Abstract
Giant cell arteritis, a common primary systemic vasculitis affecting older people, presents acutely as a medical emergency and requires rapid specialist assessment and treatment to prevent irreversible vision loss. Disruption of the health-care system caused by the COVID-19 pandemic exposed weak points in clinical pathways for diagnosis and treatment of giant cell arteritis, but has also permitted innovative solutions. The essential roles played by all professionals, including general practitioners and surgeons, in treating these patients have become evident. Patients must also be involved in the reshaping of clinical services. As an international group of authors involved in the care of patients with giant cell arteritis, we reflect in this Viewpoint on rapid service adaptations during the first peak of COVID-19, evaluate challenges, and consider implications for the future.
Introduction
The COVID-19 pandemic has had a considerable effect on all health-care services.1 At the same time, the pandemic accelerated the pace of innovation, as systemic barriers to change were lowered and new approaches were required.2 In this Viewpoint, we focus on care for patients with giant cell arteritis. This condition is one of the few medical emergencies in rheumatology because of its potential to cause irreversible vision loss. Patients with suspected giant cell arteritis need rapid assessment by a specialist and investigation with temporal artery biopsy or vascular imaging (or both). As well as immediate high-dose glucocorticoid treatment, patients need subsequent long-term specialist care, including glucocorticoid dose tapering, co-prescription of other medications, and monitoring for relapse that might require treatment escalation.3, 4 We discuss the effects of the COVID-19 pandemic on patients with this condition and reflect on implications for future clinical pathway development.
Prioritisation of emergencies
During March and April, 2020, many medical centres in cities affected by COVID-19 had to pivot to deal with the immediate crisis,5 which inevitably meant diverting resources away from routine care. There was widespread cancellation of elective surgeries and routine clinic appointments. Many hospital clinicians were either redeployed to acute care or had to self-isolate, and in the UK, general practitioners were directed to switch to telemedicine.6 Acute giant cell arteritis is a medical emergency, and so clinical pathways for suspected giant cell arteritis had to continue to function throughout this time, within the constraints of COVID-19-related restrictions. The extent of these restrictions varied greatly by location; the availability of temporal artery biopsy was particularly affected in locations with barriers to scheduling emergency surgery (table 1 ).
Table 1.
The effect of the first peak of the COVID-19 pandemic on rheumatology and auxiliary services for the management of giant cell arteritis by location
| Redeployment of personnel | Biopsy access | Ultrasound access | PET, CT, or MRI access | Clinic access | Medication access | |
|---|---|---|---|---|---|---|
| Queensland, Australia | Junior staff to COVID-19 wards | Restricted | Not used routinely before the pandemic | Restricted, case by case basis with direct discussion required | No changes in clinic volume | No change to medication access, including IL-6 inhibition |
| Dublin, Ireland | Junior staff and nurses to COVID-19 wards | Restricted, only inpatients | Not available | Restricted, only inpatients | Temporary (weeks) complete transition to telehealth | No change to medication access |
| Chicago, IL, USA | Junior staff to COVID-19 wards | Restricted | Only for emergency use | Only for emergency use | Temporary (weeks) complete transition to telehealth | No change to medication access |
| Groningen, Netherlands | Minimal loss of junior staff to COVID-19 ICU duties | Available, must have a negative swab result for SARS-CoV-2 before test | Available | Available | New urgent patients seen in person; routine follow-ups by telehealth | No change to medication access |
| Birmingham, UK | Senior and junior staff deployed to ICU and medical wards | Restricted | Only for emergency use | Restricted | Emergency patients seen in person, urgent patients by telehealth; all routine appointments cancelled | No change to medication access |
| London, UK | Most senior and junior staff deployed to COVID-19 and medical wards | Restricted, must have a negative swab result for SARS-CoV-2 before test | Not available | Available, must have a negative swab result for SARS-CoV-2 before test | Initially ambulatory care, then by telehealth | No change to medication access |
| New York, NY, USA | Most senior and all junior staff redeployed to general medicine or COVID-19 wards | Restricted | Not used routinely before the pandemic | Only for emergency use | Complete transition to telehealth for months | No change to medication access, but limitation on intravenous treatments |
| Leeds, UK | Most junior and some senior staff deployed to medical ward duties | Not available | Usual sonographers unavailable, done by rheumatologists with ultrasound training | Not available | Telephone and in person appointments for urgent and emergency cases; routine follow-ups cancelled | No change to medication access |
ICU=intensive care unit. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Presentation, recognition, referral, and diagnosis of giant cell arteritis
With a 57% decline in attendances to UK emergency departments during April, 2020,7 concern was expressed by physician and patient groups about delayed presentation of patients with non-COVID medical emergencies. Giant cell arteritis most commonly presents within the community, a context in which the pandemic imposed new constraints on physicians’ capability to assess diagnostic probability, including restrictions on the ability to do urgent blood tests or physical examinations. A marked decline in referral rates of patients with giant cell arteritis, accompanied by several cases with delayed presentation and vision loss, was reported from severely affected regions, such as Lombardy, Italy.8 In contrast, hospitals that were not overwhelmed with patients with COVID-19 reported that referrals of patients with giant cell arteritis continued or even increased, suggesting the possibility that changes in presentation were due to medical care or redirection of referrals, rather than to alterations in the true incidence of the disease in the community.9 Our own experiences also revealed diversity in the degree of disruption to referrals. As in Lombardy, referral rates of patients with suspected giant cell arteritis in Leeds, UK, initially declined during the period of highest admissions of patients with COVID-19, followed by a later rebound in referrals, including many delayed presentations. In contrast, assessment and treatment of giant cell arteritis continued largely unchanged in Queensland, Australia, where no substantial peak in hospitalised patients with COVID-19 occurred (figure 1 ).
Figure 1.
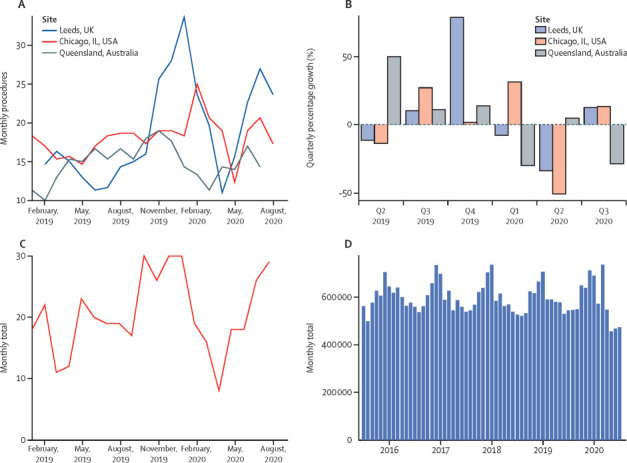
Routinely collected clinical data on health-care use for giant cell arteritis
(A) Total number (n=1030) of monthly temporal artery ultrasounds and temporal artery biopsies in 2019 and 2020 in Northwestern University, Chicago, IL, USA; Leeds, UK; and state pathology departments in Queensland, Australia. Values are expressed as 3 month rolling means. (B) Quarterly growth in temporal artery biopsies and ultrasounds. (C) New initiations of tocilizumab (n=411) for giant cell arteritis from data from National Health Service England, UK. (D) Oral prednisolone primary care prescriptions (n=36.3 million) that were dispensed by pharmacies in England, UK. Publicly available data collected from the NHS Business Service Authority downloaded in 2020 from OpenPrescribing.net, EBM DataLab, University of Oxford. These data cover prednisolone prescribing for all indications and illustrate general trends in prescribing of this medication.
