Abstract
Objective
Precise risk stratification and triage of coronavirus disease 2019 (COVID-19) patients are essential in the setting of an overwhelming pandemic burden. Clinical observation has shown a somewhat high prevalence of sick euthyroid syndrome among patients with COVID-19. This study aimed to evaluate the predictive value of free triiodothyronine (FT3) at the clinical presentation of COVID-19 for disease severity and death.
Methods
This retrospective cohort study was based on electronic medical records. The study was conducted at Sheba Medical Centre, a tertiary hospital where several acute and chronic wards have been dedicated to the treatment of patients with COVID-19. The primary outcome measure was death during hospitalization; secondary outcomes included hospitalization in intensive care, mechanical ventilation, and length of hospitalization.
Results
Of a total of 577 polymerase chain reaction-positive patients with COVID-19 hospitalized between February 27 and July 30, 2020, 90 had at least 1 measurement of thyroid-stimulating hormone, free thyroxine, and FT3 within 3 days of presentation. After applying strict exclusion criteria, 54 patients were included in the study. Patients in the lowest tertile of FT3 had significantly higher rates of mortality (40%, 5.9%, and 5.9%, P = .008), mechanical ventilation (45%, 29.4%, and 0.0%; P = .007) and intensive care unit admission (55%, 29.4%, and 5.9%, P = .006). In multivariate analyses adjusted for age, Charlson comorbidity index, creatinine, albumin, and white blood cell count. FT3 remained a significant independent predictor of death.
Conclusion
FT3 levels can serve as a prognostic tool for disease severity in the early presentation of COVID-19.
Key words: COVID-19, sick euthyroid syndrome, FT3, prognostic factors
Abbreviations: COVID-19, coronavirus disease 2019; FT3, free triiodothyronine; FT4, free thyroxine; HTN, hypertension; RT3, reverse triiodothyronine; OR, odds ratio; SES, sick euthyroid syndrome
Introduction
Precise stratification and triage of coronavirus disease 2019 (COVID-19) patients early during the course of hospitalization have become essential in the setting of emergency departments and intensive care units (ICUs) overwhelmed by the pandemic burden.1 Age and pre-existing conditions, including obesity, diabetes, cardiovascular disease, and hypertension (HTN), are associated with increased mortality.2 , 3 Additionally, laboratory markers, including D-dimer, ferritin, and lymphocyte count, have additional prognostic value.1 Clinical observations have revealed a relatively high prevalence (up to 64%) of sick euthyroid syndrome (SES) among patients with COVID-19, with some exhibiting a profound decrease in thyroid hormone levels,4 although the prognostic significance of this observation is currently unknown. SES is a physiologic adaptation to acute or chronic illness of the hypothalamic-pituitary-thyroid-(peripheral tissues) axis characterized by a decrease in thyroid hormone levels and thyroid-stimulating hormone (TSH), despite absent intrinsic thyroid dysfunction at baseline.5 The available research on SES provides considerable knowledge on etiology, correlation with disease severity, and its prognostic value in a variety of acute and chronic states.6, 7, 8, 9, 10, 11, 12, 13, 14 Specifically, free triiodothyronine (FT3) has been shown to be a robust predictor of ICU mortality. In a prospective trial involving 480 critically ill patients admitted to the ICU, FT3 levels served as an independent and powerful predictor of mortality.7 The course of SES includes a decline in serum triiodothyronine levels as early as 24 hours after disease onset, accompanied by a reciprocal increase in reverse T3 (RT3). Serum total thyroxine levels decline as the acute illness progresses,15 , 16 whereas free thyroxine (FT4) hormone levels remain normal.17 The recovery phase is characterized by a gradual increase in serum TSH levels16 and may even be prolonged for months following clinical recovery. Of all the thyroid hormones, FT3 stands out as a marker of SES because it is the most dynamic hormone in the evolution of SES and is conventionally measurable, as opposed to RT3.9 , 18 , 19
The COVID-19 pandemic burden requires accurate triage based on the early identification of individuals at risk of developing severe disease. The aim of this study was to prognostically evaluate thyroid hormone levels, specifically FT3, at COVID-19 presentation.
Methods
This retrospective was study conducted at Sheba Medical Centre, a tertiary academic hospital in Israel. During the 2020 COVID-19 pandemic, several acute and chronic wards, including internal medicine, intensive care, obstetrical, pediatric, psychiatric, and rehabilitation wards, were dedicated for the treatment of patients with COVID-19.
The study included patients aged ≥18 years with documented polymerase chain reaction (PCR)-positive COVID-19 infection. Exclusion criteria included underlying thyroid disease based on diagnosis or chronic medications; treatment with drugs that might interfere with thyroid function, including amiodarone, interferon, and glucocorticoids (other than chronic treatment with a low-dose glucocorticoid); exposure to an iodine-containing contrast medium before thyroid hormone measurement; and admission for rehabilitation after initial recovery from an acute COVID-19 infection. Medical records of PCR-positive patients with COVID-19 hospitalized between February 27 and July 30, 2020, were retrieved and searched using MDClone (mdclone.com), a query tool that provides a wide range of patient data during a predefined time frame around an index event,20 and electronic medical records (Chameleon, version 5.12.2.43395, Elad Health). The index event was defined as hospitalization with a COVID-19 diagnosis in patients aged >18 years old.
Patient data queried included demographic data, medical history, laboratory parameters related to the index event, hospitalization, transfer between wards, mechanical ventilation, discharge, and death. Thyroid function tests were performed in the hospital’s core laboratory using immunoassays (UniCel Dxl 800 Immunoassay System, Beckman Coulter Diagnostics; normal reference ranges: TSH 0.4-4.0 mIU/L, FT4 7-16 pmol/L, and FT3 3.3-7.2 pmol/L; interassay coefficients of variation ≤5%, 8.8%, and 8%, respectively; and intra-assay coefficients of variation ≤2%, 4.4%,and 6.6%, respectively).
The Charlson score was calculated for each patient. The Charlson comorbidity index, developed by Charlson21 in 1987, is based on 19 conditions found to significantly influence survival and is a reflection of the number and severity of the comorbidities that a patient has. The comorbidities present in a patient are weighted and summed to give the final score, taking into account the person’s age.
The primary outcome of the study was death during hospitalization; secondary outcomes included hospitalization in the ICU, mechanical ventilation, and length of hospitalization. Demographic, clinical, and laboratory variables were compared between FT3 tertiles and between the groups of survivors and nonsurvivors. Continuous variables that were normally distributed were compared between groups using t tests or analysis of variance as indicated, adjusting, if necessary, for inequality of variances. Continuous variables that were not normally distributed were compared using the Kruskal-Wallis rank-sum test. Categorical variables were compared using X2 or Fisher exact test according to sample size.
