Abstract
Background
COVID-19 intensive care patients can present with neurological syndromes, usually in the absence of SARS-CoV-2 in cerebrospinal fluid (CSF). The recent finding of some virus-neutralizing antibodies cross-reacting with brain tissue suggests the possible involvement of specific autoimmunity.
Design
Blood and CSF samples from eleven critically ill COVID-19 patients presenting with unexplained neurological symptoms including myoclonus, oculomotor disturbance, delirium, dystonia and epileptic seizures, were analyzed for anti-neuronal and anti-glial autoantibodies.
Results
Using cell-based assays and indirect immunofluorescence on unfixed murine brain sections, all patients showed anti-neuronal autoantibodies in serum or CSF. Antigens included intracellular and neuronal surface proteins, such as Yo or NMDA receptor, but also various specific undetermined epitopes, reminiscent of the brain tissue binding observed with certain human monoclonal SARS-CoV-2 antibodies. These included vessel endothelium, astrocytic proteins and neuropil of basal ganglia, hippocampus or olfactory bulb.
Conclusion
The high frequency of autoantibodies targeting the brain in the absence of other explanations suggests a causal relationship to clinical symptoms, in particular to hyperexcitability (myoclonus, seizures). Several underlying autoantigens and their potential molecular mimicry with SARS-CoV-2 still await identification. However, autoantibodies may already now explain some aspects of multi-organ disease in COVID-19 and can guide immunotherapy in selected cases.
Keywords: COVID-19, CSF, Autoantibody, Neurology, Encephalitis, Autoimmunity, Viral-triggered
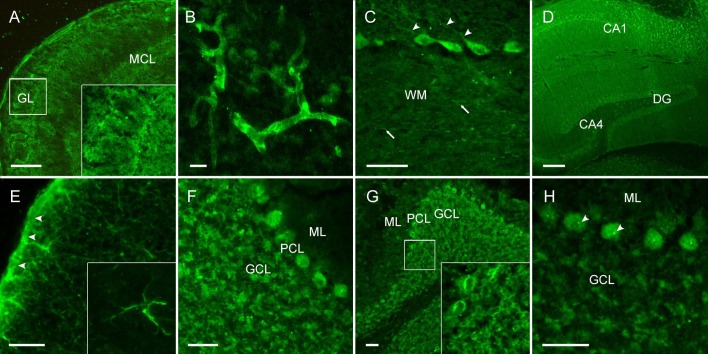
Abbreviations: CA1/4, cornu ammonis 1/4; DG, dentate gyrus; GCL, granule cell layer; GL, glomerular layer; MCL, mitral cell layer; ML, molecular layer; PCL, Purkinje cell layer; WM, white matter; Scale bars, 100 µm (A, E), 50 µm (B-C, F-H) and 250 µm (D)
1. Introduction
A broad variety of neurological symptoms has been observed in COVID-19 patients. Clinical findings comprise hyposmia and hypogeusia in mild cases and agitation, diffuse corticospinal tract signs and myoclonus (Helms et al., 2020, Liotta et al., 2020) in severe cases of COVID-19. Neurological syndromes in association with SARS-CoV-2 include many autoimmune diseases, such as Guillain-Barré syndrome (GBS), Miller-Fisher syndrome (MFS), polyneuritis cranialis, meningitis, encephalitis, stroke, epilepsy and myopathy (Helms et al., 2020, Paterson et al., 2020). SARS-CoV-2 has been detected only scarcely in cerebrospinal fluid (CSF) (Neumann et al., 2020) and there is accumulating evidence that CNS damage is not directly caused by the virus (Matschke et al., 2020). Thus, cellular or humoral autoimmunity might contribute to neurological symptoms, similar to other viral diseases. Potential mechanisms include molecular mimicry between viral proteins and neuronal autoantigens and delayed stimulation of post-viral autoimmunity similar to NMDA receptor encephalitis following herpes simplex virus encephalitis (HSE) (Armangue et al., 2018). We therefore examined the presence of a large panel of anti-neuronal and anti-glial autoantibodies in serum and CSF of COVID-19 patients with predominant neurological symptoms.
