Abstract
More than half of patients who recover from COVID-19 experience fatigue. We studied fatigue using neuropsychological and neurophysiological investigations in post-COVID-19 patients and healthy subjects. Neuropsychological assessment included: Fatigue Severity Scale (FSS), Fatigue Rating Scale, Beck Depression Inventory, Apathy Evaluation Scale, cognitive tests, and computerized tasks. Neurophysiological examination was assessed before (PRE) and 2 min after (POST) a 1-min fatiguing isometric pinching task and included: maximum compound muscle action potential (CMAP) amplitude in first dorsal interosseous muscle (FDI) following ulnar nerve stimulation, resting motor threshold, motor evoked potential (MEP) amplitude and silent period (SP) duration in right FDI following transcranial magnetic stimulation of the left motor cortex. Maximum pinch strength was measured. Perceived exertion was assessed with the Borg-Category-Ratio scale.
Patients manifested fatigue, apathy, executive deficits, impaired cognitive control, and reduction in global cognition. Perceived exertion was higher in patients. CMAP and MEP were smaller in patients both PRE and POST. CMAP did not change in either group from PRE to POST, while MEP amplitudes declined in controls POST. SP duration did not differ between groups PRE, increased in controls but decreased in patients POST. Patients' change of SP duration from PRE to POST was negatively correlated to FSS.
Abnormal SP shortening and lack of MEP depression concur with a reduction in post-exhaustion corticomotor inhibition, suggesting a possible GABAB-ergic dysfunction. This impairment might be related to the neuropsychological alterations.
COVID-19-associated inflammation might lead to GABAergic impairment, possibly representing the basis of fatigue and explaining apathy and executive deficits.
Keywords: COVID-19, Central fatigue, Peripheral fatigue, Dysexecutive syndrome, TMS
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CNS, Central Nervous System; CMAP, compound muscle action potential; FDI, first dorsal interosseous muscle; RMT, resting motor threshold; MEP, motor evoked potential; SP, silent period; CR100, Borg-Category-Ratio scale; TMS, Transcranial magnetic stimulation; FRS, Fatigue Rating Scale; CRP, C-reactive protein; IL-6, interleukine-6; HC, healthy control; FSS, Fatigue Severity Scale; BDI, Beck Depression Inventory; AES, Apathy Evaluation Scale; MoCA, Montreal Cognitive Assessment; FAB, Frontal Assessment Battery; RT, reaction time; VT, vigilance task; SIT, Stroop Interference Task; NV, Navon Task
Highlights
-
•
Neuropsychological and neurophysiological features of fatigue were studied in post-COVID-19 patients.
-
•
Apathy, deficits in executive functions and reduction in global cognition were found.
-
•
Abnormal shortening of cortical silent period and lack of MEP depression were demonstrated after a fatiguing task.
-
•
Disruption of post-exhaustion corticomotor inhibition suggests GABA-ergic dysfunction.
-
•
GABA-ergic impairment might subtend fatigue and executive deficits in COVID-19.
1. Introduction
A large number of patients who recover from the acute phase of coronavirus disease 2019 (COVID-19), caused by the novel “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2), manifest a plethora of long-lasting symptoms. Among them, a high proportion of individuals (53.1%) experience fatigue [1]. Fatigue is defined as a debilitating, non-transient feeling of physical and mental tiredness or exhaustion characterized by lack of energy, muscle weakness, slowed reactions, drowsiness, and deficit in concentration [[2], [3], [4]].
Prolonged fatigue after infections could be the consequence of biologic, behavioral, and environmental factors [5]. For decades, clinicians have referred to a controversial disorder historically defined as “post-viral fatigue syndrome” [6]. The main symptoms associated with this condition relate to muscle fatigability, aches, and pain. Nevertheless, the presence of central nervous system (CNS) abnormalities, including sleep disorders, depression, anxiety, and emotional lability, is also frequent [7]. Similar mechanisms can be envisioned for COVID-19, in which neurological, immunological and respiratory dysfunctions may finally cause fatigue [8]. Literature data converge on the assumption that fatigue is a multifaceted phenomenon with contributions of both cognitive and neuromuscular aspects.
Cognitive fatigue is defined as a decline in cognitive functioning, during sustained mental work [9]. The affected cognitive functions, overall named “cognitive control” [10], include vigilance, executive attention, working memory, judgment and long-term memory recall [9]. The feeling that people may experience during or after prolonged periods of cognitive and/or physical activity is called “mental fatigue”. Mental fatigue increases the perception of effort and worsens the performance during subsequent endurance exercise, despite it is not related to the capacity of the CNS to recruit muscles [11]. An imbalance between GABAergic and dopaminergic transmission has been postulated in “fatigue syndromes” [[12], [13], [14]]. Alterations in these neural circuits may partially account for both cognitive and mental fatigue [15].
Neuromuscular fatigue is an exercise-induced reduction in the ability of a muscle to generate force. Within certain limits, it is essential for protecting the body against damage due to excessive exercise. Neuromuscular fatigue is related with peripheral or central causes [16]. “Peripheral fatigue” depends on progressive failure of peripheral nervous system function, i.e., impaired impulse conduction along the nerve or at the neuromuscular junction, deterioration of muscle contractile properties [17,18]. “Central fatigue” is the progressive reduction in the ability of the CNS to maximally activate muscles and depends on spinal and supraspinal mechanisms. Supraspinal fatigue is, at least in part, characterized by reduced output from the motor cortex to the spinal motor neurons [19], which is due to reduced excitability of cortical motor neurons and to activation failure of structures upstream the primary motor cortex, e.g., premotor area and basal ganglia [20,21].
To date, no conclusive studies have characterized the presence of fatigue in patients with SARS-CoV-2-related neurological manifestations, who have recovered from COVID-19. Current literature lacks a neuropsychological characterization of this population and no neurophysiological studies have addressed whether fatigue is of central or peripheral origin. The present study aims to provide a comprehensive clinical, neurophysiological, and neuropsychological profile of fatigued patients suffering from neurological manifestations related to SARS-CoV-2, who recovered from the acute phase of COVID-19.
2. Materials and methods
2.1. Participants
Between April and May 2020, 12 patients (2 females; age 67 ± 9.6 years; 11 right-handers), who had recovered from the acute phase of COVID-19 (post-COVID-19 patients) and who complained of fatigue according to comprehensive medical assessment and anamnestic parameters, were enrolled in the study. Specifically, patients were asked to rate fatigue on a numeric-rating scale (Fatigue Rating Scale, FRS, 0: no fatigue; 10: extreme fatigue) [22].
