Abstract
Objectives
The aim was to evaluate the safety and effectiveness of thalidomide, an immunomodulatory agent, in combination with glucocorticoid, for the treatment of COVID-19 patients with life-threatening symptoms.
Methods
A nonrandomized comparative case series study was performed. Six patients received thalidomide 100 mg per day (with therapy lasting for ≥7 days) plus low-dose short-term dexamethasone, and 6 control patients matched with patients in the thalidomide group, received low-dose short-term treatment with dexamethasone alone. The main outcomes were: the duration of SARS-CoV-2 negative conversion from admission; length of hospital stay; and changes in inflammatory cytokine concentrations and lymphocyte subsets.
Results
The median thalidomide treatment time was 12.0 days. The median duration of SARS-CoV-2 negative conversion from admission and hospital stay length were briefer in the thalidomide group compared to the control group (respectively, 11.0 vs 23.0 days, P = 0.043; 18.5 vs 30.0 days, P = 0.043). The mean reduction rates at 7–10 days after treatment for serum interleukin-6 and interferon-γ concentrations were greater in the thalidomide group compared to the control group. Alterations in lymphocyte numbers in the subsets between the 2 groups were similar.
Conclusions
Thalidomide plus short-term glucocorticoid therapy is an effective and safe regimen for the treatment of severely ill COVID-19 patients. The mechanism of action is most likely inhibition of inflammatory cytokine production.
Keywords: Thalidomide, Glucocorticoid, COVID-19, Inflammatory cytokines, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Introduction
From December 2019, a novel coronavirus, probably of bat origin (Zhou et al., 2020), termed corona virus disease 2019 (COVID-19) caused a major respiratory illness outbreak in Wuhan, China (Guan et al., 2020) and a worldwide pandemic. The person-to-person contagious transmission of COVID-19 has been confirmed in multiple studies; the disease had infected more than 30 million individuals worldwide by September 2020 (Slack-Smith, 2020). COVID-19 induces severe acute respiratory distress syndrome (ARDS) and multiple organ failures, and has been responsible for the premature deaths of more than 1,000,000 people to date. However, at the present time, no vaccine is available to prevent infection nor a specific drug treatment regimen. Although much hope is being placed on remdesivir and hydroxychloroquine therapy, it is not clear if they are effective and safe to use in COVID-19 patients (Vinetz, 2020, Wang et al., 2020b). Two recent studies report clinical symptoms in patients suffering from a COVID-19 infection that are similar to highly pathogenic coronavirus infections such as Middle Eastern respiratory syndrome coronavirus (MERS-CoV) or severe acute respiratory syndrome coronavirus (SARS-CoV). In these patients an inflammatory cytokine storm and lymphopenia may occur (Barnes et al., 2020, Belhadjer et al., 2020, Bordoni et al., 2020, Huang et al., 2020, Li et al., 2020, Zhang et al., 2020) which can lead to ARDS or multiple organ failure (heart, kidneys, lungs). Therefore, in addition to antiviral therapy, anti-inflammatory drug therapy and the maintenance of immune homeostasis are considered to be effective treatments for severe pneumonia induced by COVID-19.
Glucocorticoids administered in high doses are often employed to reduce the surge in multiple cytokine production during inflammation. For example, glucocorticoids have been given to patients infected with SARS-CoV or MERS-CoV to reduce inflammation of the lungs and suppress immune responses (Arabi et al., 2018, Stockman et al., 2006). Unfortunately, these drugs produce serious side effects such as osteoporosis, secondary bacterial infections, inhibition of immune response and clearance of pathogens, and other adverse events (AEs). Therefore, glucocorticoids are contraindicated as therapy for patients with life-threatening COVID-19-induced pneumonia (Russell et al., 2020).
Thalidomide, which induces congenital defects in the fetus (phocomelia), has recently been introduced as a treatment for many autoimmune disorders including systemic lupus erythematosus, psoriasis, and inflammatory diseases of the gastrointestinal tract. Subsequently, the inhibitory actions of thalidomide on pro-inflammatory cytokine production, for example, interleukin-6 (IL-6), interferon-β (IFN-β), IL-12 and tumor necrosis factor alpha 2 (TNF-α,) were discovered (Millrine et al., 2016a, Moller et al., 1997, Moreira et al., 1993). Thalidomide has been used to limit injury to the lungs of mice infected with hemagglutinin type 1 and neuraminidase type 1 (H1N1) influenza virus, where it improved survival rates by reducing infiltration of inflammatory cells and by inhibiting cytokine (IL-6 and TNF-α) and chemokine production (Zhu et al., 2014). Furthermore, thalidomide has been shown to have powerful actions in stimulating T cell proliferation after CD3 is activated (Bartlett et al., 2004, LeBlanc et al., 2004).