Univariate logistic regression was performed to determine risk factors for mortality. Significant variables resulting from the univariate logistic regression were entered into multivariate logistic regression models. Models were compared using a pseudo R2 (Nagelkerke). A receiver-operating curve (ROC) analysis enabled evaluation of the predictive ability of FT3 with respect to mortality; the best cutoff point was determined using the Youden index. A Kaplan-Meier analysis assessed the probability of survival in the different FT3 groups, which were compared using the log-rank test. All analyses were performed using R.22 The study was approved by the Sheba Medical Center Institutional Review Board.
Results
Patients
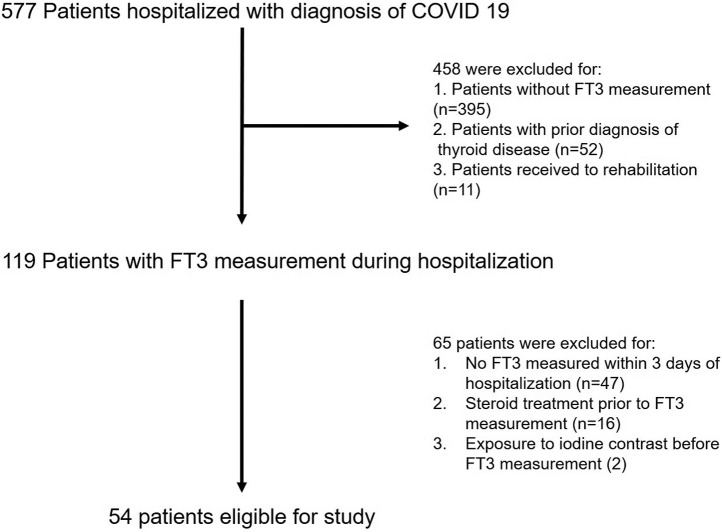
A total of 577 adult patients were diagnosed with COVID-19 and hospitalized in dedicated wards between February 27 and July 30, 2020 (Fig. 1 ). Patients without an initial FT3 measurement (n = 395), with prior thyroid disease (n = 52), or undergoing hospitalization for rehabilitation (n = 11) were excluded. Of the remaining 119 individuals, 47 patients were excluded for not having an FT3 measurement within 3 days of presentation (defined as the time window for COVID-19 presentation). An additional 16 patients who had received treatment with glucocorticoids before their first FT3 measurement (other than 1 subject on 5-mg prednisone for renal transplantation) were excluded, and 2 patients who had been exposed to iodinated contrast material prior to their thyroid hormone measurements were also excluded from the analysis. None of the remaining patients in the cohort were treated with amiodarone.
Fig. 1.
Study flowchart. FT3 = free triiodothyronine.
The remaining 54 patients included in the statistical analysis were divided into tertiles according to their FT3 levels (2.4-4.0, 4.1-4.8, and 4.9-7.4 pmol/L, respectively). Patients in the lowest tertile included patients with an FT3 value below the reference range or in the lower part of the reference range; this group of patients included those with SES. Demographic and clinical characteristics were compared between the FT3 tertiles and are shown in Table 1 .
Table 1.
Characteristics and Study Outcomes of Patients Divided into FT3 Tertiles
| Variable | FT3 tertiles |
P value | ||
|---|---|---|---|---|
| 2.4-4.0 pmol/L n =20 | 4.1-4.8 pmol/L n =17 | 4.9-7.4 pmol/L n = 17 | ||
| Age, mean (SD) | 68.75 (15.60) | 56.71 (14.88) | 48.98 (22.48) | .006 |
| Gender (%) | ||||
| Male | 15 (75.0) | 13 (76.5) | 9 (52.9) | .247 |
| Female | 5 (25.0) | 4 (23.5) | 8 (47.1) | … |
| BMI, mean (SD) | 26.49 (3.75) | 26.21 (4.56) | 26.66 (6.19) | .97 |
| Medical background | ||||
| DM (%) | 11 (55.0) | 4 (23.5) | 3 (17.6) | .033 |
| HTN (%) | 10 (50.0) | 8 (47.1) | 3 (17.6) | .093 |
| CVA (%) | 3 (15.0) | 3 (17.6) | 2 (11.8) | .89 |
| IHD (%) | 4 (20.0) | 1 (5.9) | 0 (0.0) | .095 |
| CHF (%) | 2 (10.0) | 1 (5.9) | 0 (0.0) | .416 |
| PVD (%) | 0 (0.0) | 1 (5.9) | 0 (0.0) | .33 |
| Autoimmune disease (%) | 1 (5.0) | 1 (5.9) | 0 (0.0) | .614 |
| Cognitive decline (%) | 4 (20.0) | 2 (11.8) | 4 (23.5) | .662 |
| Charlson index, mean (SD) | 5.00 (2.79) | 2.47 (1.87) | 1.65 (2.34) | <.001 |
| Smoking history (%) | 3 (15.0) | 3 (17.6) | 0 (0.0) | .205 |
| ED clinical parameters on presentation, mean (SD) | ||||
| Oxygen room air saturation (%) | 81.05 (17.92) | 92.71 (5.58) | 93.73 (7.03) | .006 |
| HR (bpm) | 94.75 (20.52) | 78.62 (15.60) | 101.30 (14.48) | .009 |
| RR (respiration/min) | 25.57 (9.06) | 22.62 (11.17) | 26.00 (12.17) | .61 |
| Temperature (°C) | 37.73 (0.73) | 37.73 (0.92) | 37.57 (0.67) | .858 |
| SBP (mm Hg) | 137.05 (21.41) | 135.79 (14.99) | 131.64 (22.48) | .767 |
| DBP (mm Hg) | 79.85 (10.45) | 76.86 (10.01) | 79.18 (10.78) | .704 |
| Laboratory results, mean (SD) | ||||
| Hb (g/dL) | 12.32 (2.30) | 13.88 (1.74) | 12.70 (2.54) | .18 |
| WBC (K/μL) | 11.04 (7.22) | 7.04 (2.76) | 7.98 (2.99) | .23 |
| PLT (K/μL) | 232.10 (89.12) | 228.35 (171.63) | 227.47 (77.68) | .55 |
| Neutrophils (K/μL) | 9.40 (7.32) | 5.21 (2.31) | 5.77 (2.47) | .17 |
| Lymphocytes (K/μL) | 0.78 (0.45) | 1.05 (0.59) | 1.36 (0.55) | .005 |
| Cr (mg/dL) | 1.30 (0.81) | 0.90 (0.33) | 0.67 (0.18) | .001 |
