient Visits and Time in ART Centers
When possible, clinics should use telehealth (either phone or secure video) in lieu of in-person clinic visits. When in-person visits cannot be avoided, the number of individuals in the facility should be limited. To achieve this, clinic and procedure schedules should be adjusted to allow adequate time to disinfect patient-care space between appointments. Clinics should minimize the number of visits required for monitoring ovarian stimulation. Access to the clinic should be restricted to patients who have appointments for a clinic visit or procedure. If the facility permits visitors, they must follow universal source-control procedures.
Universal Source Control
Preventative measures for SARS-CoV-2 transmission from asymptomatic and presymptomatic individuals require everyone in the ART center to wear facemasks, facilitate physical distancing, perform frequent hand hygiene by washing hands with soap and water or applying an alcohol-based hand sanitizer with 60%–95% alcohol, and disinfect patient-care areas between each patient. Additionally, diligent and daily disinfection of the entire ART center is required.
PPE
A description of recommended PPE for healthcare providers and patients, based on the American Society for Reproductive Medicine’s COVID-19 recommendations, is shown in Supplemental Table 2. Every fertility professional society emphasized on the need for centers to ensure that their PPE inventory meets these requirements to protect their patients and staff. Staff with potential exposures to aerosols must wear N95 masks, which provide protection from 95% of ≥0.3-μm aerosols.
Space
To facilitate physical distancing, markings of 6-feet distance should be made in registration areas, and seating should be adjusted in waiting rooms, consultation rooms, and communal staff areas to maintain the prescribed distance. If the waiting room space is limited, patients may be required to wait outside the center. Traffic patterns should be created throughout the center to allow individuals to avoid situations that might require them to be closer than 6 feet. Physical barriers should be installed in previously open staffing and reception areas (25, 26).
Disinfection
Every building occupant plays a role in disinfection. The rules of hand hygiene were outlined in detail by the Canadian Fertility and Andrology Society and SAMeR Task Force in Argentina (22, 25). In addition to the standard practices, centers were advised to increase the availability of alcohol-based hand gel stations, including one at the center’s entrance for everyone to use when entering the building (22).
SARS-CoV-2 has been shown to remain viable on surfaces for days; therefore, every surface touched by the occupants of ART centers should be considered a risk factor for contamination (30). The single-stranded ribonucleic acid of SARS-CoV-2 is enveloped by a glycoprotein membrane, making it susceptible to disinfectants, including sodium hypochlorite (0.1%), bleach (0.1%–1%), ethanol (62%–71%), hydrogen peroxide, and quaternary ammonium compounds (16, 31). Quaternary ammounium compounds are recommended for the disinfection of in vitro fertilization (IVF) laboratories because their use does not generate volatile organic compounds that may be harmful to embryos and gametes in culture (15, 21, 22, 32).
The SAMeR Task Force (22) has provided an excellent, detailed description of cleaning tasks based on area, frequency, disinfection products, and methods. In addition to disinfection, they have recommended removing extra surfaces, such as decorative items and reading materials from waiting rooms, to reduce disinfection burden. The disinfection of shared equipment, such as touch pads, pens, and keyboards, that was previously infrequently and nonroutinely cleaned is especially important for users (17, 27).
The airborne properties of SARS-CoV-2 and its potential to be circulated through the center’s heating, ventilation, and air-conditioning system should be factored into the procedure and clinic visit schedule. Hickman et al. (15) have proposed that 2 complete air exchanges are required between room uses, while another group (18) has suggested that sufficient air exchanges between room uses should be ensured, without prescribing the specific number.
There are no specific requirements for air quality in IVF procedure areas or IVF laboratories. Programs that use a controlled heating, ventilation, and air-conditioning system should avoid maintaining a positive pressure in a procedure area because the airflow can lead to an increase in the viral spread from patients shedding SARS-CoV-2. Ideally, an ART laboratory's air pressure should be positive to patient-procedure areas (17, 33). High-efficiency particulate air filters remove particulates measuring >0.3 μm from circulation but do not provide protection from smaller, aerosolized viruses. However, air handlers equipped with activated carbon filters intended to absorb volatile organic compounds have been reported to absorb viral particles (21, 34).
Mitigation of staff-staff transmission
In addition to the measures discussed to prevent patient-to-staff SARS-CoV-2 transmission, the recommendations to prevent staff-to-staff transmission include self-monitoring of symptoms among the staff before their arrival at work. All professional fertility societies have advised that practices must have policies in place for quarantine and testing requirements for employees reporting high-risk exposure or symptoms.