Based on these anti-inflammatory properties, thalidomide was introduced as an experimental therapy to treat patients with advanced COVID-19-induced pneumonia, and in particular for patients with unsatisfactory responses to conventional antiviral drugs, especially when they were in late stage disease. Thus, the main aim of the present study is to investigate the safety and efficacy of a combination of thalidomide and low-dose glucocorticoid for the treatment of patients infected with COVID-19.
Methods
Design of the study
This was a retrospective analysis of a series of patients who were confirmed to have COVID-19 infections and were recruited between January and March 2020 from the isolation ward of the First Affiliated Hospital of Wenzhou Medical University, which is a university affiliated tertiary care public hospital responsible for the treatment of critical/seriously ill COVID-19 patients referred by local authorities. The study was performed according to the Standards for the Reporting of Diagnostic Accuracy Studies guidance for observational studies. The ethics committee of the First Affiliated Hospital of Wenzhou Medical University approved the research protocols of the study (2020−006).
Criteria for inclusion or exclusion
Inclusion criteria
-
1)
Patients with life-threatening COVID-19-induced pneumonia were diagnosed based on the Guidelines of the Diagnosis and Treatment of New Coronavirus Pneumonia (ver. 7)
-
2)
Aged ≥18 years
-
3)
Both genders
-
4)
Were treated with thalidomide within 7 days of admission and took the drug for at least 5 days
-
5)
Oral consent for participation was obtained
Exclusion criteria
-
1)
Females who were pregnant or wished to get pregnant and males who desired fertility
-
2)
Patients given thalidomide within 24 weeks prior to the study
-
3)
Known allergy to thalidomide or methylprednisolone
-
4)
Patients with abnormal laboratory results including concentrations of alanine aminotransferase and aspartate aminotransferase <5 times the normal serum upper limits; glomerular filtration rate ≤30 mL/min/1.73 m2.
Interventions
Treatment regimens were: thalidomide (100 mg daily for ≥7 days) plus short-term administration of low-dose dexamethasone (40 mg IV at 12 h intervals for 3 days, when the dose was reduced and given at 24 h intervals for a further 5 days) (thalidomide group) or short-term low-dose dexamethasone therapy (control group). The controls were matched to those patients in the thalidomide group according to their baseline cytokine concentrations.
Primary outcomes
The patients’ recovery from illness and discharge from hospital was according to the following guidelines: (1) Temperature recovered to normal for ≥3 days; (2) Respiratory symptoms had markedly improved; (3) A significant reduction in lung exudation; (4) Swab specimens tested negative for SARS-Cov-2 as determined by real-time reverse transcription polymerase chain reaction (rRT-PCR) at least every other day for 2 swabs. The primary outcomes were the duration of SARS-CoV-2 negative conversion from admission, and length of hospital stay. The primary outcome measurements were a reduction in inflammatory cytokine concentrations and recovery of immune cells 7–10 days after thalidomide treatment. AEs associated with thalidomide treatment were also recorded.
Data collection
Medical records of patients concerning clinical symptoms, laboratory tests, epidemiological data, radiological characteristics, therapy protocols and outcomes were collected by the research team and maintained in an electronic database in accordance with the study protocol. Patient recent travel history, especially to Wuhan, and their contact history with infected or suspected cases was also collected. During a patient’s hospital stay, the data collected included demographics, medical and exposure history, underlying comorbidities, symptoms and therapy (antiviral therapy, anti-inflammatory, antibiotics, respiratory support, and other symptomatic and supportive treatment). Radiologic assessments were mainly based on computed tomography (CT). Laboratory tests consisted of routine blood examinations, blood chemistry, liver and renal function tests, electrolyte concentrations, C-reactive protein measurements, a coagulation test, procalcitonin, immunocyte absolute counts, and various cytokine concentration determinations.
The day the patient exhibited symptoms was considered to be the objective date of the onset of disease. Shock and ARDS were defined following the World Health Organization’s interim guidelines. Acute kidney damage was described according to previously published guidelines (Kellum et al., 2013, Lameire et al., 2013). Cardiac injury was diagnosed based on the serum concentrations of specific heart biomarkers, for example, troponin I. Secondary bacterial or fungal infections were diagnosed if hospital-acquired pneumonia or bacteremia presented together with positive infection test results for lower respiratory tract specimens, taken at least 48 h after admission.
Seriously ill patients had to meet one or more of the following criteria: (1) Shortness of breath, respiration rate (RR) ≥30 breaths/min; (2) Oxygen saturation (resting) ≤93%; (3) PaO2, fraction of inspired oxygen (FiO2) <300 mmHg. The clinical outcomes monitored included complications, prognosis, discharge, and the length of hospital stay. The duration from the onset of disease to positive SARS-Cov-2 diagnosis, hospital admission, antiviral therapy, anti-inflammatory therapy, dyspnea, ARDS, respiratory support, and discharge times were also recorded.