| Albumin (g/dL) | 3.23 (0.61) | 3.64 (0.65) | 3.79 (0.62) | .023 |
| ALT (IU/L) | 50.35 (44.79) | 32.71 (24.53) | 30.71 (14.12) | .13 |
| AST (IU/L) | 79.20 (71.52) | 49.06 (32.28) | 42.76 (19.70) | .22 |
| D-dimer (ng/mL) | 7556.88 (15450.71) | 2029.00 (2552.63) | 6697.64 (18253.27) | .09 |
| Ferritin (ng/mL) | 921.99 (773.91) | 647.61 (463.17) | 205.33 (267.29) | .002 |
| LDH (IU/L) | 492.89 (247.49) | 352.41 (154.12) | 289.88 (91.94) | .014 |
| Troponin-I HS (ng/L) | 109.79 (247.62) | 14.04 (15.17) | 39.10 (53.19) | .032 |
| CRP (mg/L) | 154.68 (90.48) | 80.70 (77.51) | 31.26 (60.73) | <.001 |
| Thyroid hormones, mean (SD) | ||||
| TSH (mIU/L)a | 2.51 (3.59) | 2.79 (3.25) | 3.12 (2.44) | .2 |
| FT4 (pmol/L)a | 13.78 (4.97) | 12.54 (3.35) | 13.21 (3.33) | .71 |
| FT3 (pmol/L)a | 3.40 (0.48) | 4.49 (0.23) | 5.56 (0.62) | <.001 |
| Min TSH (mIU/L) | 1.39 (1.88) | 1.60 (2.18) | 2.89 (2.47) | .039 |
| Min FT4 (pmol/L) | 9.24 (4.52) | 11.14 (2.22) | 12.68 (2.55) | .011 |
| Min FT3 (pmol/L) | 3.40 (0.48) | 4.49 (0.23) | 5.56 (0.62) | <.001 |
| Primary and secondary outcomes | ||||
| Death (n, %) | 8 (40.0) | 1 (5.9) | 1 (5.9) | .008 |
| Mechanical ventilation (n, %) | 9 (45.0) | 5 (29.4) | 0 (0.0) | .007 |
| ICU (n, %) | 11 (55.0) | 5 (29.4) | 1 (5.9) | .006 |
| LOS in days, mean (SD) | 40.06 (43.24) | 22.14 (23.55) | 13.98 (11.49) | .11 |
Abbreviations: ALT = alanine transaminase; AST = aspartate aminotransferase; BMI = body mass index; bpm = beats/min; CHF = congestive heart failure; Cr = creatinine; CRP = c-reactive protein; CVA = cerebrovascular accident; DBP = diastolic blood pressure; DM = diabetes mellitus; FT3 = free triiodothyronine; FT4 = free thyroxine; HR = heart rate; HTN= hypertension; Hb = hemoglobin; ICU = intensive care unit; IHD = ischemic heart disease; LDH = lactate dehydrogenase; LOS = length of stay; min = minimum; PLT = platelets; PVD = peripheral vascular disease; RR = respiratory rate; SBP = systolic blood pressure; TSH = thyroid stimulating hormone; WBC = white blood cell.
Normal reference ranges: TSH 0.4-4.0 mIU/L; FT4 7-16 pmol/L, FT3 3.3-7.2 pmol/L.
Clinical and Laboratory Characteristics According to FT3 Stratification
Participants in the lowest FT3 tertile were significantly older compared with the higher tertiles (68.7, 56.7, and 48.9 years, for the first, second, and third tertile, respectively; P = .006), had a higher Charlson index score (5.0, 2.47, and 1.65, respectively; P < .001), and a higher prevalence of diabetes mellitus (55%, 23.5%, and 17.6%, respectively; P = .033). No significant differences were found with respect to body mass index, sex, HTN, ischemic heart disease, congestive heart failure, autoimmune disease, or diagnosis of cognitive decline. Patients in the lowest FT3 tertile had significantly lower mean room air oxygen saturation on presentation (81%, 92.7%, and 93.7%, respectively; P = .006), and only patients in the lowest tertile required mechanical ventilation in the emergency department (5 patients vs 0 in both the higher tertiles; P = .009, data not shown). Patients in the lowest tertile had a higher creatinine level on presentation (1.3, 0.9, and 0.67 mg/dL, respectively; P = .001), a higher C-reactive protein level (154.8, 80.7, and 31.2 mg/L, respectively; P < .001), a lower mean lymphocyte count (0.78, 1.05, and 1.36 K/μL, respectively; P = .005), and a lower albumin level (3.23, 3.64, and 3.79 g/dL, respectively; P = .023). Other laboratory markers for severe disease were also significantly different between the groups, including LDH (492.8, 352.4, and 289.9 IU/L, respectively; P = .01), ferritin (921.9, 647.6, and 289.8 ng/mL, respectively; P = .002), and troponin (109.7, 14.0, and 39.1 ng/L, respectively; P = .032). D-dimer was not significantly different between the tertiles. No significant differences in TSH or FT4 at presentation with COVID-19 were found between the groups, but patients in the lowest FT3 tertile at presentation with COVID-19 reached a significantly lower TSH and FT4 nadir during the course of the disease (TSH: 1.3, 1.6, and 2.8 mIU/L, respectively; P = .039 and FT4: 9.2, 11.1, and 12.6 pmol/L, respectively; P = .011).