During the COVID-19 pandemic, physicians faced a new diagnostic challenge in that COVID-19 can present with acute-onset headache and systemic upset, including fever, fatigue, myalgia, or elevation of inflammatory markers—potentially mimicking the presentation of giant cell arteritis (panel 1 ). This symptomatic overlap is an issue because of the need for immediate assessment and treatment of suspected giant cell arteritis, requiring fast-track referral routes. Atypical presentations of COVID-19 are particularly common in older adults,10 and a few simple screening questions might not identify all patients with COVID-19. This new diagnostic dilemma introduces further uncertainty, raising the stakes for clinical decisions.
Panel 1. Cases of patients* with giant cell arteritis during the COVID-19 pandemic.
A 76-year-old retired health-care professional developed a new headache during the COVID-19 pandemic in the UK. In the preceding months, she had noticed increasing fatigue and early morning stiffness. She had wondered if she had giant cell arteritis, but thought a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was probable as she had no visual symptoms or jaw claudication. She felt that during lockdown she should not seek help unless absolutely necessary. Over 2 months, her headache increased in frequency and severity until it became constant and unbearable. She attended her primary care doctor who suspected giant cell arteritis, requested an ESR (the result was 90 mm/h), and commenced prednisolone (40 mg daily). 2 days later, she had two episodes of transient vision loss and attended eye casualty. An incidental branch retinal vein occlusion was documented. She was sent to the emergency department, where persistent left temporal tenderness was noted, and the physician felt this condition was not giant cell arteritis. The next day she was seen by a rheumatologist and had a temporal artery ultrasound, which was negative. The following day she was seen by a neuro-ophthalmologist who escalated the prednisolone treatment to 60 mg daily and did a temporal artery biopsy, which was positive for giant cell arteritis. Since then she has remained well on a tapering course of prednisolone.
A 62-year-old woman showed symptoms of headache, jaw claudication, scalp tenderness, and elevated inflammatory markers during the COVID-19 pandemic in Chicago, IL, USA. She was started on prednisone (60 mg daily) by her primary care physician but could not be seen face-to-face at the academic medical centre in Chicago due to COVID-19 restrictions. The specialist centre recommended tocilizumab and a 26-week prednisolone taper. The patient began treatment but wanted to confirm the diagnosis. A temporal artery ultrasound at a local clinic was negative. A temporal artery biopsy, which was delayed for 5 days until nasopharyngeal swab could rule out COVID-19, was positive for giant cell arteritis, 11 days into high-dose prednisone therapy.
A 69-year-old man showed symptoms of dry cough, fatigue, right temporal and occipital headache, scalp tenderness, anorexia, and weight loss during the COVID-19 pandemic in Ireland. The symptoms had developed when he was in New York City, NY, USA, and he initially thought he had COVID-19. On his return, he attended his primary care physician, who suspected giant cell arteritis and referred him to rheumatology. Both temporal arteries were swollen and tender, serum concentration of C-reactive protein was 60 mg/L, and ESR was 65 mm/h. He was diagnosed with giant cell arteritis and treated with prednisolone (60 mg daily) with rapid improvement. A temporal artery ultrasound and temporal artery biopsy were requested but declined due to the COVID-19 restrictions. He was subsequently treated with tocilizumab and a 26-week prednisolone taper.
*Written patient consent was obtained in all cases.
Table 2 outlines one potential approach to considering various diagnostic permutations when evaluating patients presenting with ambiguous symptoms that could reflect either giant cell arteritis or COVID-19. Table 2 was originally written as a guide for clinicians assessing patients with suspected giant cell arteritis in Leeds, UK, and further refined based on the experience of all authors. As well as the relative likelihood of each condition, the potential consequences of diagnostic error need to be considered. The relative frequency of symptoms in both diseases should be considered; for example, dry cough is an uncommon manifestation of giant cell arteritis, and shortness of breath would be a very unusual symptom of this condition alone. If there are features compatible with both diseases, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing might be useful as early as possible in the diagnostic pathway. The possibility of false negative tests both for giant cell arteritis and for SARS-CoV-2 must also be considered.
Table 2.
Possible diagnostic permutations in giant cell arteritis during the COVID-19 pandemic arising from diagnostic ambiguity between the two diseases
| First clinical suspicion | Does the patient have active giant cell arteritis? | Does the patient have COVID-19? | Possible treatment approach | |
|---|---|---|---|---|
| New giant cell arteritis | Giant cell arteritis | Yes | No | Treat with high-dose glucocorticoid therapy to prevent vision loss; close follow-up to identify adverse effects; take measures to minimise risk of patient acquiring SARS-CoV-2 during health-care contacts |
| COVID-19 misdiagnosed as giant cell arteritis | Giant cell arteritis | No | Yes | Avoid inappropriate glucocorticoid therapy; monitor for clinical deterioration; take measures to minimise risk of transmission of SARS-CoV-2 to other patients or staff |
| New giant cell arteritis and concomitant COVID-19 | Giant cell arteritis | Yes | Yes | Treat with high-dose glucocorticoid therapy to prevent vision loss; close follow-up to identify adverse effects; take measures to minimise risk of transmission of SARS-CoV-2 to other patients or staff |
| New giant cell arteritis misdiagnosed as COVID-19 | COVID-19 | Yes | No | Treat with high-dose glucocorticoid therapy to prevent vision loss; close follow-up to identify adverse effects; take measures to minimise risk of patient acquiring SARS-CoV-2 during health-care contacts |
| Giant cell arteritis relapse | Giant cell arteritis relapse | Yes | No | Escalate giant cell arteritis therapy, including adjuvant immunosuppressant, if appropriate; take measures to minimise risk of patient acquiring SARS-CoV-2 during health-care contacts |
| COVID-19 in a patient with prior diagnosis of giant cell arteritis | Giant cell arteritis relapse | No | Yes | Standard care for COVID-19; if already taking long-term, low-dose glucocorticoids for giant cell arteritis, consider short-term increase in dose to avert potential adrenal crisis, in line with recommendations for adrenal insufficiency11 |
| Giant cell arteritis relapse with concomitant COVID-19 | Giant cell arteritis relapse | Yes | Yes | Standard care for COVID-19; escalate giant cell arteritis therapy including adjuvant immunosuppressant if appropriate; take measures to minimise risk of transmission of SARS-CoV-2 to other patients or staff |
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
It is important to note that table 2 is intended as an aid to clinical reasoning and does not necessarily imply treatment recommendations. This table can also be used as a framework for risk assessments when restructuring giant cell arteritis diagnostic services in response to the pandemic, and potentially to create a checklist that could be used to assist clinical decision making and communication between members of the giant cell arteritis diagnostic team, with potential secondary uses in audit and evaluation of the diagnostic service.12 Such considerations might inform individual treatment decisions and risk assessments in relation to service adaptations for giant cell arteritis. Temporal artery ultrasound, often used as a first-line investigation for this condition, requires prolonged, close contact with a sonographer, using equipment that is difficult to clean thoroughly. However, these risks must be weighed against the rapid, non-invasive nature of ultrasound and its high clinical utility as a confirmatory test.3 Risk mitigation strategies might need to incorporate SARS-CoV-2 testing for some or all patients with suspected giant cell arteritis, depending on local test availability and turnaround times.