Staffing Schedules
All professional societies endorsed the creation of nonoverlapping shifts, with split teams of their staff to reduce the risk of exposure further if a member of the team becomes infected. The teams must be given enough time for the disinfection of the entire workspace between the shifts. Although ideal in concept, some ART programs in the United States have come to realize that this may not be possible for centers with small staff sizes or those returning to prepandemic patient volumes (27). Despite the program’s ability to split the staff into teams and strict adherence to the universal control procedures, there was a risk that multiple employees could simultaneously experience either high-risk exposure outside of work or become infected with SARS-CoV-2. In recognition of this possibility, programs were encouraged to make emergency arrangements to transfer patient care to another clinic or establish reciprocal staffing support to ensure that patient care was not interrupted (24, 25, 26, 27, 28).
Procedure Suite and Laboratory Precautions
PPE
After completing the COVID-19 triage, the procedure suite and laboratory staff should change into clean scrubs as soon as they arrive at the center (18, 27). Magguilli et al. (18) have provided a detailed graphic depicting their recommendations for PPE and garment changes for transitions from clinic entry into a changing room, anteroom, or filter zone and for movement into and out of the ART laboratory. Some of their measures exceeded those of the American Society of Reproductive Medicine and the US Center for Disease Control, including double-gloving when they are in potential contact with biological fluids and the use of FFP2 masks, which filter >94% of 0.3-μm aerosols with <8% inward leakage, providing greater protection than surgical masks and slightly less protection than N95 masks. There is no evidence that these extra precautions are necessary to protect laboratory staff, and they may pose an undue burden on precious PPE supplies.
Disinfection
Laboratory staff should rely on hand-washing and avoid using an alcohol-based hand gel for hand hygiene before entering the ART laboratory because alcohol is a source of volatile organic compounds that may adversely affect human gametes and embryos. Gloves should be worn when handling and/or wiping down packages, especially when the packages have been recently handled by delivery staff and require immediate opening (17, 27). The staff should pay extra attention to the disinfection of items brought into and out of the laboratory and before the initial refrigeration of consumable items, such as culture media (16, 17, 22, 28).
The laboratory staff must be diligent about the frequent disinfection of shared pipettes, timers, pens, microscopes (including eyepieces), and other surfaces in the laboratory that are touched (18, 22). The use of ultraviolet irradiation in combination with a chemical disinfectant in biological safety cabinets or laminar flow hoods to kill all viruses has been recommended (16, 21, 22, 28).
Mitigation of staff-cell transmission risks in the art laboratory
When the professional fertility society guidelines for the resumption of patient care were written, little was known about the risks SARS-CoV-2 posed to gametes and embryos. The knowledge that has been gained since is associated primarily with theoretical risks.
Follicular Fluid and Semen
There is limited evidence regarding the presence of SARS-CoV-2 in the follicular fluid. To date, there have been no reports of SARS-CoV-2 in the follicular fluid. As for semen, the results are mixed. A small study of semen collected from 34 patients 8–75 days after COVID-19 diagnosis reported that SARS-CoV-2 could not be detected using real-time reverse transcriptase polymerase chain reaction (RT-PCR) in any of the patients (35). This was in contrast to a study in which SARS-CoV-2 was detected using the same testing method in 4 of 15 patients (26.7%) at an acute stage of infection (6–11 days since the onset of symptoms) and 2 of 23 patients (8.7%) recovering from COVID-19 (12–16 days after the onset of symptoms) (36). These results suggest that SARS-CoV-2 is present in the semen in the early stages of the infection.
Currently, there are no reports describing SARS-CoV-2 infecting the sperm, oocytes, or embryos, but investigations of the potential for SARS-CoV-2 to bind and fuse with their cell membranes have been published (35, 37, 38). Severe acute respiratory syndrome coronavirus 2 enters cells by binding its spike glycoprotein to the host cell’s zinc metallopeptidase angiotensin-converting enzyme 2 (ACE2) receptor and relying on the cellular transmembrane protease serine (TMPRSS) to cleave the viral spike glycoprotein, allowing the viral and host cell membranes to fuse (39, 40). The inhibition of TMPRSS2 can block SARS-CoV-2 entry; therefore, the ACE2 receptor alone might not be able to make a cell vulnerable to SARS-CoV-2 (39).