Laboratory tests
SARS-CoV-2 nucleic acid levels in samples were detected using rRT-PCR and confirmed by the local Centers for Disease Control (CDC). A human Th1/2 cytokine kit II (BD Ltd., US) was used to detect plasma cytokine concentrations including TNF-α, IFN-γ, IL-2, IL-4, IL-6 and IL-10. Lymphocyte subsets were quantified using flow cytometry.
Statistical analysis
Data analyses were conducted using SPSS ver. 22.0 software (IBM, New York, US). Continuous variables are given as medians and interquartile ranges (IQRs). Potential differences between 2 groups were evaluated using the Wilcoxon matched-pairs signed-rank test. Categorical values are expressed as relative or absolute frequencies, and comparisons between the thalidomide and control groups made by employing a chi–squared or Fisher’s exact test. A two-tailed P-value <0.05 was deemed to be a significant finding.
Results
Patients’ baseline characteristics
Fifteen patients and 20 patients with critical COVID-19 symptoms were enrolled in the thalidomide and control groups, respectively. Of 15 patients in the thalidomide group, 9 were excluded because they were given thalidomide more than 7 days after admission, or given the drug for less than 5 days. Finally, the analyses were performed on 6 patients in the thalidomide group. Simultaneously, 6 patients were included in the control group who were closely matched to patients in the thalidomide group.
The baseline characteristics patients in the thalidomide and control groups are presented in Table 1 . For both cohorts, the majority of patients were male (83.3% vs 50.0%, P = 0.545), with a median age of 65.5 years (IQR: 53.3–73.3) and 71.0 years (IQR: 65.3–79.5) (P = 0.249), respectively. All patients were admitted to the isolation ward of the hospital. The median duration from first symptoms to being SARS-CoV-2 positive, having dyspnea, being admitted to hospital, and becoming seriously ill were 2.5 days (IQR, 2.0–9.5), 5.5 days (IQR, 4.3–8.5), 5.5 days (IQR, 4.8–11.0) and 5.5 days (IQR, 4.3–11.5) for the thalidomide group, and 6 days (IQR, 3.5–8.3, P = 0.414), 7.0 days (IQR, 4.0–10.0, P = 0.462), 7.5 days (IQR, 4.0–11.0, P = 0.686) and 7.0 days (IQR, 4.0–10.0, P = 0.684) for the control group, respectively. The most commonly observed symptoms were fever and a dry cough followed by fatigue for both groups. Four (66.7%) and 2 (33.3%) patients had hypertension.
Table 1.
Demographic characteristics of patients with COVID-19.
| Variable | Thalidomide group (n = 6) | Control group (n = 6) | P value |
|---|---|---|---|
| Demographic parameters | |||
| Age, years (median, IQR) | 65.5 (53.3, 73.3) | 71.0 (65.3, 79.5) | 0.249 |
| ≥65 | 3 (50.0%) | 5 (83.3%) | |
| <65 | 3 (50.0%) | 1 (16.7%) | 0.545 |
| Male Gender (n, %) | 5 (83.3%) | 3 (50.0%) | 0.545 |
| Clinical parameters | |||
| Highest temperature, ℃ (median, IQR) | 38.1 (37.6, 38.2) | 38.0 (37.1, 38.6) | 0.916 |
| Heart rate, beats/min (median, IQR) | 94 (85, 95) | 79 (75, 85) | 0.075 |
| Respiratory rate, times/min (median, IQR) | 20 (19, 22) | 26 (20, 31) | 0.114 |
| Mean arterial pressure, mmHg (median, IQR) | 92.2 (83.9, 107.3) | 88.3 (85.1, 107.2) | 0.600 |
| Duration from first symptoms to diagnosis, days (median, IQR) | 2.5 (2.0, 9.5) | 6 (3.5, 8.3) | 0.414 |
| Duration from first symptoms to admission in our hospital, days (median, IQR) | 5.5 (4.8, 11.0) | 7.5 (4.0, 11.0) | 0.686 |
| Duration from first symptoms to dyspnea, days (median, IQR) | 5.5 (4.3, 8.5) | 7.0 (4.0, 10.0) | 0.462 |
| Duration from first symptoms to server, days (median, IQR) | 5.5 (4.3, 11.5) | 7.0 (4.0, 10.0) | 0.684 |
| Comorbidities (n, %) | 4 (66.7%) | 2 (33.3%) | 0.567 |
| Chest imaging, infiltrate | |||
| Unilateral (n, %) | 0 | 0 | |
| Bilateral (n, %) | 6 (100%) | 6 (100%) | 1.000 |
| Treatment | |||
| Duration from first symptoms to antiviral (median, IQR) | 3.5 (2.0, 7.3) | 6.0 (3.5, 8.8) | 0.465 |
| Duration of antiviral therapy (median, IQR) | 9.5 (7.8, 11.8) | 8.0 (5.3, 9.5) | 0.171 |
| Durations from admission to thalidomide (median, IQR) | 3.5 (0, 7.0) | – | |
| Duration of thalidomide therapy (median, IQR) | 12.0 (7.0, 14.3) | – | |
| Clinical outcomes | |||
| Mechanical ventilation (n, %) | 1 (16.7%) | 3 (50.0%) | 0.545 |
| ARDS (n, %) | 2 (33.3%) | 3 (50.0%) | 0.558 |
| Durations from admission to virus tested negative | 11.0 (9.0, 14.5) | 23.0 (20.0, 28.0) | 0.043 |
| Hospital stay, days (median, IQR) | 18.5 (14.8, 24.5) | 30.0 (24.5, 38.5) | 0.043 |
| Mortality (n, %) | 0 | 1 (16.7%) | 0.296 |
COVID-19, corona virus disease 2019; IQR, interquartile range; ARDS, acute respiratory distress syndrome.