All patients were hospitalized at the Department of Neurorehabilitation, Hospital of Vipiteno (Vipiteno-Sterzing, BZ, Italy), because of the development of neurological complications following SARS-CoV-2 infection (see Table 1 ).
Table 1.
Demographic, clinical, and laboratory data of COVID-19 patients.
| Patient |
Sex |
Age |
Education |
Diagnosis |
Clinical features at admission in neurorehabilitation |
Time from onset of COVID-19 |
IL-6 peak level |
CRP peak level |
|---|---|---|---|---|---|---|---|---|
| [years] | [years] | [weeks] | [pg/ml] (<7) |
[mg/l] (<0.8) |
||||
| 1 | M | 65 | 8 | CINM | Flaccid tetraparesis, muscle atrophy, areflexia; deep sensory disturbances in lower limbs | 11 | 401 | 18.7 |
| 2 | M | 60 | 11 | CINM | Flaccid tetraparesis, muscle atrophy, areflexia | 10 | 555 | 15.9 |
| 3 | M | 62 | 17 | CIN | Predominantly distal tetraparesis, hyporeflexia; anosmia | 11 | 225 | 17.1 |
| 4 | M | 71 | 8 | Encephalopathy | Severe cognitive impairment; dysphagia; anosmia | 9 | 635 | 25.2 |
| 5 | M | 79 | 13 | GBS (AIDP); mild cognitive impairment |
Predominantly distal tetraparesis, areflexia; mild superficial and deep sensory disturbances; deficit in attentional processes and impulse control; anosmia | 12 | 214 | 39.3 |
| 6 | F | 75 | 13 | Stroke (rMCA) | Left hemiparesis; left hemisensory loss; left hemispatial neglect | 12 | N/A | 22.4 |
| 7 | M | 48 | 8 | Myopathy | Limb-girdle muscle atrophy and paresis; mild myalgia | 13 | 6386 | 20.1 |
| 8 | M | 56 | 11 | Myopathy | Limb-girdle muscle atrophy and paresis; myalgia; anosmia, dysgeusia | 13 | 2418 | 34.2 |
| 9 | M | 70 | 17 | GBS (AMAN) | Predominantly distal tetraparesis, areflexia | 10 | 688 | 18.9 |
| 10 | F | 61 | 11 | Encephalopathy | Behavioral changes; primary insomnia, fatigue; anosmia | 12 | 271 | 25.7 |
| 11 | M | 77 | 8 | Myopathy | Limb-girdle muscle atrophy and paresis; myalgia | 13 | 1251 | 30.4 |
| 12 | M | 80 | 17 | Encephalopathy | Severe cognitive impairment; anosmia | 12 | 129 | 23.0 |
CINM, critical illness neuropathy and myopathy; CIN, critical illness neuropathy; GBS, Guillain-Barré syndrome; AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; rMCA, right middle cerebral artery; CRP, C-reactive protein; IL-6, interleukin 6.
All patients admitted to the ward of Neurorehabilitation met the World Health Organization criteria defining the state of recovery from COVID-19. Inclusion criteria were: a) almost total resolution of the neurological symptoms resulting from COVID-19, b) FRS score ≥ 6, arbitrarily, indicating an important level of fatigue c) absence of neurological disorders prior to COVID-19, d) absence of prior or current diagnosis of psychiatric, endocrine, metabolic or cardiopulmonary conditions related to fatigue, e) absence of dyspnoea or other long-lasting sequelae of interstitial COVID-19 pneumonia, f) absence of anaemia, g) no treatment with corticosteroids, antihistaminic, antihypertensive, diuretic, or hypnotic drugs at the time of study.
A common clinical feature characterizing our post-COVID-19 patients during the acute phase of the infection was the hyper-inflammatory state, as demonstrated by both markedly elevated C-reactive protein (CRP) and interleukine-6 (IL-6) serum levels. The study was conducted at the end of the rehabilitation period. Twelve age- and sex-matched healthy subjects served as controls (4 females; age 64.3 ± 10.5 years, p = 0.541 vs. patients; all right-handers).
2.1.1. Ethic statement
The study was approved by the local Ethics Committee (“Comitato Etico del Comprensorio Sanitario di Bolzano”) (65–2020) and was in accordance with the code of Ethics of the World Medical Association (Declaration of Helsinki, 1967). All participants signed an informed written consent form for the use of their clinical data for scientific purposes.
2.2. Neuropsychological assessment
2.2.1. Fatigue assessment
Fatigue was assessed in patients and healthy controls (HC) with FRS (see above) and Fatigue Severity Scale (FSS). The FSS consists of 9 sentences related to the interference of fatigue with certain activities and rates its perceived severity on a 7-point scale (1 = “strongly disagree”; 7 = “strongly agree”).
2.2.2. Neuropsychiatric assessment
To assess the participants' affective condition, we administered the Beck Depression Inventory (BDI) [23] and Apathy Evaluation Scale (AES) [24].
2.2.3. Cognitive assessment
All participants were tested in a laboratory setting, with constant artificial light and without auditory interference. Global cognition and executive functions were evaluated with the Montreal Cognitive Assessment (MoCA) [25,26] and the Frontal Assessment Battery (FAB) [27,28], respectively. For each test, we adjusted the total scores obtained from patients based on normative data validated for the Italian population.
2.2.4. Computerized attentive tasks
We assessed decrements in cognitive function arising during sustained mental work in a controlled laboratory experiment entailing computerized tasks designed for evaluating vigilance and executive attention. Participants underwent three computerized attentive tasks: Vigilance Task (VT), Stroop Interference Task (SIT), Navon Task (NT) [[29], [30], [31], [32], [33], [34]]. For details, see the Fig. 1 .
Fig. 1.
Computerized-attentive tasks.
2.3. Neurophysiological evaluation
Neuromuscular fatigue is typically assessed via sustained isometric maximal voluntary contraction [16]. We evaluated various neurophysiological parameters 10 min before (PRE) and 2 min after (POST) a 1-min fatiguing motor task.
2.3.1. Motor task and perceived exertion
We used a pinching task of 1 min duration, in which COVID-19 patients and HC were asked to squeeze a dynamometer (Jamar, Patterson Medical, UK) with their right thumb and index finger as strongly as possible. Participants were verbally encouraged to provide maximum contractions during the whole minute. We a priori decided to evaluate the dominant right hand in all patients, also in the only ambidextrous but predominantly left-handed patient, who however preferred to perform the task with their right hand.
During the task, participants sat comfortably on a chair with their arms adducted and elbow flexed at 90°. Maximum pinch strength (kg) obtained during 1 min was considered.