Another potential host receptor for viral entry, called CD147 or basigin (BSG), was recently identified (41). Both ACE2 and BSG receptors are present in the testis, uterus, ovaries, and placenta, with the testis having the second highest number of ACE2 receptors after the lungs (19, 37, 38). Low proteomic expression of ACE2 has been reported in non-human primate primary oocytes but was undetectable by immunohistochemistry in human oocytes (37, 38). Stanley et al. (37) analyzed the transcriptome of 18 samples of human cumulus cells and found that BSG and ACE2 were widely expressed in every sample, whereas there were very low levels of expression of TMPRSS2.
The ACE2 receptor was recently detected on the trophectoderm of human blastocysts, which is concerning because an analysis of a preimplantation embryo expression dataset has revealed that both ACE2 and TMPRSS are present in the blastocyst (42). Additionally, the presence of the BSG receptor has been confirmed by immunohistochemistry on both human primary oocytes and the trophectoderm of blastocysts (38). The presence of these receptors suggests that the oocyte and blastocyst have the ability to facilitate SARS-CoV-2 entry, particularly if the zona pellucida has been breached. However, there is no evidence suggesting the presence of SARS-CoV-2 receptors on the zona pellucida.
Specimen Handling
The professional fertility society guidelines for handing follicular fluid or semen are based on universal precautions for blood-borne pathogens, with an emphasis on avoiding the risk of aerosol formation (26, 27, 28). They all recommended the use of a biological safety cabinet class II with vertical airflow. Hickman et al. (15) cautioned that when working in a laminar flow hood with horizontal airflow, laboratory staff should use additional eye protection while isolating eggs and sperm.
PPE
Before the COVID-19 pandemic, not all ART laboratories required the staff to wear face masks and gloves while working in the laboratory. Such PPE was typically reserved for patient procedures and handling samples for genetic analysis (15). Likewise, eye protection was traditionally used only while working with cryopreserved specimens. The concept of PPE use in ART laboratories was discussed during an international COVID-19 symposium for laboratory managers (15). Everyone agreed that they would continue to use gloves and masks during procedures, and most but not all planned to extend their use for the entire time spent in the laboratory. A similar conclusion was drawn from laboratory societies in the United States, recommending that laboratories not requiring extended glove use be cognizant of the need for frequent hand-washing and perform more frequent surface cleaning (27). Hickman et al. (15) noted that laboratories would need to select permissive eye protection, given the impracticality of face shields during microscopy. The adoption of additional disinfection procedures and proper use of gloves, surgical masks, and eye protection for handling cryostorage devices were also identified as a means to reduce staff-to-cell transmission of SARS-CoV-2 (15, 22).
Mitigation of cell-cell transmission
There were no recommendations for additional steps to mitigate cell-cell transmission beyond those by the guidelines that have been previously established for reducing the risk of cross-contamination for cases of sexually transmitted diseases (13, 21, 43). Pomeroy and Schiewe (20) reviewed existing good tissue practices related to IVF, embryo biopsy, and vitrification during the COVID-19 pandemic. They noted that although a “soup of microorganisms” exists in laboratories, there have been no reports of cross-contamination or vertical transmission of a disease from gametes or embryos to a patient. Although the lack of reports of cross-contamination or vertical transmission is encouraging, it is important to recognize that patient testing for sexually transmitted diseases before bringing their gametes into an IVF laboratory or liquid nitrogen storage tank is one of the defenses routinely employed by ART clinics.
Patient COVID-19 Testing
Recommendations for the incorporation of COVID-19 tests into patient evaluation before procedures vary greatly, deferring to local or national guidelines, institutional policies, and availability of tests (23, 24, 25). Most guidelines recommend testing patients during their treatment. However, neither does a positive test result consistently warrant cycle cancelation for oocyte retrieval nor should a clinic assume that a negative test result received as late as the day before a procedure guarantees that the patient will be negative when they arrive for their procedure (27, 29).
One of the more aggressive testing plans was described in the guidelines recommended by the Indian Fertility Society and were based on regional policies (28). They recommended that patients be instructed to isolate socially for 2 weeks before stimulation, requiring male and female partners to be tested by RT-PCR as early as treatment day 2 and as late as 48 hours before intrauterine insemination or IVF. A positive COVID-19 test result for the patient or their partner results in cycle cancelation. Strict testing requirements were also recommended for andrology services in Brazil, proposing that SARS-CoV-2 testing by RT-PCR or blood antibody detection be performed for all men before any andrology services were provided (19). They recommended that only PCR-negative patients or those with immunoglobulin G antibodies should be treated or have their sperm cryopreserved. It is important to note that this recommendation was made at a time when Brazil was the epicenter of the COVID-19 pandemic, illustrating an example of adjustment to the patient triage plan based on regional infection rates.