Respiratory rate, heart rate and mean blood pressure were practically identical in the 2 groups. The oxygenation indexes in both groups were similar, all below 300 mmHg; 230.8 (IQR, 193.5–270.5) in the thalidomide group and 174.5 (IQR, 109.5–225.5) in the control group (P = 0.116). Most laboratory findings were also similar for both groups (Table 2 ). Chest CT scans revealed that all enrolled patients had bilateral involvement of the lungs caused by COVID-19. One patient died on hospital day 15 in the control group.
Table 2.
Baseline laboratory test results of patients with COVID-19.
| Variable | Thalidomide group (n = 6) | Control group (n = 6) | P value |
|---|---|---|---|
| Immune cells | |||
| White blood cells, ×109/mL | 6.87 (5.66, 9.75) | 7.24 (5.00, 10.51) | 0.917 |
| Neutrophils, ×109/mL | 4.83 (3.71, 9.06) | 6.28 (3.32, 9.18) | 0.917 |
| Monocytes, ×109/mL | 0.66 (0.28, 0.83) | 0.36 (0.30, 0.62) | 0.173 |
| Lymphocytes, ×109/mL | 1.02 (0.68, 1.22) | 1.11 (0.60, 1.33) | 0.917 |
| T cell, /μL | 483.5 (343.3, 717.0) | 411.5 (244.0, 644.0) | 0.600 |
| CD4, /μL | 339.5 (230.5, 485.8) | 208.0 (164.0, 438.8) | 0.753 |
| CD8, /μL | 142.0 (96.5, 208.3) | 130.5 (72.5, 329.8) | 0.917 |
| B cell, /μL | 178.5 (138.3, 271.5) | 172.0 (82.3, 305.3) | 0.600 |
| NK cell, /μL | 228.5 (166.3, 288.3) | 186.0 (96.0, 709.5) | 0.917 |
| Cytokines | |||
| IL-2, pg/mL | 0.89 (0.64, 1.23) | 0.86 (0.57, 1.04) | 0.753 |
| IL-4, pg/mL | 0.72 (0.42, 1.09) | 0.80 (0.52, 1.48) | 0.345 |
| IL-6, pg/mL | 30.92 (5.87, 83.83) | 32.34 (3.69, 89.66) | 0.173 |
| IL-10, pg/mL | 5.84 (4.41, 16.02) | 7.39 (4.07, 12.37) | 0.600 |
| TNF-α, pg/mL | 0.10 (0.06, 0.27) | 0.24 (0.09, 1.30) | 0.249 |
| IFN-γ, pg/mL | 2.19 (0.86, 12.38) | 1.80 (0.55, 2.81) | 0.172 |
| Biochemical parameters | |||
| Total bilirubin, μmol/L | 13.5 (8.3, 21.5) | 9.5 (6.8, 22.5) | 0.596 |
| Albumin, g/L | 33.6 (29.5, 36.1) | 29.5 (27.4, 32.6) | 0.345 |
| AST, U/L | 30.5 (20.0, 36.5) | 50.5 (30.0, 98.5) | 0.058 |