At the end of the sustained pinching task, participants were asked to report their level of perceived exertion using the Borg Category Ratio (CR100) scale [35] This scale ranges from 0 to 100 (0 = “nothing at all”; 100 = “extremely strong”). The number 100 implies an extremely strong perceptual intensity, i.e. the strongest effort and exertion a person has ever experienced.
2.3.2. Peripheral nerve stimulation to assess peripheral motor excitability
Peripheral fatigue can be assessed comparing pre-to-post exercise changes in compound muscle action potentials (CMAP or M-wave) evoked by supramaximal peripheral nerve stimulation in the relaxed muscle [36,37]. The CMAP expresses the neuromuscular propagation of action potentials along the sarcolemma [37,38] and indirectly indexes membrane excitability [39].
Here we stimulated the right ulnar nerve at the wrist using a bar electrode with an interelectrode distance of 3.5 cm. Stimuli of 0.2 ms duration were delivered with a constant current stimulator (DS7A; Digitimer Ltd., Welwyn Garden City, UK), controlled by Signal 6 software. CMAPs, were recorded from relaxed first dorsal interosseous muscle (FDI) on the dominant side with self-adhesive surface electrodes attached in a belly-tendon montage. The site of stimulation that produced the highest observable mechanical twitch and CMAP amplitude was determined. Stimuli were delivered in increments of 5–10 mA until obtaining a maximum response. The stimulation intensity was then increased to 130% to ensure supramaximal stimulation.
CMAP baseline-to-peak amplitudes (corresponding to the negative component) were measured.
2.3.3. TMS to assess central motor excitability
Transcranial magnetic stimulation (TMS) of the primary motor cortex (M1) allows recording the amplitude of motor evoked potentials (MEP), a measure of cortico-spinal excitability [40]. After a fatiguing isometric exercise, MEPs evoked in the resting target muscle are depressed for about half an hour [41]. In contrast, immediately after the end of exercise, MEPs are, for 1–2 min, larger than before contraction, a phenomenon termed post-contraction facilitation [42]. The cortical silent period (SP), the electromyographic silence following MEPs evoked in the tonically contracted target muscle, is increased after a fatiguing isometric muscle effort likely with the physiological purpose to reduce corticomotor output and prevent excessive peripheral exhaustion [[43], [44], [45], [46], [47]].
Here we recorded MEP from right FDI while participants were at rest, with arms relaxed, elbows flexed at 90 degrees, forearm and supinated hand lying on an armrest. Focal TMS of the hand area of left M1 was performed with a high-power Magstim 200 (Magstim Co., Whitland, UK), which delivers monophasic pulses. We used a 7 cm figure-of-eight coil, held over the optimum scalp position to elicit motor responses in FDI, with the induced current flowing in a posterior-anterior direction [48]. Optimum coil position was defined as the site where TMS consistently resulted in the largest MEP [48]. Intensities were expressed as percentage of maximum stimulator output (% MSO). Surface electromyography signals were band-pass filtered (3–3000 Hz) and amplified with a Digitimer D440-4 amplifier (Digitimer Ltd., Welwyn Garden City, UK). Single sweeps were digitized (sampling rate 10 kHz) and recorded on computer for later analysis using a CED 1401 A/D converter and Signal 6 software (Cambridge Electronic Design, Cambridge, UK).
Resting motor threshold (RMT) was established, defined as the minimum stimulus intensity (in % MSO) that produced a liminal MEP (>50 μV in 5 of 10 trials) at rest [48]. Five MEPs were recorded from relaxed FDI following single TMS pulses (5 s inter-stimulus interval) at 120% RMT intensity. Peak-to-peak amplitude was measured and averaged off-line for each participant.
Some 30 s after evoking MEPs at rest, we investigated SP duration by evoking five MEPs at 140% RMT in right FDI during sustained isometric contraction (thumb and index finger extended and pressed against each other) of self-estimated 50% maximum voluntary contraction. SP was defined as the time elapsing from the end of the MEP until the recurrence of voluntary tonic electromyographic activity [48]. In five single sweeps, SP was measured off-line, and the obtained values were averaged for each subject.
2.3.4. Sequence of tests
At baseline (PRE), we assessed CMAP amplitude, RMT, resting MEP amplitude, and SP in this order. Following the 1-min maximum pinching task (POST), we inquired Borg CR100 score, and again assessed CMAP amplitude, resting MEP amplitude, and SP in this order. POST/PRE ratios were calculated for CMAP amplitude, resting MEP amplitude, and SP.
2.4. Statistical analysis
Distribution of obtained data was assessed applying Kolmogorov-Smirnov testing. Not all data sets were normally distributed, and some data were ordinal, therefore we applied the more conservative non-parametric testing throughout. Data of patients were compared to those obtained in HC using Mann-Whitney-U tests. CMAP, MEP, and SP data were tested with repeated-measures-ANOVA using between-subjects factor GROUP (patients, controls) and within-subjects factor TIME (PRE, POST). Significant differences were followed up with Mann-Whitney-U test for independent variables (patients-controls), and with Wilcoxon test for paired dependent variables (PRE-/POST-data) within each subject group. Correlation analysis was performed with non-parametric Spearman-rho testing to account for the relatively small number of items. We analysed possible relations among 1) FRS, FSS, AES, BDI, and Borg CR100 score; 2) MoCA, FAB, and computerized tasks; and 3) MoCA and percent change in SP duration (POST/PRE %).
2.4.1. Data availability
The authors confirm that the data supporting the results of this study are saved at the Department of Neurorehabilitation, Hospital of Vipiteno (SABES-ASDAA), Vipiteno-Sterzing, Italy. They are available upon request from the corresponding author.
3. Results
All participants tolerated the procedures well and completed all parts of the study without difficulty. Three patients did not participate in the computerized tasks, FSS, BDI, and AES, because of their severe COVID-19-associated cognitive impairment.
Demographic and clinical data of patients are reported in Table 1. They did not differ significantly from HC in age and education (p > 0.3, each).
3.1. Neuropsychological assessment
3.1.1. Fatigue assessment
Both self-evaluation scales measuring perceived fatigue, FRS and FSS, revealed significantly higher scores in post-COVID-19 patients than in HC (p < 0.001 each).
3.1.2. Neuropsychiatric assessment
With regard to neuropsychiatric symptoms, both AES and BDI showed significantly higher scores in patients than in HC (p < 0.001 each).
AES scores correlated directly to BDI scores (ρ = 0.816, p = 0.026), while no other significant correlations emerged among self-evaluation tools (FSS, FRS).