2. Methods
Between March and May 2020, during the major rise of SARS-CoV-2 infections in Germany, neurological assessment was performed on COVID-19 patients during intensive care unit (ICU) treatment in two tertiary care centers (Charité – Universitätsmedizin Berlin, Campus Virchow-Klinikum (CVK) and Campus Benjamin Franklin (CBF), and Universitätsklinikum Freiburg). In eleven patients with otherwise not explained neurological symptoms lumbar puncture was performed for autoantibody diagnostics in CSF and blood. Written informed consent for research and publication was obtained from all patients or their legal representative (ethics committee approval, Berlin: EA2/066/20, Freiburg: 153/20). Autoantibodies against intracellular and surface antigens relevant for CNS diseases were measured by line blots (EUROLINE, Euroimmun, Lübeck, Germany) for amphiphysin, CV2 (CRMP5), Hu, Ri, Yo, Ma2/Ta, Tr (DNER), by ELISA for GAD65, cardiolipin, beta2-glycoprotein and annexin (Labor Berlin GmbH, Berlin, Germany) and by cell-based assays for glutamate receptors (AMPAR1/2, NMDA), DPPX, GABAAR, GABABR, mGluR5, LGI1, Caspr2, dopamine-2 receptor, aquaporin-4 including indirect immunofluorescence tissue samples for myelin and skeletal muscle antibodies (BIOCHIP, Euroimmun, performed by Labor Berlin). In addition, indirect immunofluorescence on unfixed murine brain sections was performed to search for novel autoantibodies not included in the clinical routine assays, according to established protocols (Kreye et al., 2020). In brief, cryostat-cut unfixed 20-µm tissue sections were rinsed with PBS, blocked with PBS containing 2% Bovine Serum Albumin (Roth) and 5% Normal Goat Serum (Abcam) for 1 h at room temperature and incubated with CSF (1:1) or serum (1:400) overnight at 4 °C. After three PBS washing steps, goat anti-human IgG-Alexa Fluor 488 (Dianova) was applied for 2 h at room temperature before additional three washes and mounting using DAPI-containing Fluoroshield.
3. Results
3.1. Patient characteristics
After a median of 12 days [7–17 days] after onset of respiratory symptoms and a median of 4 days [2–8 days] after admission to ICU, 11 patients (median age 67 [54–78 years], 8 male) presented with a broad spectrum of neurological symptoms, involving down-beat nystagmus (n = 2), other oculomotor disturbances (n = 2), aphasia (n = 1), hyper- and hypoactive delirium (n = 5), partial, mainly orofacial myoclonus (n = 6), generalized stimulus-sensitive myoclonus, which improved by sedation and symptomatic treatment (n = 1), dystonia of the upper extremities (n = 1), stroke (n = 1) and epileptic seizures (n = 1). Causes for symptoms secondary to ICU treatment (e.g. hypoxia, iatrogenic induced, etc.) or explained by internal disease (e.g. sepsis, electrolyte abnormalities, etc.) were ruled out. During the observation period a total of 189 patients with COVID-19 received ICU treatment at the Charité. Patients were primarily treated by the ICU staff and neurological examinations were performed as a consultation service. Thus, only a selection of approximately 30 patients received thorough neurological work-up with CSF analysis in 11 of them. A prevalence rate of neurological manifestations in ICU-treated Covid-19 patients cannot be drawn from our cohort.
3.2. Laboratory findings
CSF and blood samples were analyzed in all patients (Table 1 ). SARS-CoV-2 PCR in CSF was negative in all patients. Mild pleocytosis was found in 3/11 patients and elevated in one with a positive varicella virus PCR. CSF protein was elevated in 4/11 and matched oligoclonal bands (OCB) present in 6/9 patients.
Table 1.
Patient characteristics and laboratory findings.