| ALT, U/L | 23.0 (13.5, 39.5) | 34.0 (21.8, 89.8) | 0.345 |
| Glucose, mmol/L | 9.9 (7.0, 13.9) | 9.5 (8.4, 10.9) | 0.463 |
| Cholesterol, mmol/L | 4.43 (3.44, 5.43) | 3.69 (3.16, 5.55) | 0.753 |
| Triglyceride, mmol/L | 1.37 (0.94, 1.55) | 1.03 (0.90, 1.36) | 0.345 |
| HDL, mmol/L | 0.85 (0.79, 1.04) | 0.92 (0.73, 1.21) | 0.753 |
| LDL, mmol/L | 2.52 (1.78, 3.39) | 2.11 (1.64, 3.17) | 0.917 |
| Blood urea nitrogen, mmol/L | 3.5 (1.3, 8.0) | 5.2 (4.3, 6.4) | 0.463 |
| Creatinine, μmol/L | 56.5 (47.5, 72.8) | 56.5 (49.5, 86.8) | 0.917 |
| CK-MB, U/L | 8.5 (3.8, 11.0) | 8.0 (5.8, 18.5) | 0.168 |
| CRP, | 54.4 (12.4, 71.2) | 75.2 (11.6, 90.0) | 0.345 |
| Serum lactate, | 2.9 (2.3, 3.5) | 2.0 (1.8, 3.6) | 0.752 |
| Coagulation function | |||
| PT, s | 13.5 (12.9, 14.0) | 13.9 (13.3, 14.3) | 0.400 |
| APTT, s | 39.2 (34.0, 48.9) | 34.8 (31.7, 44.2) | 0.600 |
| D-dimer, μg/mL | 1.02 (0.55, 1.13) | 1.77 (1.08, 4.70) | 0.046 |
| Oxygenation Index, mmHg | 230.8 (193.5, 270.5) | 174.5 (109.5, 225.5) | 0.116 |
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CK-MB, creatine kinase, MB Form; COVID-19, corona virus disease 2019; CRP, C-reactive protein; HDL, high-density lipoprotein; IL, interleukin; LDL, low-density lipoprotein; PT, prothrombin time.
Note: The variables are expressed as median and interquartile range (IQR).
Treatment and endpoint analyses
Antivirals (lopinavir/arbidol), antibiotics, low-dose short-term systematic corticosteroid and supporting treatment were given to all patients. In the thalidomide group, the median time interval from hospital admission to receiving thalidomide was 3.5 days (IQR, 0–7), 3 patients on hospital day 1, 1 patient on hospital day 6, and 2 patients on hospital day 7. The median duration for thalidomide therapy was 12.0 days (IQR, 7.0–14.3).