3.1.3. Cognitive evaluation
With respect to global cognition, MoCA revealed a significantly poorer performance in patients compared to HC (p < 0.001). The group mean score in post-COVID-19 patients was only little above the cut-off score of 15.5/30 established as normative data in the Italian population [26]. Significantly smaller values in patients compared to HC were also obtained in the FAB (p < 0.001). Here the group mean score in post-COVID-19 patients was smaller than the cut-off score of 13.4/18 indicated in normative data of the Italian population [28].
3.1.4. Computerized tasks
RTs were significantly longer in COVID-19 patients than in HC in both SIT (p < 0.015) and NT (p < 0.046), while in VT the difference did not reach statistical significance (Table 2 ).
Table 2.
Comparison of COVID-19 patients and healthy controls. Values are group mean data (standard deviation in brackets). Significant differences (Mann-Whitney-U tests) are indicated in bold.
| Test | Patients | Controls | p-values |
|---|---|---|---|
| Fatigue Rating Scale (FRS) | 8.1 (1.7) | 0.7 (0.5) | < 0.001 |
| Fatigue Severity Scale (FSS) | 31.6 (10.8) | 9.5 (0.5) | < 0.001 |
| Apathy Evaluation Scale (AES) | 39.3 (13.7) | 18.9 (1.0) | < 0.001 |
| Beck Depression Inventory (BDI) | 3.8 (2.9) | 0.0 (0.0) | < 0.001 |
| Montreal Cognitive Assessment (MoCA) | 17.8 (5.3) | 26.8 (3.1) | < 0.001 |
| Frontal Assessment Battery (FAB) | 12.3 (2.3) | 16.7 (1.2) | < 0.001 |
| RT in Vigilance Task (VT) | 341.3 (86.3) | 308.8 (44.2) | 0.541 |
| Percentage of errors in VT | 3.2 (1.0) | 0.9 (0.2) | < 0.001 |
| RT in Stroop Interference Task (SIT) | 969.4 (152.1) | 802.1 (122.0) | 0.015 |
| Percentage of errors in SIT | 4.6 (0.8) | 1.2 (0.3) | < 0.001 |
| RT in Navon Task (NT) | 1327.1 (525.3) | 850.3 (144.2) | 0.046 |
| Percentage of errors in NT | 3.8 (1.2) | 1.2 (0.3) | < 0.001 |
| Force in pinch task (kg) | 5.6 (1.9) | 7.3 (2.5) | 0.101 |
| Exertion (Borg CR100) | 75.8 (15.6) | 54.6 (9.0) | 0.001 |
| CMAP amplitude PRE (mV) | 9.4 (3.8) | 15.7 (3.6) | < 0.001 |
| CMAP amplitude POST (mV) | 9.2 (3.7) | 15.4 (3.8) | 0.001 |
| CMAP amplitude POST/PRE % | 97.5 (4.1) | 98.5 (10.5) | 0.089 |
| RMT (% MSO) | 44.6 (5.6) | 43.1 (4.8) | 0.713 |
| MEP amplitude PRE (mV) | 0.8 (0.5) | 1.9 (1.1) | 0.005 |
| MEP amplitude POST (mV) | 0.7 (0.3) | 1.3 (0.8) | 0.017 |
| MEP amplitude POST/PRE % | 90.4 (28.1) | 72.9 (20.2) | 0.242 |
| SP duration PRE (ms) | 89.7 (32.3) | 72.4 (25.5) | 0.242 |
| SP duration POST (ms) | 72.0 (33.2) | 93.5 (21.0) | 0.052 |
| SP duration POST/PRE % | 78.5 (17.0) | 138.8 (35.8) | < 0.001 |
Abbreviations: RT, reaction time; CR100, Borg Category Ratio 100 scale; CMAP, compound muscle action potential; RMT, resting motor threshold; MEP, motor evoked potential; SP, silent period.
Percentage of errors was significantly larger in patients than HC in all three computerized tasks (all p < 0.001).
RTs of all three computerized tasks correlated negatively to MoCA scores (VT: ρ = −0.710, p = 0.032; SIT: ρ = −0.728, p = 0.026; NT: ρ = −0.862, p = 0.003), while FAB scores correlated indirectly to RTs of the computerized tasks evaluating executive attention (SIT: ρ = −0.750, p = 0.020; NT: ρ = −0.700, p = 0.036).
3.2. Neurophysiological evaluation
3.2.1. Motor task and perceived exertion
Maximum group mean force in the pinching task tended to be higher in HC as compared to post-COVID-19 patients without reaching statistical significance (p = 0.101). The perceived exertion, however, expressed as Borg CR100 score, was significantly higher (range 50–100%, mean value 75.8) in post-COVID-19 patients compared to HC (range 40–70%, mean value 54.6) (p < 0.001).
3.2.2. Peripheral nerve stimulation to assess peripheral motor excitability
Repeated-measures ANOVA revealed a significant main effect on CMAP amplitude of GROUP (F1,22 = 15.776; p = 0.001; η p 2 = 0.418), but no significant effect of TIME (F1,22 = 0.827; p = 0.373; η p 2 = 0.036) nor of the interaction TIME × GROUP (F1,22 = 0.001; p = 0.974; η p 2 = 0.000). CMAP baseline-peak amplitude was significantly smaller in patients compared to HC in both PRE and POST conditions (Table 2), and did not change significantly from PRE to POST conditions in either group, i.e., CMAP was not modified by the fatiguing task (patients: p = 0.099; HC: p = 0.409). Thus, percentage change in CMAP amplitude (POST/PRE %) did not differ significantly between groups (Table 2).
3.2.3. TMS to assess central motor excitability
RMT did not differ significantly between COVID-19 patients and HC. Repeated-measures ANOVA revealed a significant main effect on MEP amplitude of GROUP (F1,22 = 8.722; p = 0.007; η p 2 = 0.284) and of TIME (F1,22 = 9.910; p = 0.005; η p 2 = 0.311), but no interaction TIME × GROUP (F1,22 = 2.827; p = 0.107; η p 2 = 0.114). Peak-to-peak amplitudes of resting MEP were significantly smaller in patients compared to HC in both PRE and POST conditions (Table 2). After the fatiguing exercise, the decline in MEP amplitude did not reach statistical significance in COVID-19 patients (p = 0.108) but was significant in HC (p = 0.003). Percentage change in MEP amplitude (POST/PRE %) did not differ significantly between groups (Table 2), likely because of the large variance of independent variables in either group.