Primary and Secondary Outcomes
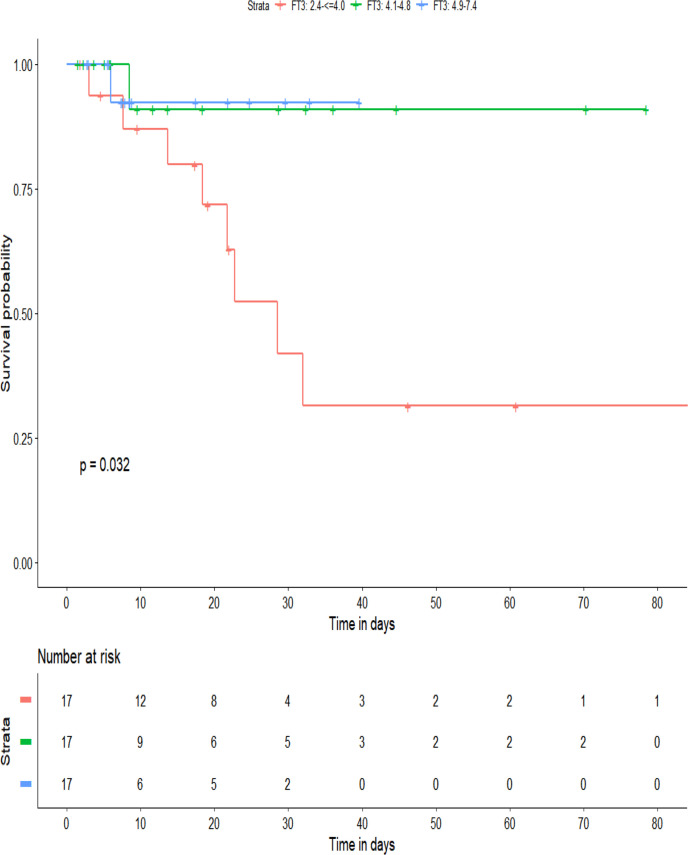
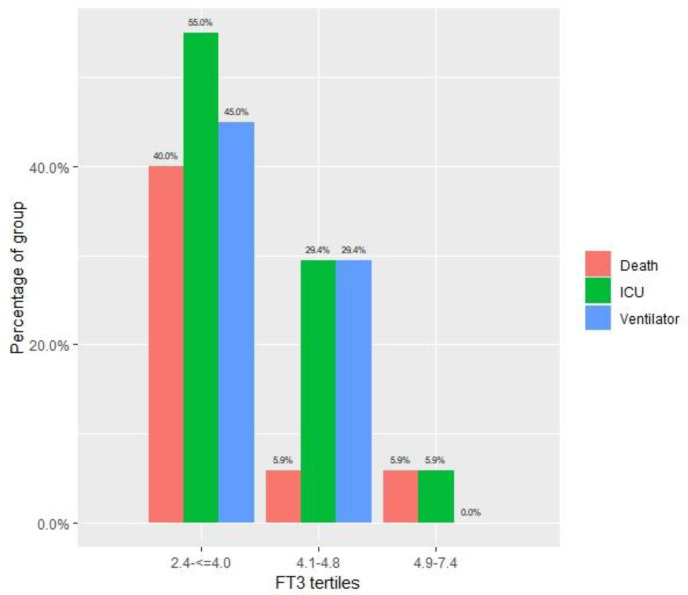
Patients in the lowest FT3 tertile had a significantly higher mortality rate (40%, 5.9%, and 5.9% in the first, second, and third tertiles, respectively; P = .008), more mechanical ventilation (45%, 29.4%, and 0.0%, respectively; P = .007), and ICU hospitalization (55%, 29.4%, and 5.9%, respectively; P = .006). The average length of hospitalization was not significantly different between the groups. A Kaplan-Meier 90-day survival analysis between the tertiles (Fig. 2 ) demonstrated the significant survival disadvantage of the lowest FT3 tertile (log-rank P = .032). Study outcomes are shown in Table 1 and Figure 3 . A full comparison of survivors with nonsurvivors is presented in Table 2 .
Fig. 2.
Kaplan-Meier survival after 90 days. FT3 = free triiodothyronine.
Fig. 3.
Primary and secondary outcomes according to FT3 tertiles. FT3 = free triiodothyronine; ICU = intensive care unit.
Table 2.
Comparison of Survivors to Nonsurvivors
| Variable | Survivors (n = 44) | Nonsurvivors (n = 10) | P value | Univariate OR (± CI) | P valuea | Multivariate ORs (+CI)b | P valuec | Rd |
|---|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 55.05 (18.43) | 74.93 (15.36) | .003 | 1.07 (1.02, 1.12) | .007 | 1.06 (1.00,1.12) | .06 | 0.47 |
| 0.21 (0.06, 0.73) | .014 | … | ||||||
| Male (%) | 31 (70.5) | 6 (60.0) | .791 | … | … | … | … | … |
| Female (%) | 13 (29.5) | 4 (40.0) | … | … | … | … | … | … |
| BMI, mean (SD) | 26.36 (4.87) | 26.84 (4.61) | .821 | … | … | … | … | … |
| Medical background | ||||||||
| DM (%) | 14 (31.8) | 4 (40.0) | .901 | … | … | … | … | |
| … | ||||||||
| HTN (%) | 14 (31.8) | 7 (70.0) | .061 | 5.0 (1.12, 22.27) | .035 | 3.81 (0.69, 21.2) | .12 | 0.43 |
| 0.18 (0.05, 0.6) | .005 | … | ||||||
| CVA (%) | 5 (11.4) | 3 (30.0) | .315 | … | … | … | … | |
| IHD (%) | 2 (4.5) | 3 (30.0) | .057 | … | … | … | … | |
| … | ||||||||
| CHF (%) | 1 (2.3) | 2 (20.0) | .15 | … | … | … | … | |
| … | ||||||||
| Charlson index, mean (SD) | 2.48 (2.45) | 6.10 (2.13) | <.001 | 1.7 (1.22, 2.37) | .0017 | 1.48 (1.03, 2.14) | .035 | 0.49 |
| 0.27 (0.07, 0.97) | .04 | … | ||||||
| Thyroid hormones | ||||||||
| TSH (mIU/L) | 2.65 (2.65) | 3.37 (4.78) | .52 | 1.07 (0.87, 1.31) | .51 | … | … | |
| FT4 (pmol/L) | 13.14 (4.11) | 13.49 (3.53) | .5 | 1.02 (0.87, 1.21) | .8 | … | … | |
| FT3 (pmol/L) | 4.65 (0.93) | 3.45 (0.77) | <.001 | 0.17 (0.05, 0.54) | .003 | … | … | |
| Laboratory results | ||||||||
| Hb (g/dL) | 13.18 (2.23) | 11.87 (2.27) | .076 | 0.78 (0.58,1.06) | .11 | … | … | |
| WBC (K/μL) | 7.50 (3.34) | 14.66 (7.63) | .001 | 1.34 (1.11, 1.63) | .0026 | 1.28 (1.05, 1.56) | .013 | 0.58 |
| 0.2 (0.05, 0.72) | .015 | … | ||||||
| Neutrophils (K/μL) | 5.59 (2.90) | 12.86 (8.21) | .001 | 1.37 (1.12, 1.67) | .0024 | 1.3 (1.05, 1.61) | .015 | 0.58 |
| 0.2 (0.05,0.73) | .015 | … | ||||||
| Lymphocytes (K/μ) | 1.09 (0.60) | 0.84 (0.35) | .3 | 0.39 (0.09, 1.7) | .21 | … | … | … |
| … | ||||||||
| Albumin (g/dL) | 3.70 (0.55) | 2.79 (0.59) | <.001 | 0.02 (0.00, 0.25) | .003 | 0.01 (0.00, 0.3) | .01 | 0.67 |
| 0.15 (0.03, 0.83) | .03 | |||||||
| Cr (mg/dL) | 0.88 (0.42) | 1.40 (0.99) | .08 | 3.28 (1.09, 9.84) | .034 | 1.2 (0.33, 4.33) | .78 | 0.38 |
| 0.19 (0.05, 0.67) | .01 | … | ||||||
| LDH (IU/L) | 357.79 (182.22) | 489.90 (229.62) | .06 | 1.0 (0.00, 1.01) | .07 | … | … | … |
| … | ||||||||
| D-dimer (ng/mL) | 4330.38 (12521.36) | 11474.00 (19677.18) | .07 | 1.0 (0.99, 1.00) | .23 | … | … | … |
| … | ||||||||
| Ferritin (ng/mL) | 617.44 (651.16) | 521.40 (571.27) | .95 | 0.99 (0.99, 1.00) | .71 | … | … | … |
| … | ||||||||
| Troponin-I HS (ng/L) | 17.26 (17.17) | 213.45 (329.72) | <.001 | 1.05 (1.01, 1.10) | .02 | … | … | … |
| … | ||||||||
| CRP (mg/L) | 82.16 (88.61) | 143.30 (98.06) | .053 | 1.01 (0.99, 1.01) | .07 | … | … | … |
| … | ||||||||
| AST (IU/L) | 52.36 (36.11) | 84.1 (88.25) | .69 | 1.01 (0.9998, 1.02) | .1 | … | … | … |
| ALT (IU/L) | 35.2 (20.93) | 53.6 (61.24) | .8 | … | … | … | … | … |
| ED clinical parameters on presentation, mean (SD) | ||||||||