The median duration for SARS-CoV-2 negative conversion from admission was 11.0 days (IQR, 9.0–14.5) and 23.0 days (IQR, 20.0–28.0) in the thalidomide and control groups (P = 0.043), respectively. The median hospital stay was shorter in the thalidomide group compared to the control group; 18.5 days (IQR, 14.8–24.5) vs 30.0 days (IQR, 24.5–38.5) (P = 0.043). One and 3 patients needed mechanical ventilation in the thalidomide group and control group (P = 0.545), respectively. The lung lesions in all patients, except for the 1 patient who died, were significantly improved.
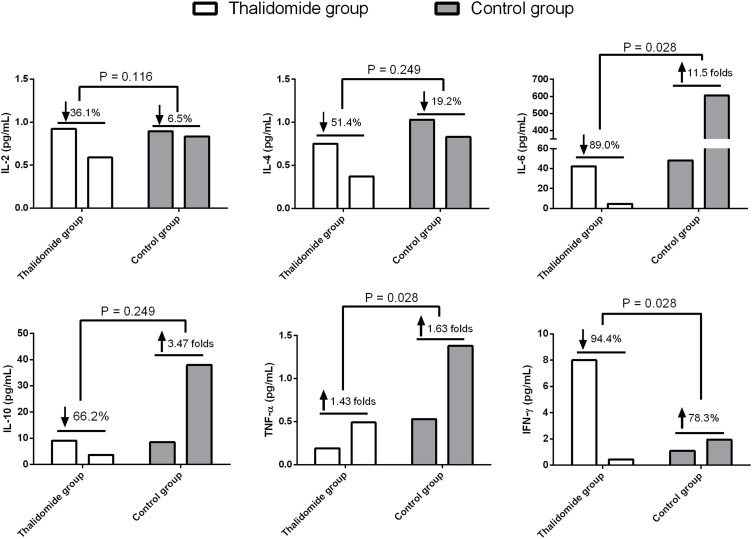
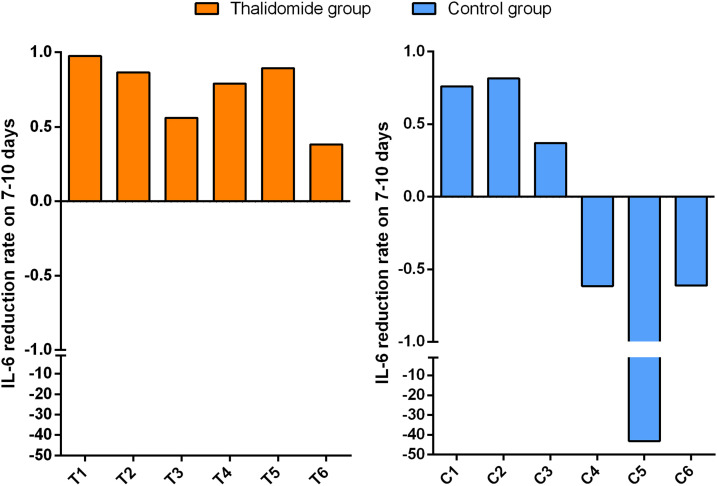
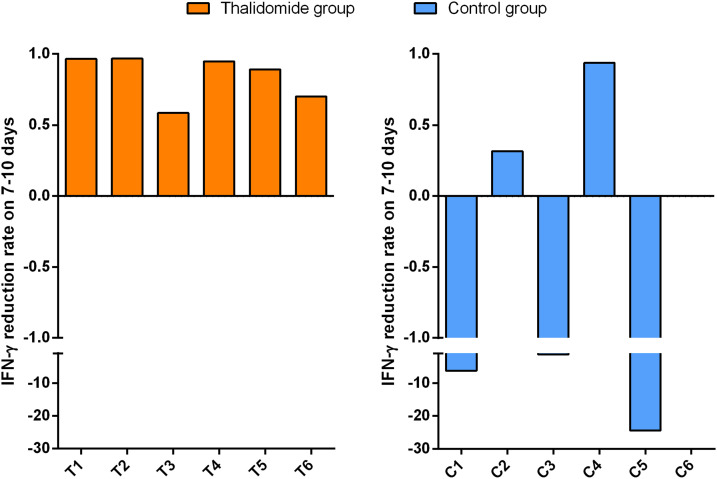
The median concentrations of serum IL-6 (30.92 vs 32.34, P = 0.173), IL-10 (5.84 vs 7.39, P = 0.600), and IFN-γ (2.19 vs 1.80, P = 0.172) of the patients taking part in the study were greater than the normal upper limit range (IL-6 < 3.00 pg/mL; IL-10 < 4.10 pg/mL; IFN-γ < 2.20 pg/mL) within 3 days of admission, but there were no significant differences when the 2 groups were compared. After treatment, the mean reduction rates at 7–10 days for IFN-γ and IL-6 serum concentrations were significantly greater in the thalidomide group (0.900 and 0.944, respectively) than in the controls (−11.5, P = 0.028; −0.783, P = 0.028; Figure 1 ). Changes in IL-6 and IFN-γ concentrations for each patient are shown in Supplementary Figures 1 and 2. However, no significant difference in the mean reduction rates at 7–10 days for serum IL-10 concentrations (0.662 vs −3.47, P = 0.249) was found in the 2 groups.
Figure 1.
Serum levels of inflammatory cytokines in COVID-19 patients treated with thalidomide or not.
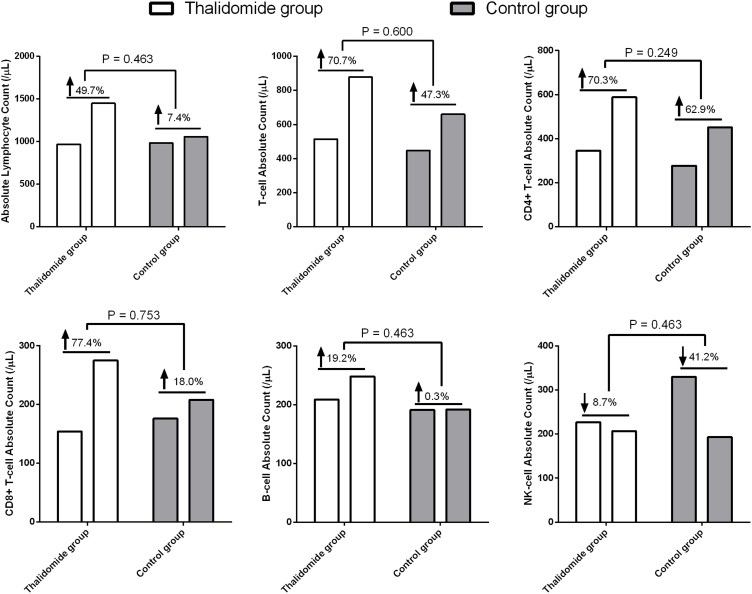
Lymphocytopenia occurred in 4 (66.7%) and 3 (50.0%) patients in the thalidomide and control (P = 0.558) groups, respectively. Although lymphocyte counts and lymphocyte subtypes recovered, there was no difference in the mean rising rate of the absolute lymphocyte count (0.497 vs 0.074, P = 0.463), T cells (0.707 vs 0.473, P = 0.600), CD4+ T cells (0.703 vs 0.629, P = 0.249), CD8+T (0.774 vs 0.180, P = 0.753), and B cells (0.192 vs 0.003, P = 0.463) (Figure 2 ).