Repeated-measures ANOVA revealed no significant main effect on SP duration of GROUP (F1,22 = 0.032; p = 0.860; η p 2 = 0.001) nor of TIME (F1,22 = 0.523; p = 0.477; η p 2 = 0.023), but revealed a notable significant interaction TIME × GROUP (F1,22 = 66.812; p = 0.000; η p 2 = 0.752). At baseline, SP duration did not differ significantly between COVID-19 patients and HC (Table 2). The fatiguing pinching task caused the expected significant SP lengthening in HC (p = 0.002), while it led to significant shortening of the SP in COVID-19 patients (p = 0.004). Thus, percent change in SP duration (POST/PRE %) differed significantly between post-COVID-19 patients and HC (Table 2). SP POST/PRE % correlated negatively to FSS (ρ = −0.711, p = 0.032).
For comprehensive neurophysiological results overview see supplementary Table 1.
4. Discussion
The present study presents evidence for abnormal neuromuscular fatigue, cognitive fatigue, apathy, and executive dysfunction in a sample of post-COVID-19 patients.
Our data demonstrate a significant impact of SARS-CoV-2 infection on both feeling of fatigue and exhaustion. We administered the multidimensional FSS for a gross, non-specific evaluation of the feeling of fatigue in daily life, while the CR100 was adopted for evaluating the perceived effort immediately following a physical engagement. Finally, the FRS allowed us to obtain a quantification of the patients' perceived fatigue at the moment of the clinical assessment. As a common denominator, the scores provided by these instruments express how people, subjectively, feel and perceive fatigue. In line with previous evidence [1,49], the results from these scales show that post-COVID-19 patients perceive physical exhaustion, and experience sense of tiredness and lack of energy affecting their daily living.
Among the twelve reported patients, eight presented the clinical sequelae of acute neuromuscular affections (e.g., critical illness neuropathy and myopathy, Guillain-Barré syndrome, see Table 1) and therefore, they were prone to abnormal fatigability linked, at least in part, to the peripheral neuromuscular dysfunction [50]. However, they shared, with other patients, clear aspects of cognitive and motivational dysregulation.
COVID-19 impacts negatively on motivational aspects. AES scores were found to be higher in patients compared to HC. Apathy is a disorder associated with the disruption of the frontal-subcortical circuit involved in the generation of motivation [51].
BDI scores were significantly higher in post-COVID-19 patients than in HC. However, no patient had evidence of major depression, and only five reported BDI scores compatible with minor depression. Fatigue and apathy have been closely related to affective disorders, including mood disturbances [52,53]. Our data showed no correlations of fatigue to depressive symptoms, nor to apathy. In contrast, a direct correlation was found between apathy and depressive symptoms. All these symptoms could be observed in neurological disorders of different aetiologies, including neurodegenerative and inflammatory conditions [[54], [55], [56], [57]]. It is also noteworthy that a significant proportion of patients suffering from psychiatric and neurological disorders exhibit a chronic, low-grade inflammation [[58], [59], [60], [61]].
Our post-COVID-19 patients manifested a hyper-inflammatory state during the acute phase of COVID-19, as demonstrated by the marked elevation of their serum IL-6 levels. IL-6 relate hyper-inflammation is considered playing a role in COVID-19 pathogenesis [62] and has been associated with central and peripheral nervous system complications: altered mental status, psychosis, affective disorders, neurocognitive disorders (dementia-like), headache, encephalitis, myelitis, stroke, myopathy and/or myositis, Guillain-Barré like syndrome (and its variants) and mono- or multineuritis) [[63], [64], [65], [66]].
Based on neuropsychological data, post-COVID-19 patients presented with cognitive deficits, particularly in the executive domain, in comparison with HC. MoCA scores were on average borderline compared to the Italian normative data cut-off [26], but they were lower than in the control group, concurring with a reduction in global cognition following COVID-19 with respect to HC. Moreover, three post-COVID-19 patients developed such a severe cognitive impairment that they were unable to participate in the computerized tests. In line with previous data [67], the abnormally low FAB scores we found in more than half of our post-COVID-19 patients clearly demonstrate evidence of a dysexecutive syndrome. The neuropsychological pattern we found, which is characterized by both dysexecutive syndrome and dysregulation of certain emotional-motivational aspects, often anticipates the development of dementia in patients suffering from neuroinflammatory and neurodegenerative diseases [68,69].
The coexistence of executive impairments and abnormal fatigue in post-COVID-19 patients is further supported by the performance in computerized tasks, which were implemented to evaluate the executive components of attention and the impact of fatigue on cognitive control [10]. The inferior performance in these tasks suggests diminished executive attention and cognitive control in post-COVID-19 patients compared to HC. Certainly, while a reduced executive attention could be the expression of the dysexecutive syndrome, the deficits in cognitive control relate to cognitive fatigue. Indeed, cognitive control tends to decrease when a subject undergoes a cognitively demanding task for a long time: this condition leads to increased distractibility, reducing the subject's capabilities to monitor the performance [[70], [71], [72]]. The impairment in executive attention emerges from RTs in both SIT and NT, which were significantly longer in post COVID-19 patients than in HC. NT and SIT evaluate the ability to inhibit inappropriate or irrelevant responses, to monitor conflicts, and to evaluate stimuli or resource allocation [73]. RTs in VT did not differ significantly between patients and HC. However, a low accuracy of performance, i.e. the percentage of errors, in all computerized tasks, leads to conclude for a decrease of cognitive control, which probably does not only depend on the dysexecutive syndrome, but is also related to fatigue.
We explored neurophysiological correlates of physical fatigue by studying the effects of a fatiguing isometric maximal muscle contraction on excitability measures of peripheral nerve and motor cortex [16]. During a fatiguing task, the CNS processes the level of perceived exertion at the primary somatosensory cortex. Sensorimotor integration modulates the activation of M1 and its corticospinal output. These mechanisms regulate the work rate and the need for rest [16]. However, this model does not explain why fatigue can be present at rest. In patients with “abnormal” fatigue, the amplified sense of fatigue might be due to pathological changes in the motor system, disruption of feedback to the primary somatosensory cortex and/or changes in patients' motivation.
Post-COVID-19 patients perceived the sustained pinching task as much more fatiguing as compared to HC (as demonstrated by higher scores in the CR100), despite lower force production.