Geographical variations in access to temporal artery biopsy during the first peak of the pandemic were observed, broadly correlating with the extent to which locations were affected (table 1). In severely affected locations, such as New York, NY, USA, cancellation of all elective surgery was justified by the redeployment of surgeons, anaesthetists, and other key staff to acute services, the unavailability of operating rooms and ventilators, and other structural factors. By contrast, emergency surgery was permitted. Urgent diagnostic surgical procedures, such as temporal artery biopsy, which do not require an anaesthetist or a ventilator, are part of the services that were still permitted in many severely affected locations. In areas where access to temporal biopsies was affected by COVID-19, the level of availability (ie, completely unavailable or only available on a case-by-case basis) and duration of reduced availability varied by location (table 1).
We also found that reopening surgical services required arriving at a shared understanding of the role of temporal artery biopsies among everybody involved in making decisions on how to reopen services. The surgical literature might be interpreted as suggesting that giant cell arteritis can be diagnosed with the 1990 American College of Rheumatology (ACR) classification criteria,13, 14, 15, 16, 17 a view that implies that a negative temporal artery biopsy has no diagnostic use, or that there is minimal value in doing a biopsy after a few weeks of steroid therapy because of false negatives.15 In contrast, the rheumatology and ophthalmology literature suggests that the 1990 ACR classification criteria are not suitable for clinical diagnosis of giant cell arteritis.18, 19 A negative biopsy substantially downgrades the probability of the patient having the condition, because of reasonable test sensitivity coupled with very high specificity,20, 21, 22 and temporal artery biopsies often show inflammatory infiltrates for weeks23, 24 or months25 into steroid therapy. Where health systems, such as National Health Service (NHS) England, require confirmation of giant cell arteritis by biopsy or imaging before prescribing tocilizumab, surgeons play an essential role, not only in confirming the clinical diagnosis, but also in ensuring access to treatments.
Treatments for giant cell arteritis during the pandemic
When discussing the risks and benefits of treatment for giant cell arteritis during the COVID-19 pandemic, there are three important questions to consider. First, does a treatment for giant cell arteritis increase susceptibility to SARS-CoV-2 infection? Second, if a patient with giant cell arteritis established on treatment develops COVID-19, should the dose be maintained, reduced, or increased? Third, in patients with COVID-19, does treatment improve or worsen important disease outcomes, including mortality?
Glucocorticoids
Since the start of the pandemic, there has been concern from patients and clinicians that glucocorticoid therapy might increase COVID-19 susceptibility or severity. A joint statement was issued by multiple British professional societies, including the British Society for Rheumatology and the Royal College of General Practitioners, about the potential risks of oral and parenteral glucocorticoids during the pandemic.26 Clinicians were advised to “think before starting oral steroids”; however, it was also emphasised that organ-threatening disease, including vasculitis, should still be treated as usual.26 Clinicians were advised to taper glucocorticoid doses if clinically safe to do so, in line with usual practice and to use the lowest possible dose of oral steroids for the shortest period of time.26 The two largest major rheumatology associations, the European League against Rheumatism (EULAR) and the ACR, also recommended that patients with rheumatic diseases who do not have COVID-19 should continue their glucocorticoid or immunosuppressant treatment (or both)27, 28 and that high-dose steroids can be given for active vasculitis, including giant cell arteritis.28
Data on prednisolone prescriptions issued by general practitioners in England, publicly available from OpenPrescribing.net,29 showed an increase in dispensation of prednisolone prescriptions during March, 2020 (figure 1). This increase might have been driven in part by advice to patients to ensure they had an adequate supply of medication at home, a particular issue for those on long-term prednisolone therapy, which should not be stopped abruptly. However, during May, June, and July, 2020, fewer prednisolone prescriptions were dispensed in the community than usual (figure 1); such a pattern of a rise and then a decline was not seen for methotrexate, alendronic acid, or codeine–paracetamol (co-codamol) (codes in OpenPrescribing.net: 1001030U0, 0606020A0, 0407010F0). We speculate that the subsequent decline in prednisolone prescriptions might reflect ongoing challenges for patients in accessing care for non-urgent conditions, coupled with physician awareness of the risks of glucocorticoid therapy. The explicit recommendation to practise shared decision making in relation to glucocorticoid prescribing26 might have influenced this trend, as many patients were also concerned about the risks of glucocorticoid therapy.
This clinical concern remains. The fact that systemic glucocorticoid therapy, via its suppressive effect on innate and adaptive immunity, might increase susceptibility to COVID-19 is biologically plausible. Observational studies suggested that patients receiving higher doses of prednisolone are at risk of poorer outcomes from COVID-19.30, 31, 32 However, in such observational studies, it is difficult to eliminate confounding by indication. In patients with inflammatory diseases, oral glucocorticoid therapy might be frequently prescribed for those with comorbidity, frailty, or socioeconomic barriers to access to newer immunosuppressant therapies. Therefore, the magnitude of additional risk of glucocorticoid therapy in patients with rheumatic diseases, including giant cell arteritis, remains unclear. It should be noted that patients established on long-term (more than 4 weeks) glucocorticoid therapy (oral prednisolone of 5 mg or more) for rheumatic disease are at risk of hypothalamic–pituitary–adrenal axis suppression (tertiary adrenal insufficiency) and steroid dependence. These patients are thus at risk of adrenal crisis in the event of acute stressors, including sepsis.11 Current endocrinology consensus recommends that, given the potential for rapid deterioration of patients with the severe systemic upset of COVID-19, patients at risk of adrenal insufficiency who develop COVID-19 might require extra stress doses of glucocorticoid to avert potential adrenal crisis.33
The question of whether administering glucocorticoid therapy for COVID-19 itself is beneficial or harmful has been addressed in pragmatic platform trials. In the adaptive, open-label, randomised RECOVERY trial,34 2104 patients with COVID-19 were allocated to receive high-dose glucocorticoid therapy for up to 10 days, and 4321 patients were allocated to the standard of care. A significant survival benefit of dexamethasone was observed in severe COVID-19 (35% reduction in mortality in patients requiring mechanical ventilation; 20% reduction in mortality in those requiring supplemental oxygen).34 In response to the announcement of these results, three other trials of systemic glucocorticoids were halted early, and as a result were underpowered to show changes in mortality. Meta-analysis of all these trials to date showed an overall benefit of systemic glucocorticoids.35 Accordingly, WHO changed its guidance to advise systemic glucocorticoid therapy for severely or critically ill patients with COVID-19.36
For patients taking long-term glucocorticoid therapy for giant cell arteritis, carefully titrating the glucocorticoid dose down to the lowest possible dose compatible with symptom control remains crucial, given the concern that glucocorticoid therapy might impair antiviral defences. This principle must be balanced against the awareness that major relapse of giant cell arteritis might require further face-to-face specialist consultations or substantial glucocorticoid dose escalation (or both). Therefore, considering adjuvant immunosuppression at an early stage (after diagnosis of GCA or after first relapse) is important.