Specimen Handling
Regardless of each ART center’s patient-testing policies and despite the lack of evidence that SARS-CoV-2 is present in the follicular fluid, embryologists have been advised to remove cumulative complexes from the follicular fluid as quickly as possible. This practice follows guidelines for reducing viral load by incorporating additional washing of the cumulus complexes and oocytes after cumulus digestion to dilute any contaminants (13, 16, 17, 18, 20, 21, 22, 24, 27).
Laboratories may want to double wrap or clean the outside of the specimen containers before handling semen (21, 22). Sperm isolation methods aimed to process and minimize viral contaminants in the semen of a patient who has tested positive should be used in all patients, and the centrifugation of raw semen should be avoided (20). To achieve this goal, the use of a density gradient was commonly prescribed (16, 21, 22).
Other considerations for specimen handling included the use of filtered micropipette tips and additional rinsing steps before cryopreservation or embryo transfer (16, 20, 21, 22). There is no evidence of SARS-CoV-2 infection due to waste; however, several groups have recommended that additional steps be taken to assure that containers housing seminal plasma, supernatants from semen centrifugation, and follicular fluid are well sealed before disposal (17, 21, 24).
Cryopreservation
There is a good chance, albeit no direct evidence, that SARS-CoV-2, like many viruses, can survive the low temperatures of liquid nitrogen and liquid nitrogen vapors. Despite the number of pathogens that linger in liquid nitrogen storage tanks, there has been no direct evidence of disease transmission from a transferred cryopreserved embryo in humans or animals (44). Cobo et al. (45) were unable to detect viral ribonucleic acid or deoxyribonucleic acid sequences in spent culture media after embryo culture or in liquid nitrogen used for oocyte or embryo vitrification in patients seropositive for human immunodeficiency virus, hepatitis C virus, or hepatitis B virus undergoing IVF cycles. The lack of evidence to support such a strong theoretical risk may be due to the relatively low viral load associated with the washed oocyte or embryo, especially when the viral load is further reduced by loading the oocyte or embryo onto a vitrification device in a very small volume. Although these previous studies are reassuring, the pathogens studied did not spread via aerosols. Risk assessment by Maggiulli et al. (18) ranked cryostorage as the most sensitive step of the entire IVF process during the COVID-19 pandemic. They determined that there is a high risk of failure if the staff fails to use proper PPE while entering the storage tank and unknowingly shedding SARS-CoV-2 into the liquid nitrogen.
Several groups have recommended using a closed, high-security system for semen cryopreservation (15, 16, 17, 19, 20, 21, 22). This was also the recommendation of some but not all groups for the vitrification of oocytes and embryos because many ART laboratories are accustomed to using an open system for oocyte and embryo vitrification. Laboratory managers that participated in the international COVID symposium agreed that the use of a closed system merited consideration, but at the time of the symposium, they did not reach a unanimous opinion because of a reported increase in the success rates associated with open systems (15). The use of a closed system for vitrification and avoiding a breach of the zona pellucida before vitrification were proposed as methods to mitigate the risk of SARS-CoV-2 infection of embryos and oocytes, with some restrictions to SARS-CoV-2-positive patients (20, 21, 24, 28). Additional proposed infection mitigation strategies for cryopreservation included the use of unique liquid nitrogen supplies for each patient during vitrification and warming, use of separate storage tanks for all patients or SARS-CoV-2-positive patients, employing vapor storage, and use of ultraviolet-sterilized liquid nitrogen.
In the absence of specific directives for changes to good tissue practices by regulatory agencies, specifically cryostorage, during the COVID-19 pandemic, the ART community has been left to conduct their own cost and risk analysis when considering adaptations in their cryopreservation programs (46). This is difficult when much of the risk is theoretical. Pomeroy and Schiewe (20) summarized the situation nicely when they said, “We must think hard about the worst-case scenario and then ask if we have the right to gamble with our patient’s future based on protocols for the best-case scenario?” The cryopreservation of gametes and embryos is a critical service in assisted reproduction. We need to ensure that we deliver the best care for our patients’ future family-building.