| Patient N° | Age | Sex | Neurological symptoms | SARS-CoV-2 PCR |
CSF |
OCB |
Autoantibody panela |
CSF indirect Immuno-fluorescence (IgG) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swab | CSF | Cell count [<5/µl] | Glucose [mg/dl] | Lactate [<22 mg/dl] | Protein [<450 mg/l] | NfL [pg/ml] | Serum | CSF | Serum | CSF | |||||
| #1 | 69 | m | Downbeat nystagmus, orofacial myoclonus, delirium | pos. | neg. | 8 | 123 | 21 | 594 | >10,000 | pos. | pos. | NMDAR IgG 1:1000 | neg. | Medium-sized blood vessels |
| #2 | 76 | m | Downbeat nystagmus, generalized stimulus-sensitive myoclonus | pos. | neg. | 1 | 76 | 18,9 | 314 | 5,062 | pos. | pos. | Cardiolipin, beta2 glycoprotein | neg. | Densely clustered pan-neuronal binding |
| #3 | 67 | m | Oculomotor disturbance | pos. | neg. | 2 | 113 | 22 | 336 | 1,049 | pos. | pos. | neg. | neg. | Cerebellum granule cells, brainstem neuropil |
| #4 | 58 | f | Delirium | pos. | neg. | 5 | 93 | 27,4 | 368 | 1,535 | pos. | pos. | Myelin 1:100 | Neg. | Perinuclear binding |
| #5 | 76 | m | Right-sided stimulus-sensitive myoclonus | pos. | neg. | 177 | 83 | 43,8 | 937 | 9,491 | pos. | pos. | neg. | neg. | Cerebellum granule cells, nucleoli of Purkinje neurons |
| #6 | 58 | f | Right-sided orofacial myoclonus | pos. | neg. | 17 | 52 | 16,6 | 281 | 2,215 | neg. | neg. | Yo | Yo | Nuclear binding |
| #7 | 54 | m | Delirium, myoclonus, epileptic seizures | pos. | neg. | 1 | 75 | 28,1 | 256 | n.d. | n.d. | n.d. | Myelin 1:100 | neg. | Proximal dendrites of Purkinje cells, myelin |
| #8 | 77 | m | Right-sided faciobrachial myoclonus, multifocal strokes | pos. | neg. | 1 | 145 | 32,3 | 682 | >10,000 | pos. | pos. | Cardiolipin | n.d. | Blood vessels, glia limitans, astrocytes |
| #9 | 48 | m | Oculomotor paresis, transient generalized myoclonus, prolonged awakening | pos. | neg. | 1 | 80 | 16,9 | 741 | n.d. | neg. | neg. | Annexin, cardiolipin | n.d. | Cerebellum granule cells |
| #10 | 78 | m | Dystonia right > left upper limb, delirium | pos. | neg. | 1 | 94 | 26,1 | 437,8 | n.d. | n.d. | n.d. | neg. | neg. | Neuropil olfactory bulb, cerebellum hippocampus |
| #11 | 75 | f | Aphasia, neglect, encephalopathy, stupor, impaired consciousness | pos. | neg. | 1 | n.d. | 27 | 168 | n.d. | neg. | neg. | neg. | neg. | Blood vessels, astrocytes, glia limitans |
aIncludes antibodies against amphiphysin, CV2 (CRMP5), GAD65, Hu, Ri, Yo, Ma2/Ta, Tr (DNER), GAD65, glutamate receptors (AMPAR1/2, NMDA), DPPX, GABAAR, GABABR, mGluR5, LGI1, myelin, Caspr2, dopamine-2 receptor, aquaporin-4, skeletal muscle and phospholipids (cardiolipin, beta2-glycoprotein, annexin), Abbreviations: CSF, cerebrospinal fluid; f, female; m, male; n.d., not determined; neg, negative; OCB, oligoclonal bands; pos, positive (>3 bands).
Using routine diagnostics, one patient showed Yo antibodies in serum and CSF and two patients myelin antibodies in serum. One patient had high-level serum IgG NMDA receptor antibodies. Neurofilament light chain (NfL) levels in CSF were increased in all tested patients (7/7). Testing for serum antibodies of SARS-CoV-2 was not generally available in the first weeks of the pandemic in Germany. Archived CSF samples, available from four patients of this cohort were analyzed for the detection of anti-SARS-CoV-2 antibodies (SARS-CoV-2-S1 ELISA, Euroimmun, Germany). One of four specimen contained high-level IgA and IgG SARS-CoV-2 antibodies.
3.3. Screening assay for novel CSF autoantibodies
CSF analysis for the presence of anti-neuronal autoantibodies not included in commercial routine assays using indirect immunofluorescence on unfixed mouse brain sections reproducibly showed strong IgG binding in most patients. IgG staining patterns included vessel endothelium, perinuclear antigens, astrocytic proteins and neuropil of basal ganglia, hippocampus or olfactory bulb (Fig. 1 ). Although antigenic epitopes are currently unknown, the intense staining indicates high specificity to certain neuronal, astrocytic and vascular proteins and is reminiscent of the brain tissue binding recently observed with certain human monoclonal SARS-CoV-2 antibodies (Kreye et al., 2020).