Adjuvant immunosuppression
The IL-6 receptor antagonist tocilizumab is licensed for the treatment of giant cell arteritis. When tocilizumab cannot be given, other disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, are sometimes used, particularly when relapses or glucocorticoid toxicity are a concern.3 Leflunomide is occasionally prescribed for giant cell arteritis but has not been tested in formal clinical trials.37 Whether long-term treatment with any biological or non-biological DMARD affects COVID-19 susceptibility or severity in patients with rheumatic diseases remains unclear.
During the initial phase of the pandemic, initiation of adjuvant immunosuppression was sometimes deferred due to concerns about the risks of attending centres for blood test monitoring and uncertainty about potential effects of immunosuppressant drugs on COVID-19 susceptibility. Data from international registries have not yet suggested that patients prescribed either conventional synthetic DMARDs or biological DMARDs for rheumatic diseases are at higher risk of admission to hospital or death, should they develop intercurrent COVID-19.30 An analysis of the dataset published by the COVID-19 Global Rheumatology Alliance did not reveal a statistically significant association of methotrexate use with hospitalisation status (n=600, adjusted odds ratio 0·90, 95% CI 0·59–1·37). Of potential relevance to an eventual availability of COVID-19 vaccines, it has been shown that discontinuing methotrexate therapy for 2 weeks after influenza vaccination appears to improve the ability to raise an antibody response to influenza.38
On the basis of these preliminary registry data, and the clear evidence that tocilizumab therapy can reduce glucocorticoid requirements in patients with giant cell arteritis,39 there appears to be no reason to change guidance3, 4 on the role for tocilizumab in treating this condition. In England, UK, clinicians initiating high-cost drugs, including tocilizumab, for giant cell arteritis must register patients on a central database called Blueteq. Although the database showed there was a reduction in initiation of tocilizumab therapy during the first peak of the pandemic, subsequent rates of initiation have risen to at least pre-pandemic rates (figure 1). These data must be viewed in the context of the funding restrictions for tocilizumab in the NHS. To qualify for funding, patients must have refractory giant cell arteritis, or relapsing giant cell arteritis with the diagnosis confirmed by either temporal artery biopsy or vascular imaging.40 Reduced access to these diagnostic tests, coupled with reduced patient access to rheumatology services at the height of the first peak of the pandemic, might have restricted access to tocilizumab initially. However, the available data suggest a return to previous prescribing rates. In the case of SARS-CoV-2 infection, recommendations from NHS and ACR advise temporarily stopping all non-glucocorticoid immunosuppressants, except for hydroxychloroquine, sulfasalazine,41 and perhaps IL-6 receptor inhibitors,28 whereas EULAR recommendations emphasise the importance of individualised decisions on a case-by-case basis, taking the patient's own views into account.27
Initial proposals that IL-6 pathway blockade using drugs such as tocilizumab might be a potential short-term treatment for COVID-19-related cytokine storm led to widespread publicity. However, randomised clinical trials of these drugs in patients with COVID-19 have not supported their widespread adoption in clinical practice; although peer-reviewed publications are still awaited, early reports suggest negative results.42, 43, 44 Only one press release has suggested that a trial's primary endpoint was met,45 but interpretation must be very guarded until all these trials have been reported in full.
There has been some interest in the use of leflunomide for treating COVID-19 because of its possible antiviral activity in vitro and in patients with other viral infections,46 but whether this postulated antiviral activity is relevant to doses used in rheumatology is unclear. Two small, open-label studies from China,47, 48 one of which has been published as a preprint only,48 suggested a reduced length of hospital stay in patients given leflunomide compared with non-treated patients. Disappointing results from large-scale trials of other repurposed drugs for COVID-19, such as hydroxychloroquine,49 indicate caution in overinterpreting preliminary data or in referring to repurposed drugs as antiviral agents. Overhyping the potential of repurposed medications in COVID-19 might lead to a false sense of security for patients who are prescribed these drugs for their rheumatic disease; if in response, patients were to relax vigilance in handwashing and physical distancing, their risk of COVID-19 could increase.
Health-care redesign
Different clinical units had different responses to the challenges of urgent diagnosis and treatment of giant cell arteritis, depending on the local effects of the pandemic and accessibility to tests. In locations, such as New York, NY, USA, where the pandemic was overwhelming, care redesign was not possible. In other locations, such as Queensland, Australia, that were minimally affected by COVID-19, there was no perceived need to redesign services. However, in locations that had sufficient clinical capacity, service redesign and innovation were possible (table 1). Perhaps the best way forward for rheumatology units during future peaks of the COVID-19 pandemic might be to consider the weaknesses in care pathways identified during the first peak, and then to continue to develop innovations that were originally introduced from necessity, which might have far-reaching benefits for future care.
Case study of health-care redesign during the peak
In Leeds, UK, the COVID-19 pandemic arrived during an ongoing quality improvement project that used Lean methodology to improve the diagnostic pathway for giant cell arteritis; key metrics of the service were already being closely tracked.50 To increase efficiency, the giant cell arteritis referral pathway had been adapted such that referrals from general practitioners within working hours were directed to a rapid-access specialist clinic with onsite temporal artery ultrasound. From there, patients were referred for temporal artery biopsy, if necessary.
During the first peak of the pandemic in Leeds, UK, COVID-19 restrictions meant that temporal artery biopsies became largely unavailable, and redeployment and shielding (ie, additional protective measures taken by individuals who are clinically extremely susceptible to COVID-19) meant that new staff had to be identified to cover every step in the pathway, each with a nominated backup in case of an unexpected absence. The pathway was redesigned according to the guidance that recommended minimising the time spent by patients on hospital premises.41 Early in the pandemic, a few patients referred with suspected giant cell arteritis were subsequently diagnosed with COVID-19. In response to these diagnoses, all patients referred with suspected giant cell arteritis were initially assessed by telephone consultation before attending a hospital to stratify patients according to the likelihood of either having giant cell arteritis or COVID-19 (table 2). Patients then had a single one-stop hospital visit, which included, as appropriate, measurement of vital signs, blood tests, chest radiograph, nasopharyngeal swab for SARS-CoV-2, a face-to-face visit with the specialist for further history and physical examination, temporal artery ultrasound, and dispensing of medication from the onsite pharmacy. During the initial piloting of this service, many patients found these multiple activities bewildering; therefore, in response, a short schedule of activities with a hospital map, including COVID-19 measures, was provided in advance by email, if possible. After their first visit, patients were followed up, primarily by telemedicine, supported by written summaries to patients and general practitioners, including a schedule of dates and doses of glucocorticoid therapy to enable them to taper according to guidelines, and clear directions as to what to do in the event of ocular or non-ocular giant cell arteritis relapse. As the COVID-19 restrictions in Leeds began to ease, the vascular surgeons began to offer day-case temporal artery biopsy; 2 days before the procedure, patients were required to have a nasopharyngeal swab for SARS-CoV-2. In line with current guidance,3 it was agreed that only patients with ongoing diagnostic uncertainty would be referred for biopsy.