Patients showed a post-exercise decline neither in CMAPs nor in MEPs amplitude. This may be due to smaller amplitudes at baseline, which may have made further depression, both of CMAPs and MEPs, less likely after sustained muscular effort. However, CMAPs did neither decline in patients nor in HC, which may indicate that we were not able to detect a significant peripheral failure after the fatiguing task with the chosen neurophysiological measure. Conversely, the lack of post-exercise depression of MEPs in patients only could be attributed, as an alternative hypothesis, to altered corticomotor excitability changes after fatiguing muscle contraction. Conceptually, this data bonds well with the abnormal post-exhaustion reduction of SP duration in patients, while in HC SP was prolonged, in line with previous data [[74], [75], [76], [77], [78], [79]].
Unlike spinal inhibitory circuitry, the cortical part of the SP is dependent on the integrity of GABAB-ergic circuits [80]. SP duration did not differ significantly between patients and HC at baseline, before the fatiguing pinching task. This result indicates that “under normal circumstances” the tonic activity of GABAB-ergic neurons within M1 exhibits a normal function. The fatiguing motor task, however, unmasked a reduced inhibition of corticomotor neurons in post-COVID-19 patients.
This phenomenon could be interpreted as a compensatory attempt of the central motor circuits to counteract a reduced peripheral capacity to generate force, and could thus be related to the already low pre-exercise mean MEP amplitudes in the patient group.
More intriguingly, the shortened cortical SP and the lack of MEP decline after the fatiguing exercise express an altered central functioning of sensory-motor circuits in controlling the muscle workload. The negative correlation of POST/PRE percentage change of SP duration with FSS in patients concurs with this interpretation. Interestingly, lack of post-exercise depression of MEPs was also observed in multiple sclerosis patients with chronic fatigue [22].
In our sample of patients, the reduced activity of intracortical GABAergic circuits, reflected in post-exercise shortening of SP, was previously demonstrated by means of paired-pulse TMS techniques (data submitted). Patients presented, as compared to HC, markedly reduced short-interval intracortical inhibition (SICI), and disruption of long-interval intracortical inhibition (LICI) assessed in the FDI at rest. SICI is thought to represent GABAA-receptor-mediated fast inhibitory post-synaptic potentials (IPSPs) in corticospinal neurons [81] and LICI is considered to be dependent on slow IPSPs mediated through GABAB-receptors [81]. Moreover, short-latency afferent inhibition (SAI) was slightly diminished in these patients. SAI evaluates motor cortex inhibition induced by sensory afferents (through inhibitory connections from the primary somatosensory cortex to M1). SAI is modulated by excitatory cholinergic thalamocortical projections to the inhibitory GABAergic network in M1 and is reduced by muscarinic and GABAA agonist administration [81]. SAI was decreased during repetitive non-fatiguing movements inducing MEP depression [82] and was significantly activated during cognitive tasks [83]. Taken together, these findings point out to a general reduction of cortical GABAergic and - to a lesser extent - cholinergic activity in COVID-19 patients. This could underlie both the reduced cognition and the abnormal fatigue perception and could represent one of the possible mechanisms of COVID-19-related neurotoxicity.
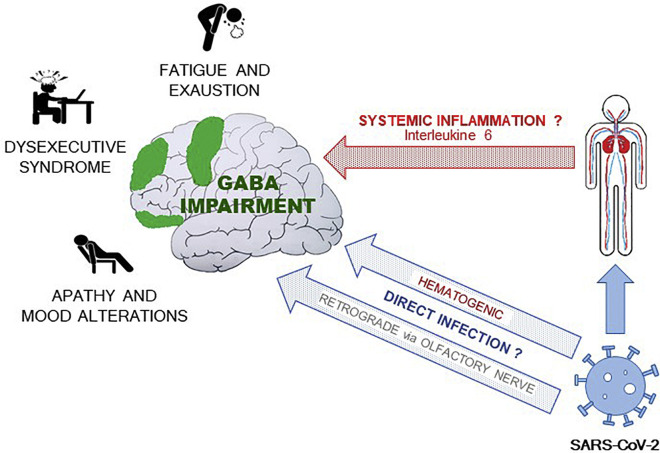
Previous studies from animal models suggest that IL-6 hyperinflammatory-induced state may decrease the density of functional GABA receptors and shifts the balance between synaptic inhibition and excitation [84]. This imbalance could be responsible for alterations of neurophysiological responses [85] and for the misprocessing of information that largely regulate emotionally salient information and cognitive functions [86,87]. Neuroinflammation may induce central GABAergic impairment, representing a common denominator for neuromotor and cognitive fatigue, executive deficits, and apathy in post-COVID-19 patients (see Fig. 2 ).
Fig. 2.
Direct and indirect hyper-inflammatory-inducing mechanisms driven by SARS-CoV-2 infection. The ensuing GABAergic impairment explains the neuropsychological and neuromotor features of patients (see the text).
It is conceivable that fatigue and stress-related exhaustion may be of major importance for the reduced cognitive performance in these patients [88].
This study has some limitations that need to be acknowledged. First, we did not follow-up our patients during a prolonged period and are unable to predict, whether or not the described disturbances tend to disappear over time or to become chronic. Further, we did not explore fatigue either in a sample of post COVID-19 patients who did not sustain neurological (especially neuromuscular) complications or in a control group of patients with similar neurological affections unrelated to COVID-19. This would have made our findings on fatigue and cognitive dysfunction related directly to COVID-19 after-effects. Further studies will broaden the initial knowledge resulting from the present study.
5. Conclusions
This is the first study linking neuropsychological with neurophysiological data in a sample of post-COVID-19 patients with neurological complication. We demonstrated the presence of central neuromotor and cognitive fatigue, apathy, and executive dysfunction. A cortical impairment of GABAergic neurotransmission could underlie these findings. It needs to be investigated whether such a mechanism may also be present in other post-viral chronic fatigue syndromes.
The following is the supplementary data related to this article.
Neurophysiological data in COVID-19 patients and healthy controls.
Funding
This study was not supported by any fund or grant.
Declaration of Competing Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We would like to thank all our patients who participated in this study and their families.