| Oxygen room air saturation (%) | 88.31 (14.40) | 85.90 (13.07) | .47 | … | … | … | … | … |
| HR (bpm) | 90.15 (17.48) | 95.50 (26.12) | .46 | … | … | … | … | … |
| RR (respirations/min) | 23.84 (10.26) | 27.33 (8.19) | .34 | … | … | … | … | … |
| Temperature (°C) | 37.73 (0.80) | 37.53 (0.67) | .5 | … | … | … | … | … |
| SBP (mm Hg) | 134.37 (18.35) | 138.70 (24.27) | .54 | … | … | … | … | …. |
| DBP (mm Hg) | 77.74 (10.49) | 82.30 (8.90) | .22 | … | … | … | … | … |
Abbreviations: ALT = alanine transaminase; AST = aspartate aminotransferase; BMI = body mass index; bpm = beats/min; CHF = congestive heart failure; Cr = creatinine; CRP = c-reactive protein; CVA = cerebrovascular accident; DBP = diastolic blood pressure; DM, Diabetes mellitus; FT3 = free triiodothyronine; FT4 = free thyroxine; Hb = hemoglobin; HR = heart rate; HS = high sensitivity; HTN, Hypertension; IHD = ischemic heart disease; LDH = lactate dehydrogenase; RR = respiratory rate; SBP = systolic blood pressure; TSH = thyroid stimulating hormone; WBC = white blood cell.
P value for univariate model.
In the Multivariate OR (CI) column, the first OR (CI) is for the covariate and the second is for FT3
P values for multivariate model (with FT3). The P value for FT3 is the second one for each model.
Nagelkerke R for Multivariate model (with FT3). R2 for a model with FT3 alone is 0.38.
Comparison of Survivors with Nonsurvivors
Of the 54 patients in our cohort, there were 10 deaths, of which 8 were in the lowest tertile of FT3. These patients had an average FT3 on presentation, which was significantly lower than that of the survivors (3.45 vs 4.65 pmol/L; P < .001). Patients who died were significantly older (74.9 vs 55.0 years; P = .03) and had a higher Charlson index score (6.1 vs 2.48; P < .001). There were no significant differences regarding body mass index and pre-existing comorbidities. Heart rate, mean room air saturation, respiratory rate, temperature, and blood pressure were not significantly different at presentation. Laboratory markers that were significantly different between nonsurvivors and survivors included the white blood cell (WBC) count (14.6 vs 7.5 K/μL; P = .001), absolute neutrophil count (12.8 vs 5.5 K/μL; P = .001), and albumin (2.79 vs 3.70 g/dL; P < .001). Several markers for severe disease, including creatinine, LDH, and D-dimer, were borderline significant between the 2 groups. In a univariate analysis, baseline characteristics that were significantly associated with a higher risk of death included older age (odds ratio [OR] 1.07, 95% CI: 1.02-1.12) and a higher Charlson index (OR 1.7, 95% CI: 1.22-2.37). When analyzing the association of thyroid hormones at presentation and death, lower FT3 levels were significantly associated with death, but neither TSH or FT4 levels were significant for mortality (OR for FT3: 0.17, 95% CI: 0.05-0.54). Other laboratory markers significantly associated with death were low albumin (OR 0.02, 95% CI: 0.00-0.25), low WBC count (OR 1.34, 95% CI: 1.11-1.63), and low neutrophil count (OR 1.37, 95% CI: 1.12-1.67). The ORs for FT3 and albumin were low because, unlike other variables associated with death, a higher FT3 and higher albumin levels are associated with a decreased risk of death.
In multivariate analyses adjusted for age, Charlson index, WBC count, neutrophil count, and albumin as separate covariates, FT3 remained a statistically significant predictor of death. However, after adjustment for FT3, age, HTN, and creatinine were no longer significant (P = .06, .12, and .78, respectively), while low albumin, Charlson index score, WBC count, and neutrophil count retained their significance as predictors of mortality (P = .01, .035, .012, and .013, respectively).
FT3 alone (in a univariate analysis) was found to be a significant predictor of mortality (R2 = 0.38), and the addition of 1 of the other covariates in a model with FT3 strongly increased joint predictive ability (Table 2). These other covariates included age (R2 = 0.47), Charlson index (R2 = 0.49), WBC count (R2 = 0.58), neutrophil count (R2 = 0.58), and albumin levels (R2 = 0.67).