Figure 2.
Lymphocyte counts in COVID-19 patients treated with thalidomide or not.
Adverse events
No severe AEs related to thalidomide therapy were detected and no secondary infections developed in the 2 groups.
Discussion
Thalidomide, with known actions as an immunomodulatory drug, has shown great promise for the treatment of autoimmune disorders. However, as far as the authors are aware there have been no reports for the treatment of acute severe inflammatory disease with thalidomide. Our study is the first to evaluate the effects of thalidomide on critically ill COVID-19 patients. The results revealed that thalidomide significantly accelerated the negative conversion of SARS-CoV-2 and shortened the hospital stay for affected patients. In addition, thalidomide reduced the requirement for mechanical ventilation in patients with critical COVID-19 infections. These findings indicated that combination therapy of thalidomide plus low dose glucocorticoid was effective in improving the prognosis of patients with this disease.
Neutrophilia increased serum inflammatory cytokine levels and lymphopenia, which is related to the cytokine ‘storm’ induced by severe viral infections, are thought to be linked to the disease severity including SARS-CoV and MERS-CoV infections in COVID-19 patients (Wang et al., 2020a, Ye et al., 2020). Thus, antiviral therapy is only the first-line treatment for COVID-19 patients, especially in critical cases. Alleviating the immune response would be the key factor in improving the prognosis of critically ill COVID-19 patients. Glucocorticoids have been widely used in the clinic to suppress immune responses and to inhibit inflammatory responses. For instance, glucocorticoids have been employed during the outbreaks of SARS-CoV and MERS-CoV to limit lung inflammation and suppress immune system responses. Unfortunately, many side effects are associated with this therapy including osteoporosis and secondary bacterial infections (Soo et al., 2004). Large doses of a glucocorticoid are not recommended to treat severe SARS-CoV-2 pneumonia because they inhibit the immune system and the clearance of pathogens (Russell et al., 2020). During the progression of critical COVID-19, many inflammatory cytokines are produced by overactive immune responses that can lead to lung injury and rapid progression of the disease. Therefore, there is an urgent need to develop an anti-inflammatory strategy to treat critically ill COVID-19 patients.
Thalidomide treatment of one patient in this study produced complete resolution of major inflammatory skin lesions. In the last decade or so, thalidomide has been prescribed to treat many autoimmune disorders because of its potent anti-inflammatory and immunomodulatory effects. A recent study showed that thalidomide could reduce injury to the lungs of mice infected with H1N1 influenza virus, improve survival, reduce inflammatory cell infiltration, and inhibit the production of cytokines (Zhu et al., 2014). A retrospective analysis concerning drug therapy of SARS patients found that the rational use of glucocorticoids (low or medium doses) could reduce the mortality of severely ill SARS patients and shorten hospital stays, without producing secondary infections or other complications. Therefore, short-term use of a low dose of glucocorticoid can be used in patients with COVID-19. Combined therapy with thalidomide and a glucocorticoid is considered to be a novel therapeutic strategy to limit dangerous responses of the immune system. In the present study, this novel therapeutic regimen was found to exert powerful anti-inflammatory effects. The data showed major reductions in serum concentrations of IL-6 and IFN-γ on days 7–10 of treatment in the thalidomide group and fewer patients required mechanical ventilation. Furthermore, the time periods of admission to a negative virus test and of hospital stay were less for the thalidomide group than for the control group.
Importantly, a massive cytokine storm, such as excessive production of IL-6, can drive intense cytokine production, which subsequently can lead to tissue damage and major organ failure (Teijaro et al., 2011). Levels of IL-6 are markedly increased in a chimeric antigen receptor T cell immunotherapy-induced cytokine storm (Norelli et al., 2018), which can be avoided by blocking the binding of IL-6 to its receptor (Neelapu et al., 2018). Thus, although some other underlying mechanisms may be involved, it is reasonable to suppose that thalidomide may improve the progression of critical COVID-19 through its effects on the cytokine milieu. Studies that have addressed this question also reveal an inhibitory action of thalidomide on the pro-inflammatory cytokines IL6 and type-I IFN via suppression of the TRIF/IRF3 pathway (Millrine et al., 2016a), Rabex-5 (Millrine et al., 2016b).