References
- 1.Carfi A., Bernabei R., Landi F., C.-P.-A.C.S.G. Gemelli Against Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagberg M. Muscular endurance and surface electromyogram in isometric and dynamic exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981;51(1):1–7. doi: 10.1152/jappl.1981.51.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Marcora S.M., Staiano W., Manning V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009;106(3):857–864. doi: 10.1152/japplphysiol.91324.2008. (1985) [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri A., Behan P.O. Fatigue in neurological disorders. Lancet. 2004;363(9413):978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 5.Katz B.Z., Collin S.M., Murphy G., Moss-Morris R., Wyller V.B., Wensaas K.A., Hautvast J.L.A., Bleeker-Rovers C.P., Vollmer-Conna U., Buchwald D., Taylor R., Little P., Crawley E., White P.D., Lloyd A. The international collaborative on fatigue following infection (COFFI) Fatigue. 2018;6(2):106–121. doi: 10.1080/21641846.2018.1426086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David A.S., Wessely S., Pelosi A.J. Postviral fatigue syndrome: time for a new approach. Br. Med. J. (Clin. Res. Ed.) 1988;296(6623):696–699. doi: 10.1136/bmj.296.6623.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behan P.O., Behan W.M.H., Bell E.J. The postviral fatigue syndrome — an analysis of the findings in 50 cases. J. Inf. Secur. 1985;10(3):211–222. doi: 10.1016/s0163-4453(85)92488-0. [DOI] [PubMed] [Google Scholar]
- 8.Oliviero A., de Castro F., Coperchini F., Chiovato L., Rotondi M. COVID-19 pulmonary and olfactory dysfunctions: is the chemokine CXCL10 the common denominator? Neuroscientist. 2020:1–8. doi: 10.1177/1073858420939033. (Epub ahead of print, PMID: 32659199) [DOI] [PubMed] [Google Scholar]
- 9.Trejo L., Kochavi R., Kubitz K., Montgomery L.D., Rosipal R., Matthews B. SPIE Defense + Commercial Sensing. 2005. Measures and models for predicting cognitive fatigue. [Google Scholar]
- 10.Ridderinkhof K.R., van den Wildenberg W.P., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Pageaux B., Marcora S.M., Rozand V., Lepers R. Mental fatigue induced by prolonged self-regulation does not exacerbate central fatigue during subsequent whole-body endurance exercise. Front. Hum. Neurosci. 2015;9:67. doi: 10.3389/fnhum.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno R.L., Creange S.J., Frick N.M. Parallels between post-polio fatigue and chronic fatigue syndrome: a common pathophysiology? Am. J. Med. 1998;105(3):66S–73S. doi: 10.1016/s0002-9343(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 13.Dobryakova E., Genova H.M., DeLuca J., Wylie G.R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front. Neurol. 2015;6:52. doi: 10.3389/fneur.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomares F.B., Roy S., Funck T., Feier N.A., Thiel A., Fitzcharles M.-A., Schweinhardt P. Upregulation of cortical GABAA receptor concentration in fibromyalgia. PAIN. 2020;161(1):74–82. doi: 10.1097/j.pain.0000000000001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boksem M.A., Tops M. Mental fatigue: costs and benefits. Brain Res. Rev. 2008;59(1):125–139. doi: 10.1016/j.brainresrev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Gandevia S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 17.Bigland-Ritchie B., Woods J.J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7(9):691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- 18.Allen D.G., Lamb G.D., Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 19.Brasil-Neto J.P., Pascual-Leone A., Valls-Solé J., Cammarota A., Cohen L.G., Hallett M. Postexercise depression of motor evoked potentials: a measure of central nervous system fatigue. Exp. Brain Res. 1993;93(1):181–184. doi: 10.1007/BF00227794. [DOI] [PubMed] [Google Scholar]
- 20.Taylor J.L., Butler J.E., Allen G.M., Gandevia S.C. Changes in motor cortical excitability during human muscle fatigue. J. Physiol. 1996;490(Pt 2):519–528. doi: 10.1113/jphysiol.1996.sp021163. (Pt 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri A., Behan P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000;179(S 1–2):34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 22.Mordillo-Mateos L., Soto-Leon V., Torres-Pareja M., Peinado-Palomino D., Mendoza-Laiz N., Alonso-Bonilla C., Dileone M., Rotondi M., Aguilar J., Oliviero A. Fatigue in multiple sclerosis: general and perceived fatigue does not depend on corticospinal tract dysfunction. Front. Neurol. 2019;10:339. doi: 10.3389/fneur.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 24.Marin R.S., Biedrzycki R.C., Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Santangelo G., Siciliano M., Pedone R., Vitale C., Falco F., Bisogno R., Siano P., Barone P., Grossi D., Santangelo F., Trojano L. Normative data for the montreal cognitive assessment in an Italian population sample. Neurol. Sci. 2015;36(4):585–591. doi: 10.1007/s10072-014-1995-y. [DOI] [PubMed] [Google Scholar]
- 27.Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 11, 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 28.Appollonio I., Leone M., Isella V., Piamarta F., Consoli T., Villa M.L., Forapani E., Russo A., Nichelli P. The frontal assessment battery (FAB): normative values in an Italian population sample. Neurol. Sci. 2005;26(2):108–116. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 29.Ridderinkhof K.R., van der Molen M.W. Mental resources, processing speed, and inhibitory control: a developmental perspective. Biol Psychol. 1997;45:241–261. doi: 10.1016/s0301-0511(96)05230-1. [DOI] [PubMed] [Google Scholar]
- 30.Scarpina F., Tagini S. The stroop color and word test. Front. Psychol. 2017;8:557. doi: 10.3389/fpsyg.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caffarra P., Vezzadini G., Francesca D., Zonato F., Venneri A. A short version of the Stroop test: normative data in an Italian population sample. Nuova Rivista Neurol. 2002;12:111–115. [Google Scholar]
- 32.Navon D. Forest before trees: the precedence of global features in visual perception. Cogn. Psychol. 1977;9(3):353. [Google Scholar]
- 33.Ratcliff R. Methods for dealing with reaction time outliers. Psychol. Bull. 1993;114(3):510. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- 34.Ratcliff R., Hans P.A.V.D. Diffusion model for one-choice reaction-time tasks and the cognitive effects of sleep deprivation. Proceed. Nat. Acad. Sci. PNAS. 27, 2011;108:11285–11290. doi: 10.1073/pnas.1100483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borg E., Borg G. A comparison of AME and CR100 for scaling perceived exertion. Acta Psychol. 2002;109(2):157–175. doi: 10.1016/s0001-6918(01)00055-5. [DOI] [PubMed] [Google Scholar]