In conclusion, the COVID-19 pandemic has presented ART centers with a unique opportunity to assess every element of their practice. Many of the actions recommended for defense against SARS-CoV-2 infection in ART centers were made in the absence of scientific evidence but based on a general understanding of the disease’s transmission and theoretical risks. The use of additional PPE, increased disinfection, limiting person-to-person contact, and extended use of gloves and face coverings are logical ways to increase safety in our laboratories. Patient and partner testing, if available, may increase awareness among laboratory personnel and affect cryopreservation protocols. As the pandemic continues, our understanding of its behavior and knowledge about how to best contain its spread will grow and result in additional forthcoming recommendations. In the meantime, ART centers must be attentive to the need to maintain staff, supplies, and clean space to deliver safe and effective care while dodging the persistent and invisible SARS-CoV-2.
Footnotes
A.E.T.S. has nothing to disclose. J.D.K. has nothing to disclose.
Supplementary data
References
- 1.World Health Organization Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Available at:
- 2.Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 3.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China of novel coronavirus – infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J. A familiar cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–524. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Hogan C.A., Sahoo M.K., Huang C.H., Garamani N., Stevens B., Zehnder J. Five-minute point-of-care testing for SARS-CoV-2: not there yet. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Society for Reproductive Medicine Patient Management and clinical recommendations during the Coronavirus (COVID-19) pandemic. https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforce.pdf Available at:
- 11.Assisted reproduction and COVID-19 A statement from ESHRE for phase 1 – guidance on fertility services during pandemic. https://www.eshre.eu/Press-Room/ESHRE-News#COVID19P2 Available at:
- 12.Infection control in healthcare personnel: infrastructure and routine practices for occupational infection prevention and control services. Centers for Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/pdf/guidelines/infection-control-HCP-H.pdf Available at:
- 13.ASRM Practice Committee Recommendations for reducing the risk of viral transmission during fertility treatment with the use of autologous gametes: a committee opinion. Fertil Steril. 2013;99:340–346. doi: 10.1016/j.fertnstert.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Veiga A., Gianaroli L., Ory S., Horton M., Feinberg E., Penzias A. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Fertil Steril. 2020;114:484–485. doi: 10.1016/j.fertnstert.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman C., Rogers S., Huang G., MacArthur S., Meseguer M., Nogueira D., et al. Managing the IVF laboratory during a pandemic: international perspectives from laboratory managers. Reprod Biomed Online. 2020;41:141–150. doi: 10.1016/j.rbmo.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zivkovic S.V., Baricevic M., Cavlovic K., Cerina M., Cukusi Kalajzic A., Dundovic M., et al. Croatian Society of Clinical Embryologist – guidelines on the epidemiological framework for the implementation of medically assisted reproduction (MAR) procedures during the COVID-19 pandemic regarding the safety of patients and medical health workers. Mol Exp Biol Med. 2020;3:9–16. [Google Scholar]
- 17.Andrabi S.W., Jaffar M., Arora P.R. COVID-19: new adaptation for IVF laboratory protocols. JBRA Assist Reprod. 2020;3:358–361. doi: 10.5935/1518-0557.20200054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggiulli R., Giancani A., Fabozzi G., Dovere L., Tacconi L., Amendola M.G., et al. Assessment and management of the risk of SARS-CoV-2 infection in an IVF laboratory. Reprod Biomed Online. 2020;41:385–394. doi: 10.1016/j.rbmo.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallak J., Esteves S.C. Concise practice recommendations for the provision of andrological services and assisted reproductive technology for male infertility patients during the SARS-CoV-2 in Brazil. Int Braz J Urol. 2020;46:1082–1089. doi: 10.1590/S1677-5538.IBJU.2020.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomeroy K.O., Schiewe M.C. Cryopreservation and IVF in the time of Covid-19: what is the best good tissue practice (GTP)? J Assist Reprod Genet. 2020;37:2393–2398. doi: 10.1007/s10815-020-01904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choucair F., Younis N., Hourani A. IVF laboratory COVID-19 pandemic response plan: a roadmap. Middle East Fertil Soc J. 2020;25:1–7. doi: 10.1186/s43043-020-00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alaluf M.G., Pasqualini A., Fiszbajn G., Botti G., Estofan G., Ruhlmann C., et al. COVID-19 risk assessment and safety management operational guidelines for IVF center reopening. J Assist Reprod Genet. 2020;37:2669–2686. doi: 10.1007/s10815-020-01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Society for Reproductive Medicine Patient Management and clinical recommendations during the Coronavirus (COVID-19) pandemic. https://www.asrm.org/news-and-publications/covid-19/statements/patient-management-and-clinical-recommendations-during-the-coronavirus-covid-19-pandemic/ Available at:
- 24.ESHRE guidance on recommencing ART Treatments. ESHRE COVID-19 Working Group. https://www.eshre.eu/Press-Room/ESHRE-News#COVID19P2 Available at:
- 25.Fertility care during the COVID-19 pandemic: guiding principles to assist Canadian ART clinics to resume services and care. https://cfas.ca/CFAS_Communication_on_COVID-19.html Available at:
- 26.BFS & ARCS U.K. best practice guidelines for reintroduction of routine fertility treatments during the COVID-19 pandemic. https://www.britishfertilitysociety.org.uk/category/covid-19/ Available at:
- 27.Laboratory guidance for commencing or continuing ART operations during the ongoing COVID-19 pandemic. Guidance from the SART, SRBT and CRB. https://www.sart.org/globalassets/__sart/covid-19/labguidanceupdate1.pdf Available at:
- 28.Joint IFS-ISAR-ACE Recommendations on resuming/opening up ART services. https://www.indianfertilitysociety.org/joint-ifs-isar-ace-recommendations-on-resuming-opening-up-art-services/ Available at:
- 29.Papthanasiou A. COVID-19 screening during fertility treatment: how do guidelines compare against each other? J Assist Reprod Genet. 2020;37:1831–1835. doi: 10.1007/s10815-020-01885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cimolai N. Environmental and decontamination issues for human coronaviruses and their potential surrogates. J Med Virol. 2020;92:2498–2510. doi: 10.1002/jmv.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization Cleaning and disinfection of environmental surfaces in the context of COVID-19. https://www.who.int/publications/i/item/cleaning-and-disinfection-of-environmental-surfaces-inthe-context-of-covid-19 Available at:
- 32.Li R., Yin T., Fang F., Li Q., Chen J., Wang Y. Potential risks of SARS-CoV-2 infection on reproductive health. Reprod Biomed Online. 2020;41:89–95. doi: 10.1016/j.rbmo.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortimer D., Cohen J., Mortimer S.T., Fawzy M., Mcculloh D.H., Morbeck D.E., et al. Cairo consensus on the IVF laboratory environment and air quality: report of an expert meeting. Reprod Biomed Online. 2018;36:658–674. doi: 10.1016/j.rbmo.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Reza M.S., Hasan A.K., Afroze S., Bakar M.S.A., Taweekun J., Azad A.K. Analysis on preparation, application, and recycling of activated carbon to aid in COVID-19 protection. Int J Integrated Eng. 2020;12:233–244. [Google Scholar]
- 35.Pan F., Xiao X., Guo J., Song Y., Li H., Patel D.P. No evidence of severe acute respiratory syndrome – coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley K.E., Thomas E., Leaver M., Wells D. Coronovirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114:33–42. doi: 10.1016/j.fertnstert.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Essahib W., Verheyen F., Tournaye H., Van de Velde H. SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J Assist Reprod Genet. 2020;37:2657–2660. doi: 10.1007/s10815-020-01952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W., Yuan P., Yang M., Yan Z., Kong S., Yan J., et al. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal-fetal interface. Engineering. 2020;6:1162–1169. doi: 10.1016/j.eng.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fourie J.M., Loskutoff N., Huyser C. Semen decontamination for the elimination of seminal HIV-1. Reprod BioMed Online. 2015;30:296–302. doi: 10.1016/j.rbmo.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Bielanski A. A review of the risk of contamination of semen and embryos during cryopreservation and measures to limit cross-contamination during banking to prevent disease transmission in ET practices. Theriogenology. 2012;77:467–482. doi: 10.1016/j.theriogenology.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 45.Cobo A., Bellver J., de los Santos M.J., Remohí J. Viral screening of spent culture media and liquid nitrogen samples of oocytes and embryos from hepatitis B, hepatitis C, and human immunodeficiency virus chronically infected women undergoing in vitro fertilization cycles. Fertil Steril. 2012;97:74–78. doi: 10.1016/j.fertnstert.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Alteri A., Pisaturo V., Somigliana E., Vigano P. Cryopreservation in reproductive medicine during the COVID-19 pandemic: rethinking policies and European safety regulations. Hum Reprod. 2020;35:2650–2657. doi: 10.1093/humrep/deaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.