Fig. 1.
CSF of COVID-19 patients shows strong IgG autoreactivity on unfixed mouse brain sections. Representative images of indirect immunofluorescence demonstrate autoantibody binding to circumscribed anatomical structures including (A) neuropil of the olfactory bulb, (B) medium-sized vessels in the brain, (C) proximal dendrites of Purkinje neurons (arrowheads) and myelinated fibers (arrows) in the cerebellum, (D) neuropil in the hippocampus, (E) glia limitans (arrowheads) and astrocytes (enlarged box) throughout the brain. Several autoantibodies target intracellular antigens, such as (F) densely clustered intraneuronal epitopes, (G) perinuclear antigens or (H) nucleoli (arrowheads) as part of an anti-nuclear antibody response.
3.4. Neuroimaging
All patients received CT scans of the brain; additionally, in four patients an MRI scan of the brain was performed. Neuroimaging of one patient (#8) detected an ischemic lesion of the right middle cerebral artery (MCA) region. One patient (#7) showed marked edema of the fornix. One patient (#6) additionally received a PET-CT, which showed evidence for florid encephalitis with tracer increase in the basal ganglia and limbic system as well as in the cerebellar region of the inferior cerebellar artery. All other neuroimaging findings did not present any abnormalities.
4. Discussion
We report autoantibody findings in eleven critically ill COVID-19 patients presenting with a variety of neurological symptoms with unexplained etiology. The high frequency of CSF anti-neuronal and anti-glial autoantibodies is remarkable, as is the confinement to specific immunofluorescence patterns (Fig. 1). Although more than one patient each had IgG autoantibodies targeting neuropil, astrocytes or medium-sized blood vessels, it will require larger patient cohorts for linking a given autoantibody pattern to clinical symptoms. Similar findings of as yet undetermined anti-neuronal autoantibodies in COVID-19 patients are increasingly observed, including specific IgG binding to fibre tracts on rat brain slices (Delamarre et al., 2020). The responsiveness to immunotherapy in these patients suggests that these novel autoantibodies might participate in the disease cascade.
Interestingly, the binding pattern seen with indirect immunofluorescence of patients’ CSF on mouse brain sections resembled the tissue distribution we recently detected with some monoclonal human SARS-CoV-2 antibodies (Kreye et al., 2020). While these antibodies could strongly neutralize the spread of authentic virus and largely prevented lung disease in hamsters, a fraction of them cross-reacted with self-epitopes, often in the brain. Further experimental work is needed to clarify whether these cross-reactive autoantibodies can confer human disease, however, the present patient series strongly supports this hypothesis. In particular, the neuropil pattern in some patients suggests binding to surface receptors or ion channels and thus pathogenicity (Fig. 1A, D), similar to the rapidly growing group of antibody-mediated encephalitides (Dalmau and Graus, 2018). Likewise, the astrocyte pattern in two patients (Fig. 1E) is reminiscent of the relatively common form of GFAP antibody encephalitis (Fang et al., 2016), even though GFAP was excluded as the target antigen. The high prevalence of autoantibodies may result from a virus-induced inflammation in response to the infection with SARS-CoV-2 in analogy to the high prevalence of NMDAR autoantibodies after brain infection with herpes viruses (Armangue et al., 2018).
Due to the retrospective nature of this study, our findings could not guide treating physicians to initiate immunomodulatory therapy. Clinical improvement of COVID-19 patients with GBS has been reported after therapy with intravenous immunoglobulins (ivIG) and after steroids and plasma exchange in COVID-19 patients with encephalitis (Paterson et al., 2020), indicating that immunotherapy should be considered in future cases of CSF autoantibody-positive COVID-19 patients.
In most patients, increased CSF protein, lactate or white blood cells with negative SARS-CoV-2 PCR indicated inflammatory changes compatible with autoimmune encephalitis. NfL was markedly elevated (>5000 pg/ml) in the CSF of four COVID-19 patients and might reflect direct tissue destruction from viral replication or from inflammatory damage as already demonstrated (Eden et al., 2020). Whether this is a transient elevation or a continuous transformation into a degenerative phenotype in some patients is yet to be determined (Heneka et al., 2020) and could be dependent on a persisting stimulus, such as autoantibodies. Evaluations at different timepoints in patients recovered from COVID-19 are required to shed light on that matter.