Lymphocytopenia is a common laboratory abnormality found in COVID-19 patients. In addition to reducing inflammatory responses by inhibiting the production of cytokines, thalidomide also plays an immunomodulatory role by regulating CD4+ T cells (Kim et al., 2017, Vergara et al., 2017). Therefore, the lymphocyte recovery rate was measured in the present study. Although there was no difference in the mean rising rate of lymphocyte counts and lymphocyte subtypes, the mean rising rates of lymphocytes in the thalidomide group were greater than in the control group. This apparent effect may be associated with insufficient cases in the 2 groups of patients. Further studies with a greater cohort of patients will be necessary to verify this action of thalidomide.
One possibility is that the therapeutic benefits of thalidomide on COVID-19 patients may be its anti-emetic and sedative properties, calming an anxious patient, thus reducing oxygen consumption and alleviating symptoms of digestive tract dysfunctions.
Of course, there are several limitations to the present study. First, it was a nonrandomized trial that analyzed a limited number of patients in a single hospital. It will be necessary to conduct a multi-center, prospective randomized controlled trial in a future study. Second, this was a retrospective study, so it requires us to further confirm the findings.
In summary, this study investigated the effects of thalidomide in combination with short-term low-dose glucocorticoid therapy to treat severe COVID-19 symptoms. The results established that this drug regimen had excellent efficacy and safety in reducing inflammation, inhibiting the production of inflammatory cytokines, and enhancing immunomodulation in seriously ill COVID-19 patients. However, additional studies are warranted to validate these effects.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
None.
Acknowledgements
Not applicable.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.12.023.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J.B., Dredge K., Dalgleish A.G. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- Bordoni V., Sacchi A., Cimini E., Notari S., Grassi G., Tartaglia E., et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa577. ciaa577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Kovalic A.J., Graber C.J. Prognostic value of leukocytosis and lymphopenia for coronavirus disease severity. Emerg Infect Dis. 2020;26(8):1839–1841. doi: 10.3201/eid2608.201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum J.A., Lameire N., Group K.A.G.W. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Lee J.G., Kim E.J.Y., Song S.H., Joo D.J., Huh K.H., et al. Enhanced immune-modulatory effects of thalidomide and dexamethasone co-treatment on T cell subsets. Immunology. 2017;152(4):628–637. doi: 10.1111/imm.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameire N., Kellum J.A., Group K.A.G.W. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2) Crit Care. 2013;17(1):205. doi: 10.1186/cc11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc R., Hideshima T., Catley L.P., Shringarpure R., Burger R., Mitsiades N., et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103(5):1787–1790. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- Li S., Jiang L., Li X., Lin F., Wang Y., Li B., et al. Clinical and pathological investigation of severe COVID-19 patients. JCI Insight. 2020 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millrine D., Miyata H., Tei M., Dubey P., Nyati K., Nakahama T., et al. Immunomodulatory drugs inhibit TLR4-induced type-1 interferon production independently of Cereblon via suppression of the TRIF/IRF3 pathway. Int Immunol. 2016;28(6):307–315. doi: 10.1093/intimm/dxw005. [DOI] [PubMed] [Google Scholar]
- Millrine D., Tei M., Gemechu Y., Kishimoto T. Rabex-5 is a lenalidomide target molecule that negatively regulates TLR-induced type 1 IFN production. Proc Natl Acad Sci U S A. 2016;113(38):10625–10630. doi: 10.1073/pnas.1611751113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller D.R., Wysocka M., Greenlee B.M., Ma X., Wahl L., Flockhart D.A., et al. Inhibition of IL-12 production by thalidomide. J Immunol. 1997;159(10):5157–5161. [PubMed] [Google Scholar]
- Moreira A.L., Sampaio E.P., Zmuidzinas A., Frindt P., Smith K.A., Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177(6):1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L., et al. Chimeric antigen receptor T-cell therapy–assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Smith L. The COVID-19 pandemic. Gerodontology. 2020;37(2):99. doi: 10.1111/ger.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K., et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F., et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara T.R.C., Samer S., Santos-Oliveira J.R., Giron L.B., Arif M.S., Silva-Freitas M.L., et al. Thalidomide is associated with increased T cell activation and inflammation in antiretroviral-naive HIV-infected Individuals in a randomised clinical trial of efficacy and safety. EBioMedicine. 2017;23:59–67. doi: 10.1016/j.ebiom.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinetz J.M. Lack of efficacy of hydroxychloroquine in covid-19. BMJ. 2020;369:m2018. doi: 10.1136/bmj.m2018. [DOI] [PubMed] [Google Scholar]
- Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J., et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020;80(5):e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Shi X., Ju D., Huang H., Wei W., Dong X. Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in mice. Inflammation. 2014;37(6):2091–2098. doi: 10.1007/s10753-014-9943-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.