Many service innovations occurred at other centres, albeit not specific to giant cell arteritis. For example, in Birmingham, UK, to minimise the need for hospital attendance, blood tests could be done at one of the city's large railway stations. Different approaches might be appropriate in different contexts, depending on the severity of constraints and the organisation's preferred approach to service development. In retrospect, the Leeds team would have preferred more general practitioner and patient involvement in the redesign process. The team have now reached out to local general practitioners and patient groups using virtual meetings to build relationships and to understand each other's perspectives and enable better collaboration in the future.
The effect of the COVID-19 pandemic on patients
All patients living with chronic disease have been affected by the COVID-19 pandemic. Patient groups were among the first to document these effects, including on physical activity, mental health, loneliness, and isolation.51 Anecdotally, similar patterns were observed by helpline volunteers for the charities Polymyalgia Rheumatica and Giant Cell Arteritis (PMR GCA) UK and PMR GCA Scotland. Patients reported that healthy diet and exercise routines were difficult to maintain, and help from others for essential tasks—from cleaning and shopping to personal care—was frequently compromised. The uncertain health risks of COVID-19, combined with disruption to health services, exacerbated health anxiety for many patients. Social isolation increased vulnerability and depression. Limitations in safe transport and the ability to access local newspapers further increased isolation. Although these effects were felt to some extent by all patients with rheumatic diseases, patients with giant cell arteritis are especially vulnerable because of older age and accompanying comorbidities, the effects of vascular inflammation, and the physical and emotional effects of glucocorticoid therapy.
A telephone survey of 79 patients with giant cell arteritis done 4 weeks into the French lockdown52 documented many physical and mental effects of the pandemic on patients, including weight gain, physical deconditioning, anxiety, and depression. These effects might not be evident to clinicians via telemedicine, since patients might not choose to report them. In turn, these physical and mental effects have the potential to exacerbate the risk of glucocorticoid-associated adverse effects, such as diabetes, bone fragility, and hypertension. These glucocorticoid-associated adverse effects might be invisible to both the patient and clinician unless monitoring tests can be done.
Concerningly, in the same survey, seven of 79 patients with giant cell arteritis reported symptoms suggesting relapse during the lockdown period.52 By contrast, in a telephone survey of 95 patients with giant cell arteritis done in Lombardy 6 weeks after the outbreak, no patients reported relapse.53 Multiple factors might be at play in variations in giant cell arteritis relapse rates, including treatment decisions (eg, tapering of glucocorticoids and initiation of adjuvant immunosuppression) and changes in physical and mental health that might affect disease activity. We speculate that COVID-19 might mimic giant cell arteritis relapse via diagnostic confusion, or that SARS-CoV-2 infection might directly induce giant cell arteritis relapse via COVID-19-associated endothelial dysfunction.54, 55 Further research is needed to unravel this complex network of possible causes.
Giant cell arteritis care in the age of telemedicine
Like many patients with rheumatic diseases, patients with giant cell arteritis have felt the effects of losing face-to-face contact with their treating physician. Telemedicine has many advantages, notably the ability to arrange frequent appointments at short notice, especially for patients who have difficulty travelling. Telemedicine can substantially increase convenience for patients, some of whom might find it easier to talk on the phone from their own home than in a clinical environment, and others who find transport to clinical centres challenging. At the same time, telemedicine cannot convey the depth and nuance of non-verbal communication, which helps physicians build rapport and manage the emotional effects of disease. Video-based and internet-based telemedicine solutions might be less accessible for older people, who also have a higher rate of sensory and cognitive impairments, which might further frustrate telemedicine encounters.
For urgent giant cell arteritis presentations, physical examination yields important diagnostic information.56 Switching to letter and telephone follow-up communications with patients has highlighted the importance of providing patients with high-quality information about their disease and its treatment. Much of the standard written information about rheumatology drugs, such as methotrexate and tocilizumab, was originally developed for patients with inflammatory arthritis and might not seem relevant to patients with giant cell arteritis. Drug counselling via telemedicine might fulfil minimal requirements of communicating information about risk, but it can be stripped of the ability to convey non-verbal signals, and a clinician's ability to reassure patients and help them feel safe can be compromised.
One critique of the rapid adaptations of clinical services to the pandemic has been the limited amount of patient involvement.2 Innovative solutions for patient involvement might be needed for conditions, such as giant cell arteritis, that tend to affect individuals who are clinically susceptible to infection due to age, disease, and treatments. Here, the global community of patients with giant cell arteritis could play a role. On Sept 3, 2020, in response to a request for information that would help in the rebuilding of services for this patient group, PMR GCA UK initiated an online poll for patients worldwide with polymyalgia rheumatica, giant cell arteritis, and large-vessel vasculitis, via their publicly accessible forum on the HealthUnlocked website. As of Sept 15, 2020, 452 responses were recorded. In parallel, the same survey was translated into Dutch and directly disseminated via the Dutch Vasculitis Foundation (Vasculitis Stichting) to all its members with large vessel vasculitis (giant cell arteritis or Takayasu arteritis). This survey received 142 responses between Sept 9 and Sept 14, 2020. Although neither survey can be considered representative of all patients, the results were strikingly concordant and have been combined and summarised in figure 2 . Respondents to the PMR GCA UK survey also posted many free-text comments; a summary of suggestions for clinicians based on these comments is shown in panel 2 .
Figure 2.
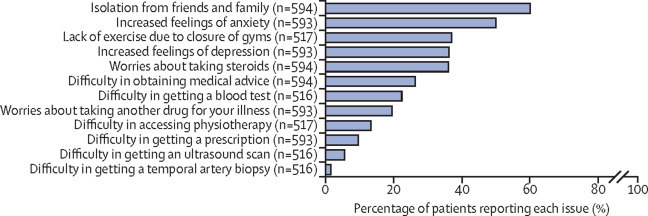
The effect of the COVID-19 pandemic on patients: results from an online survey by PMR GCA UK and the Dutch Vasculitis Foundation
The PMR GCA UK survey was opened on the HealthUnlocked website from Sept 3, 2020, and analysed on Sept 15, 2020. The PMR GCA UK survey was posted on a forum used by patients with polymyalgia rheumatica, giant cell arteritis, or large vessel vasculitis; the forum also reaches patients from outside the UK (eg, USA and Australia). The Dutch survey was done in SurveyMonkey from Sept 9 to Sept 14, 2020, and was directly sent by the Dutch Vasculitis Foundation to all members with large vessel vasculitis (giant cell arteritis or Takayasu arteritis). The number of respondents for each question is given. PMR GCA UK=Polymyalgia Rheumatica and Giant Cell Arteritis UK.
Panel 2. A list of suggestions for clinicians involved with care for patients with giant cell arteritis.