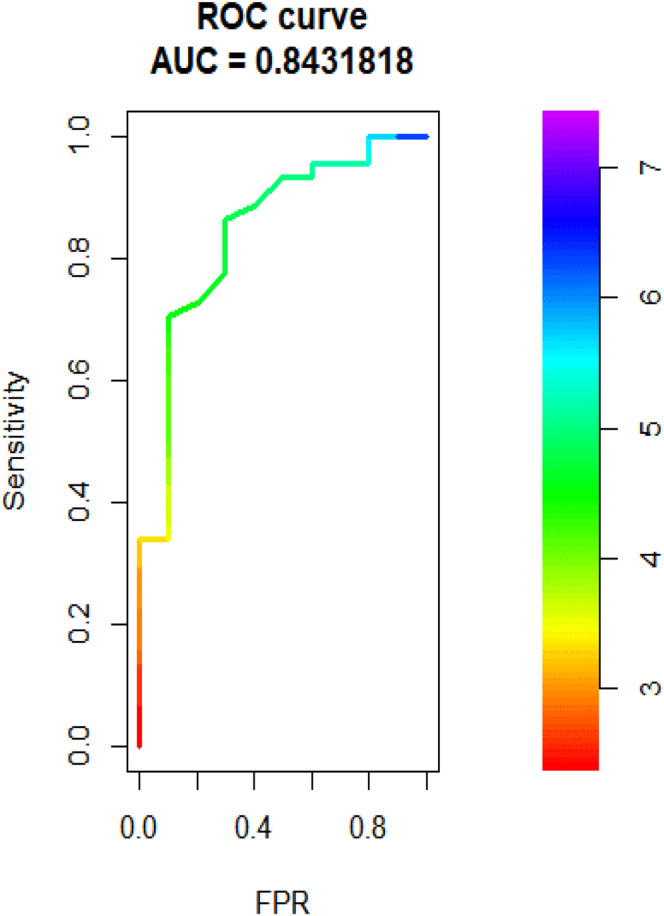
An ROC curve for the association between FT3 levels and death is shown in Figure 4 . With a cutoff value of 4.15 pmol/L, the area under the curve (AUC) was 0.843 (95% CI: 0.705-0.981), the sensitivity was 90%, and the specificity was 70%. When compared with other variables that were found to be significantly associated with death, FT3 was superior to age and WBC count (AUC = 0.79 and 0.83, respectively) and inferior to albumin levels and Charlson index score (AUC = 0.89 and 0.86, respectively).
Fig. 4.
ROC curve of association between FT3 levels and death, AUC = 0.84. FT3 = free triiodothyronine; ROC = receiver-operating curve.
Comparison Between the Study Cohort and the General Population of Patients with COVID-19
The study cohort was similar to the general population of patients hospitalized with COVID-19 with respect to age, sex, and medical history, with the exception of diabetes (33.3% vs 19.2%; P = .027), but differed in laboratory markers for severe disease. These markers included the WBC count (8.85 vs 7.93 K/μL; P < .001) and the levels of albumin (3.54 vs 3.8 g/dL; P = .002), LDH (382.7 vs 313.4 IU/L; P = .018), and troponin (62.1 vs 55.2 ng/L; P < .001). As far as study outcomes were concerned, the study cohort had a significantly higher mortality rate (18.5% vs 3.5%; P > .001), use of mechanical ventilation (25.9% vs 0.8%; P > .001), ICU admission (31.5% vs 3.5%; P < .001), and length of hospitalization (26.2 vs 7.4 days; P < .001). A detailed comparison is shown in Table 3 .
Table 3.
Comparison Between the Study Cohort and Hospitalized Patients with Coronavirus 2019 Without Free Triiodothyronine Measurements
| Variable | Study cohort (n = 54) | Hospitalized patients not in study cohorta (n = 395) | P value |
|---|---|---|---|
| Age (mean (SD)) | 58.73 (19.40) | 57.29 (18.73) | .64 |
| Gender (%) | |||
| Male | 37 (68.5) | 228 (57.7) | .172 |
| Female | 17 (31.5) | 167 (42.3) | … |
| BMI, mean (SD) | 26.43 (4.78) | 27.41 (5.32) | .36 |
| Medical background | |||
| DM (%) | 18 (33.3) | 76 (19.2) | .027 |
| HTN (%) | 21 (38.9) | 135 (34.2) | .596 |
| CVA (%) | 8 (14.8) | 30 (7.6) | .13 |
| IHD (%) | 5 (9.3) | 30 (7.6) | .59 |
| CHF (%) | 3 (5.6) | 19 (4.8) | .74 |
| PVD (%) | 1 (1.9) | 13 (3.3) | 1 |
| Autoimmune disease (%) | 2 (3.7) | 23 (5.8) | .76 |
| Cognitive decline (%) | 10 (18.5) | 37 (9.4) | .07 |
| Charlson index, mean (SD) | 3.15 (2.77) | 2.66 (2.67) | .19 |
| Laboratory tests, mean (SD) | |||
| Hb (g/dL) | 12.94 (2.28) | 13.25 (1.88) | .52 |
| WBC (K/μL) | 8.85 (5.20) | 7.93 (26.73) | <.001 |
| Neutrophils (K/μL) | 6.94 (5.14) | 4.69 (3.08) | <.001 |
| Lymphocytes (K/μL) | 1.05 (0.57) | 1.19 (0.64) | .11 |
| PLT (K/μL) | 229.46 (116.47) | 194.95 (77.99) | .026 |
| Albumin (g/dL) | 3.54 (0.66) | 3.80 (0.51) | .002 |
| Cr (mg/dL) | 0.98 (0.59) | 0.90 (0.48) | .97 |
| AST (IU/L) | 58.24 (50.35) | 40.67 (28.42) | .003 |
| ALT (IU/L) | 38.61 (32.32) | 30.80 (26.57) | .02 |
| LDH (IU/L) | 382.72 (196.64) | 313.41 (128.85) | .018 |
| D-dimer (ng/mL) | 5691.07 (14154.82) | 1741.45 (5117.51) | .072 |
| Ferritin (ng/mL) | 601.80 (633.44) | 485.06 (600.04) | .5 |
| Troponin-I HS (ng/L) | 62.11 (172.03) | 55.24 (342.78) | <.001 |
| CRP (mg/L) | 93.69 (92.68) | 71.27 (75.10) | .19 |
| Study outcomes | |||
| Death cases (%) | 10 (18.5) | 14 (3.5) | <.001 |
| Mechanical ventilation (%) | 14 (25.9) | 3 (0.8) | <.001 |
| ICU (%) | 17 (31.5) | 14 (3.5) | <.001 |
| LOS (d), mean (SD) | 26.21 (31.67) | 7.41 (9.21) | <.001 |
Abbreviations: ALT = alanine transaminase; AST = aspartate aminotransferase; BMI = body mass index; CHF = congestive heart failure; Cr = creatinine; CRP = c-reactive protein; CVA = cerebrovascular accident; DM = diabetes mellitus; Hb = hemoglobin; HTN = hypertension; ICU = intensive care unit; IHD = ischemic heart disease; LDH = lactate dehydrogenase; LOS = length of stay; PLT = platelets; PVD = peripheral vascular disease.