CSF was not routinely taken in COVID-19 patients, but only if there were symptoms pointing to nervous system complications. Therefore, a control group consisting of COVID-19 patients without neurological symptoms was not available. In our experience with routine and research diagnostics using indirect immunofluorescence in pre-selected ICU cohorts, CSF anti-neuronal antibody binding to unfixed murine brain sections is present in less than 25% of cases, indicating that the here determined high frequency of anti-neuronal antibodies is related to the current infection with SARS-CoV-2.
Support for autoimmune mechanisms in “Neuro-Covid” also comes from a recent post-mortem case series reporting neuropathological changes predominantly in the brainstem and cerebellum, compatible with autoimmune encephalitides (Matschke et al., 2020). Although SARS-CoV-2 was detected in almost half of the examined brains, the severity of CNS damage did not correlate with the presence of virus, thus arguing against direct virus-induced damage (Matschke et al., 2020).
Post-viral humoral autoimmunity is an emerging concept best studied for NMDA receptor encephalitis developing in almost 30% of cases post HSE. Tissue destruction may lead to the release of brain-restricted ‘neo-antigens’ such as NMDA receptors, and viral material might provide costimulatory signals to antibody-producing cells (Suurmond and Diamond, 2015), leading to antibody induction even without mechanisms of molecular mimicry. Recent findings suggest that several viral infections can lead to secondary autoimmune encephalitis, including EBV, HHV-6, enterovirus, adenovirus, hepatitis C or HIV infections (Prüss, 2017). Thus, the present work indicates that SARS-CoV-2 is no exception to this general principle. One of four CSF specimen contained high-level IgA and IgG SARS-CoV-2 antibodies, indicating that these antibodies can reach the brain.
A limitation of our study is the absence of matched COVID-19 patients without neurological symptoms because of the lack of CSF. Due to logistic reasons we assessed only a small number of patients within the observation period, therefore prevalence of neurological manifestations in ICU treated COVID-19 patients cannot be derived. Some of the detected antibodies were located intracellularly (Fig. 1F-H), indicating that the humoral immune response is secondary to other immune mechanisms, including CNS damage from cytotoxic T cells and innate autoimmunity. In these cases, autoantibodies may be biomarkers of the activated immune system rather than direct drivers of the disease. Likewise, the role of myelin antibodies, annexin antibodies and serum-only NMDAR antibodies as well as their potential pathogenicity is currently unclear.
The following months will require intensive work to determine the here described neuronal surface autoantigens. This will help to judge whether CSF autoantibodies in COVID-19 are pathogenic or not, whether some of them are self-reactive virus-neutralizing antibodies and whether such autoreactivity can cause persisting neurological morbidity even after clearance of SARS-CoV-2 and remission of COVID-19. The findings will then clarify whether virus-induced “Neuro-Covid” or neurological “Long-Covid” is a modern equivalent of the unexplained severe ‘encephalitis lethargica’ – commonly with postencephalitic parkinsonism – in more than a million patients of the influenza pandemic in 1918 (Hoffman and Vilensky, 2017).
5. Conclusions
The high frequency of autoantibodies targeting the brain in the absence of other explanations suggests a causal association with clinical symptoms, in particular with hyperexcitability (myoclonus, seizures). Several underlying autoantigens and their potential molecular mimicry with SARS-CoV-2 still await identification. However, the presence of autoantibodies may already now explain some aspects of multi-organ disease in COVID-19 and can guide immunotherapy in selected cases.