These suggestions are based on findings from the Polymyalgia Rheumatica and Giant Cell Arteritis (PMR GCA) UK and Dutch Vasculitis Foundation surveys:
-
•
Acknowledge the anxiety and uncertainties that patients might have regarding COVID-19 but emphasise that there is a growing body of knowledge that will aid in decision making
-
•
Decide whether to use face-to-face, telephone, or video appointments guided by hospital situations and patient-specific factors (ie, sensory and cognitive impairments, and access to technology or relatives that can help); in case of potential new cranial symptoms, face-to-face consultation is highly recommended
-
•
Discuss the needs and benefits of the treatment for giant cell arteritis, and discuss the understanding of the effect of immunosuppressive treatment on COVID-19 susceptibility and severity; emphasise that glucocorticoid treatment should not be stopped abruptly, as it might be dangerous (ie, cause relapse or adrenal insufficiency)
-
•
Discuss the patient's extent of self-isolation; identify opportunities to remain active; for instance, walking in remote or quiet areas
-
•
Discuss the urgency of tests that have been postponed due to COVID-19; for instance, it might be perfectly safe if a dual-energy x-ray absorptiometry scan is postponed
-
•
Provide written information following an appointment, which might include laboratory results, information regarding the disease state (remission or active), therapy plan (tapering scheme), date of next appointment, and instructions on when to contact the rheumatology advice line
-
•
Promote patient organisations for support, advice, and signposting to relevant information; for instance, PMR GCA UK, PMR GCA Scotland, Vasculitis Foundation, and Dutch Vasculitis Foundation
-
•
Promote electronic access to laboratory results whenever available
-
•
Promote self-testing of blood pressure, and glucose concentrations, if needed
Overcoming these challenges has required care and effort from providers. Clear, unambiguous information in a well written letter can be used to augment telemedicine encounters. Patients must also be clearly informed about how and when to seek medical advice. This need is particularly evident when initiation of tocilizumab therapy has been delayed by the peak of the pandemic, or in the UK where tocilizumab therapy is only funded for patients with relapsing or refractory disease. These relapses might only have come to clinical attention as outpatient appointments resumed.
Personalised medical advice from clinicians might be complemented by patient groups that aim to provide general information and peer support. During March, 2020, there was an increased number of calls to PMR GCA Scotland and PMR GCA UK telephone helplines, and posts to the PMR GCA UK online forum on HealthUnlocked (data on helpline calls was provided by PMR GCA UK and PMR GCA Scotland). During the UK lockdown, calls and forum posts declined, but with easing of lockdown restrictions, the number of calls and forum posts increased, as patients were faced with new choices to make in relation to risk and vulnerability.
Future perspectives
At many clinical centres, the experience of assessing patients for giant cell arteritis under new constraints, including reduced access to temporal artery biopsy, has increased the impetus to develop an ultrasound diagnostic service, and has increased appreciation of the value of other imaging modalities, such as PET and CT, to make a rapid diagnosis of giant cell arteritis.
Beyond the effects of the pandemic, one of the most pressing research questions for giant cell arteritis remains how fast steroids can safely be tapered. Even the fast taper used in the GiACTA trial39 tapered relatively slowly until a dose of 20 mg daily was reached. The possibility of faster steroid taper has been suggested for some patients with giant cell arteritis.57 Trials of steroid tapering strategies are urgently needed. Furthermore, tocilizumab is the only non-steroidal agent licensed for giant cell arteritis. In the UK, a rapid policy statement was issued allowing a temporary extension beyond 1 year of tocilizumab for patients who met specific criteria to avoid many patients stopping therapy during a possible further peak of the pandemic, and while there are winter pressures on the NHS.58 Globally, not all patients can receive tocilizumab, either due to clinical contraindication, inability to self-inject or attend for infusions, or absence of funding and reimbursement for this high-cost drug. Trials of more affordable drugs, including methotrexate59 or leflunomide,37 are urgently needed to prevent giant cell arteritis relapse and minimise exposure to steroids in patients who cannot receive biological DMARDs.
In academic centres, many non-COVID-19 research trials had to be paused or stopped; trial capacity and capability assessments must now consider physical distancing requirements in relation to all aspects of trial protocols. These restrictions have affected many clinical trial support services, including radiology. The pandemic has prompted discussions about how future clinical research can be made COVID-19 safe, while preserving scientific validity and regulatory compliance. The success of open-label, pragmatic, platform trials, such as RECOVERY,34 might perhaps usher in a new era of well powered, pragmatic clinical trials, with patient-friendly participant information sheets and efficient data collection methods that harness the power of routinely collected clinical data.
Looking forward, we can visualise an optimistic best-case COVID-19 scenario (low-level outbreaks controlled by vaccination and public health measures), a pessimistic worst-case scenario (further severe peaks affecting health-care delivery, as in the first peak), or most likely, something in between. There remains a high rate of uncertainty as to what will happen next. In rebuilding clinical services that will be resilient to future stresses, we must prepare for all three scenarios.
Under the optimistic scenario, we must heed what the pandemic has taught us about the weaknesses of current care for giant cell arteritis, including inadequate information about the disease and its treatment, variable availability of temporal artery ultrasound, variable understanding among surgeons regarding the diagnostic utility of temporal artery biopsy, infrequent follow-up appointments, and short duration of funding for therapies that have been shown to reduce relapses and steroid requirements. Under the pessimistic scenario, centres must implement plans to prevent or mitigate a repeat of the effects of the first peak: delayed presentations to medical care exacerbated by poor public awareness of giant cell arteritis; difficulties of referring doctors in identifying possible giant cell arteritis in the community; and restrictions in the availability of diagnostic tests. Compromised diagnostic ability would have secondary consequences of prolonged, inappropriate glucocorticoid use by patients without giant cell arteritis, alongside delays in starting appropriate treatment for patients with giant cell arteritis. However, in the intermediate scenarios in which enough rheumatology capacity remains to redesign services, we hope that we might see a so-called sweet spot of accelerated innovations for the diagnostic evaluation of giant cell arteritis and giant cell arteritis relapse, and a plurality of consultation modalities that can be tailored to individual patient needs.
Looking forward, we hope that we will learn from history, and that future changes will be planned and implemented with input and expertise from all relevant professionals involved, including primary care physicians, nurses, pharmacists, rheumatologists, ophthalmologists, surgeons who do temporal artery biopsies, radiologists and sonographers, and many others. Most importantly, patients must be involved; the voices of people living with this disease must be heard. Giant cell arteritis remains a medical emergency. Even during a global pandemic, patients require access to specialist advice and treatment. As physicians who lead and coordinate the care of patients with giant cell arteritis, rheumatologists have a duty to ensure patients receive the care they need.
Search strategy and selection criteria
A comprehensive search in any language of all articles published before Aug 25, 2020, was designed and done by an experienced research librarian with input from the study investigators. The following databases were included: Ovid MEDLINE and Epub ahead of print, Web of Science, Scopus, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, bioRxiv, and LitCOVID from the US National Center for Biotechnology Information. Search terms included terms for COVID-19 and terms for giant cell arteritis (appendix). After deduplication, 14 articles from preprint databases and ten peer reviewed articles were identified. All articles were read in their entirety and included if their content was relevant to the discussion of giant cell arteritis and COVID-19.