Excluded patients who were excluded from the study and study cohort.
Discussion
Clinical observations have revealed a relatively high prevalence of SES among patients with COVID-19.4 These observations have raised the question of whether FT3 levels represent an integrative indicator of disease severity and a patient's reserve early in the course of COVID-19 disease. We analyzed a cohort of 54 PCR-confirmed patients with COVID-19 who had a full thyroid function profile upon disease presentation. Patients with a low FT3 (in the lowest tertile of FT3 values) had a markedly higher disease severity and increased mortality (40% mortality rate) compared with patients with a higher FT3 (5% mortality rate in the higher tertiles). Low FT3 at presentation remained a robust predictor of mortality in multivariate analyses that included all other significant predictors: age, Charlson index, albumin level, WBC count, and neutrophil count. The FT3 ROC curve proved that FT3 was an excellent predictor of mortality (AUC = 0.84), superior to age (AUC = 0.79), and only slightly inferior to albumin levels (AUC = 0.89) and the Charlson index (AUC = 0.86).
SES is recognized as a nonspecific adaptive mechanism for illness and an indirect marker of disease severity in various conditions, including acute coronary syndrome,10 hospitalization in the critical care setting,7 cancer,23 burns,9 and brain surgery.8 The underlying mechanisms for SES include multiple and complex alterations in iodothyronine deiodinases, TSH secretion, thyroid hormone binding to plasma proteins, thyroid hormone transport and activity in peripheral tissues, and expression of thyrotropin-releasing hormone in the hypothalamus.24. Cytokines are central mediators of endocrine changes related to systemic illness, with specific effects on the thyroid gland. They have been shown to inhibit thyroid iodide uptake,25 inhibit iodine organification,26, 27, 28 suppress thyroglobulin synthesis,29 , 30 and decrease thyroid hormone secretion.15 , 31, 32, 33, 34, 35 Administration of thyroid hormone to restore normal serum thyroid hormone levels is controversial, and currently available data do not provide clear evidence of a benefit.36, 37, 38
The course and severity of COVID-19 are closely linked to the action of several cytokines and the presence of a cytokine storm induced by the virus. Proinflammatory cytokines, including IL-6 and TNF-α, which are known to interact with thyroid function, as described above, lead to acute respiratory distress syndrome aggravation and widespread tissue damage resulting in multiorgan failure.39 , 40 The rise in inflammatory cytokines occurs before the clinical deterioration in patients with COVID-19. Thus, the suppression of FT3 may serve as a simple indicator of a clinically significant increase in cytokines. Moreover, the rise in cortisol in the setting of acute infection may also exert a suppressive effect on TSH secretion, FT4 to FT3 conversion, and an increase in the conversion of FT4 to RT3. Low FT3 is likely to be an integrative marker for the host response to COVID-19 infection.
This study has several limitations. Due to the retrospective data collection, thyroid function tests were unavailable for all patients on admission. This might reflect diverse policies of laboratory assessment in different wards or the decision of medical staff to perform a more thorough initial laboratory work-up when assessing the patient. The study population is relatively small due to the meticulous cohort selection performed to evaluate FT3 as a predictor of mortality early in the course of hospitalization and without potential confounders, such as glucocorticoid treatment, which is very common among these patients. This stringent selection method limited the cohort size but was necessary for the clarity and significance of the results. We measured FT3 by a conventional automated clinical method rather than by equilibrium dialysis, which could overcome potential interference in the laboratory assay due to alterations in thyroid hormone-binding capacity. Given the scarce availability of these methods in most medical centers and the availability of FT3 immunoassays, the latter is a more feasible biomarker for COVID-19 risk stratification.
In conclusion, our findings suggest that FT3 provides a robust prognostic value that can serve as a valuable stratification tool for newly diagnosed patients with COVID-19.
Acknowledgments
Disclosure
The authors have no multiplicity in interest to declare.
Author Contributions
Y.S. and R.P. are co-primary authors.
References
- 1.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 doi: 10.1136/bmj.m1091. 368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Chen M., Zhou W., Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2020;31(1):8–11. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
- 5.Moura Neto A., Zantut-Wittmann D.E. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol. 2016 doi: 10.1155/2016/2157583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Pan W., Wang H., Wang S., Pan S., Ge J. Relationship between thyroid function and ICU mortality: a prospective observation study. Crit Care. 2012;16(1):R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutch M., Kumar S., Gupta K.K. Prognostic value of thyroid profile in critical care condition. Indian J Endocrinol Metab. 2018;22(3):387. doi: 10.4103/ijem.IJEM_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunevicius A., Deltuva V., Tamasauskas S., Tamasauskas A., Laws E.R., Bunevicius R. Low triiodothyronine syndrome as a predictor of poor outcomes in patients undergoing brain tumor surgery: a pilot study. J Neurosurg. 2013;118(6):1279–1287. doi: 10.3171/2013.1.JNS121696. [DOI] [PubMed] [Google Scholar]
- 9.Gangemi E.N., Garino F., Berchialla P. Low triiodothyronine serum levels as a predictor of poor prognosis in burn patients. Burns. 2008;34(6):817–824. doi: 10.1016/j.burns.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Pavlou H.N., Kliridis P.A., Panagiotopoulos A.A., Goritsas C.P., Vassilakos P.J. Euthyroid sick syndrome in acute ischemic syndromes. Angiology. 2002;53(6):699–707. doi: 10.1177/000331970205300611. [DOI] [PubMed] [Google Scholar]