References
- Armangue T., Spatola M., Vlagea A., Mattozzi S., Carceles-Cordon M., Martinez-Heras E., Llufriu S., Muchart J., Erro M.E., Abraira L., Moris G., Monros-Gimenez L., Corral-Corral I., Montejo C., Toledo M., Bataller L., Secondi G., Arino H., Martinez-Hernandez E., Juan M., Marcos M.A., Alsina L., Saiz A., Rosenfeld M.R., Graus F., Dalmau J., Spanish Herpes Simplex Encephalitis Study G. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropper A.H., Dalmau J., Graus F. Antibody-mediated encephalitis. N. Engl. J. Med. 2018;378(9):840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- Delamarre L., Gollion C., Grouteau G., Rousset D., Jimena G., Roustan J., Gaussiat F., Aldigé E., Gaffard C., Duplantier J., Martin C., Fourcade O., Bost C., Fortenfant F., Delobel P., Martin-Blondel G., Pariente J., Bonneville F., Geeraerts T. COVID-19–associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J. Neurol. Neurosurg. Psychiatry. 2020;91(9):1004–1006. doi: 10.1136/jnnp-2020-323678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.M., Lindh M., Price R.W., Zetterberg H., Gisslen M. CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology. 2020 doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- Fang B., McKeon A., Hinson S.R., Kryzer T.J., Pittock S.J., Aksamit A.J., Lennon V.A. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alz. Res. Therapy. 2020;12(1) doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, L.A., Vilensky, J.A., 2017. Encephalitis lethargica: 100 years after the epidemic. Brain 140, 2246–2251. [DOI] [PubMed]
- Kreye, J., Reincke, S.M., Kornau, H.C., Sánchez-Sendin, E., Corman, V.M., Liu, H., Yuan, M., Wu, N.C., Zhu, X., Lee, C.D., Trimpert, J., Höltje, M., Dietert, K., Stöffler, L., von Wardenburg, N., van Hoof, S., Homeyer, M.A., Hoffmann, J., Abdelgawad, A., Gruber, A.D., Bertzbach, L.D., Vladimirova, D., Li, L.Y., Barthel, P.C., Skriner, K., Hocke, A.C., Hippenstiel, S., Witzenrath, M., Suttorp, N., Kurth, F., Franke, C., Endres, M., Schmitz, D., Jeworowski, L.M., Richter, A., Schmidt, M.L., Schwarz, T., Müller, M.A., Drosten, C., Wendisch, D., Sander, L.E., Osterrieder, N., Wilson, I.A., Prüss H. 2020 Nov 12. A Therapeutic Non-self-reactive SARS-CoV-2 Antibody Protects from Lung Pathology in a COVID-19 Hamster Model. Cell. 183 (4), 1058–1069.e19. doi: 10.1016/j.cell.2020.09.049. Epub 2020 Sep 23. PMID: 33058755; PMCID: PMC7510528. [DOI] [PMC free article] [PubMed]
- Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., Koralnik I.J. Frequent neurologic manifestations and encephalopathy‐associated morbidity in Covid‐19 patients. Ann. Clin. Transl. Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, B., Schmidbauer, M.L., Dimitriadis, K., Otto, S., Knier, B., Niesen, W.D., Hosp, J.A., Gunther, A., Lindemann, S., Nagy, G., Steinberg, T., Linker, R.A., Hemmer, B., Bosel, J., Pandemic, the, I.S.G., 2020. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci 418, 117090. [DOI] [PMC free article] [PubMed]
- Paterson, R.W., Brown, R.L., Benjamin, L., Nortley, R., Wiethoff, S., Bharucha, T., Jayaseelan, D.L., Kumar, G., Raftopoulos, R.E., Zambreanu, L., Vivekanandam, V., Khoo, A., Geraldes, R., Chinthapalli, K., Boyd, E., Tuzlali, H., Price, G., Christofi, G., Morrow, J., McNamara, P., McLoughlin, B., Lim, S.T., Mehta, P.R., Levee, V., Keddie, S., Yong, W., Trip, S.A., Foulkes, A.J.M., Hotton, G., Miller, T.D., Everitt, A.D., Carswell, C., Davies, N.W.S., Yoong, M., Attwell, D., Sreedharan, J., Silber, E., Schott, J.M., Chandratheva, A., Perry, R.J., Simister, R., Checkley, A., Longley, N., Farmer, S.F., Carletti, F., Houlihan, C., Thom, M., Lunn, M.P., Spillane, J., Howard, R., Vincent, A., Werring, D.J., Hoskote, C., Jager, H.R., Manji, H., Zandi, M.S., Neurology, U.C.L.Q.S.N.H.f., Neurosurgery, C.-S.G., 2020. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. [DOI] [PMC free article] [PubMed]
- Prüss H. Postviral autoimmune encephalitis: manifestations in children and adults. Curr. Opin. Neurol. 2017;30(3):327–333. doi: 10.1097/WCO.0000000000000445. [DOI] [PubMed] [Google Scholar]
- Suurmond J., Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 2015;125(6):2194–2202. doi: 10.1172/JCI78084. [DOI] [PMC free article] [PubMed] [Google Scholar]