This online publication has been corrected. The corrected version first appeared at thelancet.com/rheumatology on December 23, 2020
Acknowledgments
Acknowledgments
We thank all our colleagues and patients who made this Viewpoint possible. In particular, we thank Richard Wakefield for his helpful comments on the manuscript and Peter Verhoeven, chair of the Dutch vasculitis patient organisation (Vasculitis Stichting), for distribution of the effect of the COVID-19 pandemic patient questionnaire in the Netherlands. We thank Pathology Queensland for supplying the temporal artery biopsy data for Queensland, Australia. We thank NHS England and NHS Improvement for providing the data on initiations of tocilizumab for giant cell arteritis. We would like to thank Ellie Taylor and Rayna Bhogal, medical students at the University of Leeds, UK, for assistance with reviewing statements in the medical literature about the role of the ACR 1990 classification criteria in the diagnosis of giant cell arteritis. We would like to acknowledge Helen Simpson for reviewing our interpretation of the current guidance about the management of patients with secondary adrenal insufficiency in the event of COVID-19 infection. No funding was received for the writing of the manuscript or the decision to submit it for publication. No payment was received by any of the authors for the writing of this Viewpoint. Individual authors take responsibility for the integrity of case histories and service use data they have contributed, as listed in the Contributors section; original source data cannot be shared due to institutional information governance restrictions.
Contributors
SLM and PM conceived the original idea for the manuscript, which was iteratively refined by ongoing discussion with all authors during the writing of the Viewpoint. SLM, MP, and PCR collected the data on clinical service use. SPM, RC, and MP provided the case histories (panel 1). MP prepared figure 1 and KSMvdG prepared figure 2 and panel 2. All authors were involved in the drafting and critical revision of the manuscript and gave final approval for publication.
Declaration of interests
KSMvdG reports personal fees from Roche, outside the submitted work. EB reports personal fees from Roche (2017 and 2018) for speaker and consulting fees, outside the submitted work. PCR reports personal fees from AbbVie, Eli Lilly, Gilead, and Roche, grants and personal fees from Janssen, Pfizer, Novartis, and UCB Pharma, and non-financial support from Bristol Myers Squibb, outside the submitted work. SLM was supported by Roche to attend EULAR 2019; has acted as an investigator on clinical trials in giant cell arteritis for Sanofi, Roche, and GlaxoSmithKline (GSK); reports consultancy fees from Roche and Sanofi that were paid to her institution, outside the submitted work; is patron of the UK charity PMR GCA UK; and receives infrastructure support from the UK Medical Research Council (MRC) TARGET Partnership Grant (MR/N011775/1/MRC_/Medical Research Council/United Kingdom) and is also supported by the the UK National Institute for Health Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care. SPM reports advisory board fees and speaker fees from Chugai-Roche and Roche, advisory board fees from Jannsen and Invex therapeutics, speaker fees from Allergan and Santen, and consulting fees from Neurodiem, outside the submitted work. PM is an MRC–GSK EMINENT clinical training fellow with project funding, outside the submitted work; receives co-funding by the NIHR University College London Hospitals Biomedical Research Centre; and is on a scientific advisory board for the Swedish Orphan Biovitrum. MP receives funding from the Rheumatology Research Foundation Scientist Development Award and is supported in part by Grant Number T32 AR007611-13 from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). SES reports funding from the Vasculitis Clinical Research Consortium (VCRC)–Vasculitis Foundation Fellowship. The VCRC is part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Science (NCATS). The VCRC is funded through a collaboration between NCATS and NIAMS (US4 AR057319). LN is a trustee of the charity PMR GCA Scotland. RC declares no competing interests.
Supplementary Material
References
- 1.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172:756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swaithes L, Dziedzic K, Sharp CA, Ellis B, Walsh N. Context, context, context: how has COVID-19 changed implementation globally and how can we ‘lock in' learning? Rheumatology (Oxford) 2020;59:1804–1807. doi: 10.1093/rheumatology/keaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackie SL, Dejaco C, Appenzeller S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology (Oxford) 2020;59:e1–23. doi: 10.1093/rheumatology/kez672. [DOI] [PubMed] [Google Scholar]
- 4.Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. doi: 10.1136/annrheumdis-2019-215672. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro L. Pivoting during a pandemic, preparing for a new normal. May 13, 2020. https://www.usnews.com/news/healthiest-communities/articles/2020-05-13/how-a-specialty-hospital-in-new-york-city-pivoted-during-covid-19
- 6.The Health Foundation How might COVID-19 have affected people's ability to see their GP? May 1, 2020. https://www.health.org.uk/news-and-comment/charts-and-infographics/how-might-covid-19-have-affected-peoples-ability-to-see-GP
- 7.The Health Foundation How is COVID-19 changing the use of emergency care? May 15, 2020. https://www.health.org.uk/news-and-comment/charts-and-infographics/how-is-covid-19-changing-the-use-of-emergency-care
- 8.Monti S, Delvino P, Bellis E, Milanesi A, Brandolino F, Montecucco C. Impact of delayed diagnoses at the time of COVID-19: increased rate of preventable bilateral blindness in giant cell arteritis. Ann Rheum Dis. 2020;79:1658–1659. doi: 10.1136/annrheumdis-2020-217915. [DOI] [PubMed] [Google Scholar]
- 9.Lecler A, Villeneuve D, Vignal C, Sené T. Increased rather than decreased incidence of giant-cell arteritis during the COVID-19 pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218343. published online Aug 7. [DOI] [PubMed] [Google Scholar]
- 10.Gan JM, Kho J, Akhunbay-Fudge M, et al. Atypical presentation of COVID-19 in hospitalised older adults. Ir J Med Sci. 2020 doi: 10.1007/s11845-020-02372-7. published online Sept 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson H, Tomlinson J, Wass J, Dean J, Arlt W. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin Med (Lond) 2020;20:371–378. doi: 10.7861/clinmed.2019-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P, Sattui SE, van der Geest K, et al. Giant cell arteritis and COVID-19: similarities and discriminators, a systematic literature review. J Rheumatol. 2020 doi: 10.3899/jrheum.200766. published online Oct 15. [DOI] [PubMed] [Google Scholar]
- 13.Chew BJW, Khajuria A, Ibanez J. The impact of temporal artery biopsy at a UK tertiary plastic surgery unit. Plast Reconstr Surg Glob Open. 2019;7 doi: 10.1097/GOX.0000000000002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies C, Frost B, Eshan O, McLain AD, Shandall A. Temporal artery biopsy...who needs one? Postgrad Med J. 2006;82:476–478. doi: 10.1136/pgmj.2005.043646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieri A, Milligan R, Hegde V, Hennessy C. Temporal artery biopsy: are we doing it right? Int J Health Care Qual Assur. 2013;26:559–563. doi: 10.1108/IJHCQA-06-2012-0055. [DOI] [PubMed] [Google Scholar]
- 16.Hussain O, McKay A, Fairburn K, Doyle P, Orr R. Diagnosis of giant cell arteritis: when should we biopsy the temporal artery? Br J Oral Maxillofac Surg. 2016;54:327–330. doi: 10.1016/j.bjoms.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Goslin BJ, Chung MH. Temporal artery biopsy as a means of diagnosing giant cell arteritis: is there over-utilization? Am Surg. 2011;77:1158–1160. [PubMed] [Google Scholar]