- 11.Özcan K.S., Osmonov D., Toprak E. Sick euthyroid syndrome is associated with poor prognosis in patients with ST segment elevation myocardial infarction undergoing primary percutaneous intervention. Cardiol J. 2014;21(3):238–244. doi: 10.5603/CJ.a2013.0108. [DOI] [PubMed] [Google Scholar]
- 12.Nafae R.M., Mohammed M.A., Morsi A.F., Ibrahim D.A. Thyroid function in respiratory failure patients. Egypt J Chest Dis Tuberc. 2014;63(2):513–521. [Google Scholar]
- 13.Chuang C.P., Jong Y.S., Wu C.Y., Lo H.M. Impact of triiodothyronine and N-terminal pro-B-type natriuretic peptide on the long-term survival of critically ill patients with acute heart failure. Am J Cardiol. 2014;113(5):845–850. doi: 10.1016/j.amjcard.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 14.De Alfieri W., Nisticò F., Borgogni T. Thyroid hormones as predictors of short-and long-term mortality in very old hospitalized patients. J Gerontol - Ser A Biol Sci Med Sci. 2013;68(9):1122–1128. doi: 10.1093/gerona/glt012. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Berghe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol. 2000;143(1):1–3. doi: 10.1530/eje.0.1430001. [DOI] [PubMed] [Google Scholar]
- 16.Plikat K., Langgartner J., Buettner R. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56(2):239–244. doi: 10.1016/j.metabol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Adler S.M., Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am. 2007;36(3):657–672. doi: 10.1016/j.ecl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Falkowski B., Rogowicz-Frontczak A., Grzelka A. Higher free triiodothyronine concentration is associated with lower prevalence of microangiopathic complications and better metabolic control in adult euthyroid people with type 1 diabetes. Endocrine. 2018;60(3):458–465. doi: 10.1007/s12020-018-1582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer S., Schuetz P., Wieland M., Nusbaumer C., Mueller B., Christ-Crain M. Low triiodothyronine syndrome: a prognostic marker for outcome in sepsis? Endocrine. 2011;39(2):167–174. doi: 10.1007/s12020-010-9431-4. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg I. Insulin detemir use is associated with higher occurrence of hypoglycemia in hospitalized patients with hypoalbuminemia. Diabetes Care. 2018;41(4):e44–e46. doi: 10.2337/dc17-1957. [DOI] [PubMed] [Google Scholar]
- 21.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2017 https://www.R-project.org Accessed November 11, 2020. [Google Scholar]
- 23.EkremCengiz S., Cetinkaya E., Altin S. Nutritional and prognostic significance of sick euthyroid syndrome in non-small cell lung cancer patients. Intern Med. 2008;47(4):211–216. doi: 10.2169/internalmedicine.47.0423. [DOI] [PubMed] [Google Scholar]
- 24.Lee S., Farwell A.P. Euthyroid sick syndrome. Compr Physiol. 2011;6(2):1071–1080. doi: 10.1002/cphy.c150017. [DOI] [PubMed] [Google Scholar]
- 25.Pang X.P., Hershman J.M., Chung M., Pekary A.E. Characterization of tumor necrosis factor-α receptors in human and rat thyroid cells and regulation of the receptors by thyrotropin. Endocrinology. 1989;125(4):1783–1788. doi: 10.1210/endo-125-4-1783. [DOI] [PubMed] [Google Scholar]
- 26.Ashizawa K., Yamashita S., Nagayama Y. Interferon-y inhibits thyrotropin-induced thyroidal peroxidase gene expression in cultured human thyrocytes. J Clin Endocrinol Metab. 1989;69(2):475–477. doi: 10.1210/jcem-69-2-475. [DOI] [PubMed] [Google Scholar]
- 27.Asakawa H., Hanafusa T., Kobayashi T., Takai S., Kono N., Tarui S.E. Interferon-γ reduces the thyroid peroxidase content of cultured human thyrocytes and inhibits its increase induced by thyrotropin. J Clin Endocrinol Metab. 1992;74(6):1331–1335. doi: 10.1210/jcem.74.6.1592878. [DOI] [PubMed] [Google Scholar]
- 28.Sato K., Satoh T., Shizume K. Inhibition of 125I organification and thyroid hormone release by interleukin-1, tumor necrosis factor-α, and interferon-γ in human thyrocytes in suspension culture. J Clin Endocrinol Metab. 1990;70(6):1735–1743. doi: 10.1210/jcem-70-6-1735. [DOI] [PubMed] [Google Scholar]
- 29.Poth M., Tseng Y.C., Wartofsky L. Inhibition of TSH activation of human cultured thyroid cells by tumor necrosis factor: an explanation for decreased thyroid function in systemic illness? Thyroid. 1991;1(3):235–240. doi: 10.1089/thy.1991.1.235. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki K., Yamada E., Kanaji Y. Interleukin-6 (IL-6) inhibits thyroid function in the presence of soluble IL-6 receptor in cultured human thyroid follicles. Endocrinology. 1996;137(11):4857–4863. doi: 10.1210/endo.137.11.8895357. [DOI] [PubMed] [Google Scholar]
- 31.Kraiem Z., Sobel E., Sadeh O., Kinarty A., Lahat N. Effects of γ-interferon on DR antigen expression, growth, 3, 5, 3′-triiodothyronine secretion, iodide uptake, and cyclic adenosine 3', 5'-monophosphate accumulation in cultured human thyroid cells. J Clin Endocrinol Metab. 1990;71(4):817–824. doi: 10.1210/jcem-71-4-817. [DOI] [PubMed] [Google Scholar]
- 32.de Vries E.M., Fliers E. Boelen A The molecular basis of the non-thyroidal illness syndrome. J Endocrinol. 2015;225(3):R67–R81. doi: 10.1530/JOE-15-0133. [DOI] [PubMed] [Google Scholar]
- 33.Fliers E., Guldenaar S.E.F., Wiersinga W.M., Swaab D.F. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab. 1997;82(12):4032–4036. doi: 10.1210/jcem.82.12.4404. [DOI] [PubMed] [Google Scholar]
- 34.Papanicolaou D.A. Euthyroid sick syndrome and the role of cytokines. Rev Endocr Metab Disord. 2000;1(1-2):43. doi: 10.1023/a:1010060303031. [DOI] [PubMed] [Google Scholar]
- 35.Feelders R.A., Swaak A.J.G., Romijn J.A. Characteristics of recovery from the euthyroid sick syndrome induced by tumor necrosis factor alpha in cancer patients. Metabolism. 1999;48(3):324–329. doi: 10.1016/s0026-0495(99)90080-x. [DOI] [PubMed] [Google Scholar]
- 36.Gardner D.F., Kaplan M.M., Stanley C.A., Utiger R.D. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979;300(11):579–584. doi: 10.1056/NEJM197903153001102. [DOI] [PubMed] [Google Scholar]
- 37.Brent G.A., Hershman J.M. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63(1):1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- 38.Glinoer D. Comment on dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab. 1999;84(6):2261–2262. doi: 10.1210/jcem.84.6.5809-7. [DOI] [PubMed] [Google Scholar]
- 39.McGonagle D., Sharif K., O’Regan A. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]