- 18.Grossman C, Barshack I, Bornstein G, Ben-Zvi I. Is temporal artery biopsy essential in all cases of suspected giant cell arteritis? Clin Exp Rheumatol. 2015;33(suppl 89):S-84–S-89. [PubMed] [Google Scholar]
- 19.Murchison AP, Gilbert ME, Bilyk JR, et al. Validity of the American College of Rheumatology criteria for the diagnosis of giant cell arteritis. Am J Ophthalmol. 2012;154:722–729. doi: 10.1016/j.ajo.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Hall S, Persellin S, Lie JT, O'Brien PC, Kurland LT, Hunder GG. The therapeutic impact of temporal artery biopsy. Lancet. 1983;2:1217–1220. doi: 10.1016/s0140-6736(83)91269-2. [DOI] [PubMed] [Google Scholar]
- 21.Vilaseca J, González A, Cid MC, Lopez-Vivancos J, Ortega A. Clinical usefulness of temporal artery biopsy. Ann Rheum Dis. 1987;46:282–285. doi: 10.1136/ard.46.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackie SL, Brouwer E. What can negative temporal artery biopsies tell us? Rheumatology (Oxford) 2020;59:925–927. doi: 10.1093/rheumatology/kez628. [DOI] [PubMed] [Google Scholar]
- 23.Achkar AA, Lie JT, Hunder GG, O'Fallon WM, Gabriel SE. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med. 1994;120:987–992. doi: 10.7326/0003-4819-120-12-199406150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Narváez J, Bernad B, Roig-Vilaseca D, et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum. 2007;37:13–19. doi: 10.1016/j.semarthrit.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Maleszewski JJ, Younge BR, Fritzlen JT, et al. Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol. 2017;30:788–796. doi: 10.1038/modpathol.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.British Society for Rheumatology. British Association of Orthopaedics. British Association of Spinal Surgeons. Royal College of General Practitioners. British Society of Interventional Radiology. Faculty of Pain Medicine. British Pain Society. Chartered Society of Physiotherapy Management of patients with musculoskeletal and rheumatic conditions who: are on corticosteroids; require initiation of oral/IV corticosteroids; require a corticosteroid injection. June 16, 2020. https://www.rheumatology.org.uk/Portals/0/Documents/COVID-19/MSK_rheumatology_corticosteroid_guidance.pdf
- 27.Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 28.Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheum. 2020;72:1241–1251. doi: 10.1002/art.41301. [DOI] [PubMed] [Google Scholar]
- 29.EBM DataLab. University of Oxford Prednisolone (0603020T0) https://openprescribing.net/chemical/0603020T0/
- 30.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haberman RH, Castillo R, Chen A, et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheum. 2020 doi: 10.1002/art.41456. published online July 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481. doi: 10.1053/j.gastro.2020.05.032. 91.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arlt W, Baldeweg SE, Pearce SHS, Simpson HL. Endocrinology in the time of COVID-19: management of adrenal insufficiency. Eur J Endocrinol. 2020;183:G25–G32. doi: 10.1530/EJE-20-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. New Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO WHO updates clinical care guidance with corticosteroid recommendations. Sept 2, 2020. https://www.who.int/news-room/feature-stories/detail/who-updates-clinical-care-guidance-with-corticosteroid-recommendations
- 37.Hočevar A, Ješe R, Rotar Ž, Tomšič M. Does leflunomide have a role in giant cell arteritis? An open-label study. Clin Rheumatol. 2019;38:291–296. doi: 10.1007/s10067-018-4232-x. [DOI] [PubMed] [Google Scholar]
- 38.Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence Tocilizumab for treating giant cell arteritis: technology appraisal guidance [TA518] April 18, 2018. https://www.nice.org.uk/guidance/ta518
- 41.National Institute for Health and Care Excellence COVID-19 rapid guideline: rheumatological autoimmune, autoinflammatory and metabolic bone disorders: NICE guideline [NG167] May 21, 2020. https://www.nice.org.uk/guidance/ng167 [PubMed]
- 42.Rosas I, Brau N, Waters M, et al. Tocilizuamb in hospitalised patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.08.27.20183442. published online Sept 12. (preprint) [DOI] [Google Scholar]
- 43.Sanofi Sanofi and Regeneron provide update on Kevzara (sarilumab) phase 3 US trial in COVID-19 patients. July 2, 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00
- 44.Sanofi Sanofi provides update on Kevzara (sarilumab) phase 3 trial in severe and critically ill COVID-19 patients outside the US. Sept 1, 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-09-01-07-00-00
- 45.Roche Roche's phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. Sept 18, 2020. https://www.roche.com/media/releases/med-cor-2020-09-18.htm
- 46.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu K, Wang M, Zhao Y, et al. A small-scale medication of leflunomide as a treatment of COVID-19 in an open-label blank-controlled clinical trial. Virol Sin. 2020 doi: 10.1007/s12250-020-00258-7. published online July 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Guo H, Li Y, et al. Efficacy and safety of leflunomide for refractory COVID-19: an open-label controlled study. medRxiv. 2020 doi: 10.1101/2020.05.29.20114223. published online June 2. (preprint) [DOI] [Google Scholar]
- 49.Sattui SE, Liew JW, Graef ER, et al. Swinging the pendulum: lessons learned from public discourse concerning hydroxychloroquine and COVID-19. Expert Rev Clin Immunol. 2020;16:659–666. doi: 10.1080/1744666X.2020.1792778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackie S, Barr A, Cracknell A, et al. Tracking the effects on a clinical service of introducing ultrasound for diagnosis of giant cell arteritis: design of a service evaluation using Lean methodology. Ann Rheum Dis. 2020;79(suppl) (abstr). [Google Scholar]
- 51.Versus Arthritis Versus Arthritis initial assessment of public health impacts of COVID-19 on people with arthritis. April 30, 2020. https://www.versusarthritis.org/media/22346/initial-assessment-of-public-health-impacts-of-covid-19-on-people-with-arthritis-apr20.pdf
- 52.Praliaud R, Greigert H, Samson M, et al. Impact of the COVID-19 lockdown on the management and control of patients with GCA. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218360. published online Aug 7. [DOI] [PubMed] [Google Scholar]
- 53.Tomelleri A, Sartorelli S, Campochiaro C, Baldissera EM, Dagna L. Impact of COVID-19 pandemic on patients with large-vessel vasculitis in Italy: a monocentric survey. Ann Rheum Dis. 2020;79:1252–1253. doi: 10.1136/annrheumdis-2020-217600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huertas A, Montani D, Savale L, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Geest KSM, Sandovici M, Brouwer E, Mackie SL. Diagnostic accuracy of symptoms, physical signs, and laboratory tests for giant cell arteritis: a systematic review and meta-analysis. JAMA Intern Med. 2020;180:1295–1304. doi: 10.1001/jamainternmed.2020.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karabayas M, Dospinescu P, Locherty M, et al. Stratified glucocorticoid monotherapy is safe and effective for most cases of giant cell arteritis. Rheum Adv Pract. 2020;4 doi: 10.1093/rap/rkaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.NHS England Tocilizumab for giant cell arteritis (GCA) during the COVID-19 pandemic (RPS 2007) July 30, 2020. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/07/C0620_Rapid-policy-statement-for-Giant-Cell-Arteritis-during-COVID_30July.pdf
- 59.Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–2797. doi: 10.1002/art.22754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