- 36.Millet G.Y., Martin V., Martin A., Vergès S. Electrical stimulation for testing neuromuscular function: from sport to pathology. Eur. J. Appl. Physiol. 10, 2011;111:2489–2500. doi: 10.1007/s00421-011-1996-y. [DOI] [PubMed] [Google Scholar]
- 37.Millet G.Y., Bachasson D., Temesi J., Wuyam B., Féasson L., Vergès S., Lévy P. Potential interests and limits of magnetic and electrical stimulation techniques to assess neuromuscular fatigue. Neuromuscul. Disord. 2012;22(Suppl. 3):S181–S186. doi: 10.1016/j.nmd.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Enoka R.M., Stuart D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992;72(5):1631–1648. doi: 10.1152/jappl.1992.72.5.1631. (1985) [DOI] [PubMed] [Google Scholar]
- 39.Milner-Brown H.S., Miller R.G. Muscle membrane excitation and impulse propagation velocity are reduced during muscle fatigue. Muscle Nerve. 1986;9(4):367–374. doi: 10.1002/mus.880090415. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell J.C., Thompson P.D., Day B.L., Boyd S., Marsden C.D. Stimulation of the human motor cortex through the scalp. Exp. Physiol. 1991;76(2):159. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- 41.Brasil-Neto J.P., Cohen L.G., Hallett M. Central fatigue as revealed by postexercise decrement of motor evoked potentials. Muscle Nerve. 1994;17(7):713–719. doi: 10.1002/mus.880170702. [DOI] [PubMed] [Google Scholar]
- 42.Mathis J., Gurfinkel V.S., Struppler A. Facilitation of motor evoked potentials by postcontraction response (Kohnstamm phenomenon) Electroencephalogr. Clin. Neurophysiol./ Electromyogr. Motor Control. 1996;101(4):289–297. doi: 10.1016/0924-980x(96)95599-x. [DOI] [PubMed] [Google Scholar]
- 43.Mills K.R., Thomson C.C.B. Human muscle fatigue investigated by transcranial magnetic stimulation. Neuroreport. 15, 1995;6:1966–1968. doi: 10.1097/00001756-199510010-00004. [DOI] [PubMed] [Google Scholar]
- 44.McKay W.B., Stokic D.S., Sherwood A.M., Vrbova G., Dimitrijevic M.R. Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle Nerve. 1996;19(8):1017–1024. doi: 10.1002/mus.880190803. [DOI] [PubMed] [Google Scholar]
- 45.Taylor J.L., Butler J.E., Gandevia S.C. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp. Brain Res. 1999;127(1):108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- 46.Hilty L., Lutz K., Maurer K., Rodenkirch T., Spengler C.M., Boutellier U., Jäncke L., Amann M. Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp. Physiol. 2011;96(5):505–517. doi: 10.1113/expphysiol.2010.056226. [DOI] [PubMed] [Google Scholar]
- 47.Arias P., Robles-García V., Corral-Bergantiños Y., Madrid A., Espinosa N., Valls-Solé J., Grieve K.L., Oliviero A., Cudeiro J. Central fatigue induced by short-lasting finger tapping and isometric tasks: a study of silent periods evoked at spinal and supraspinal levels. Neuroscience. 2015;305:316–327. doi: 10.1016/j.neuroscience.2015.07.081. [DOI] [PubMed] [Google Scholar]
- 48.Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R., Di Lazzaro V., Ferreri F., Fitzgerald P.B., George M.S., Hallett M., Lefaucheur J.P., Langguth B., Matsumoto H., Miniussi C., Nitsche M.A., Pascual-Leone A., Paulus W., Rossi S., Rothwell J.C., Siebner H.R., Ugawa Y., Walsh V., Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goërtz Y.M.J., Van Herck M., Delbressine J.M., Vaes A.W., Meys R., Machado F.V.C., Houben-Wilke S., Burtin C., Posthuma R., Franssen F.M.E., van Loon N., Hajian B., Spies Y., Vijlbrief H., Van’t Hul A.J., Janssen D.J.A., Spruit M.A. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ. Open. Res. 2020;6(4) doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schillings M.L., Kalkman J.S., Janssen H.M., van Engelen B.G., Bleijenberg G., Zwarts M.J. Experienced and physiological fatigue in neuromuscular disorders. Clin. Neurophysiol. 2007;118(2):292–300. doi: 10.1016/j.clinph.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Bonelli R.M., Cummings J.L. Frontal-subcortical circuitry and behavior. Dialogues Clin. Neurosci. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demyttenaere K., De Fruyt J., Stahl S.M. The many faces of fatigue in major depressive disorder. Int. J. Neuropsychopharmacol. 2005;8(1):93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- 53.Husain M., Roiser J.P. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat. Rev. Neurosci. 2018;19(8):470–484. doi: 10.1038/s41583-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 54.Braley T.J., Chervin R.D. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep. 2010;33(8):1061–1067. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman J.H., Beck J.C., Chou K.L., Clark G., Fagundes C.P., Goetz C.G., Herlofson K., Kluger B., Krupp L.B., Lang A.E., Lou J.S., Marsh L., Newbould A., Weintraub D. Fatigue in Parkinson's disease: report from a mutidisciplinary symposium. NPJ. Parkinsons. Dis. 2016;2 doi: 10.1038/npjparkd.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cumming T.B., Packer M., Kramer S.F., English C. The prevalence of fatigue after stroke: a systematic review and meta-analysis. Int. J. Stroke. 2016;11(9):968–977. doi: 10.1177/1747493016669861. [DOI] [PubMed] [Google Scholar]
- 57.Cudeiro-Blanco J., Onate-Figuérez A., Soto-León V., Avendaño-Coy J., Mordillo-Mateos L., Brocalero-Camacho A., Esclarin-Ruz A., Rotondi M., Aguilar J., Arias P., Oliviero A. Prevalence of fatigue and associated factors in a spinal cord injury population: data from an internet-based and face-to-face surveys. J. Neurotrauma. 15, 2017;34:2335–2341. doi: 10.1089/neu.2016.4950. [DOI] [PubMed] [Google Scholar]
- 58.Barbosa I.G., Bauer M.E., Machado-Vieira R., Teixeira A.L. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural. Plast. 2014;2014:360481. doi: 10.1155/2014/360481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felger J.C., Treadway M.T. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42(1):216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 12, 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides tocilizumab? SGP130Fc. Cytokine X. 2020;2(2):100029. doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filosto M., Piccinelli S. Cotti, Gazzina S., Foresti C., Frigeni B., Servalli M.C., Sessa M., Cosentino G., Marchioni E., Ravaglia S., Briani C., Castellani F., Zara G., Bianchi F., Del Carro U., Fazio R., Filippi M., Magni E., Natalini G., Palmerini F., Perotti A.M., Bellomo A., Osio M., Scopelliti G., Carpo M., Rasera A., Squintani G., Doneddu P.E., Bertasi V., Cotelli M.S., Bertolasi L., Fabrizi G.M., Ferrari S., Ranieri F., Caprioli F., Grappa E., Broglio L., De Maria G., Leggio U., Poli L., Rasulo F., Latronico N., Nobile-Orazio E., Padovani A., Uncini A. Guillain-Barré syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. J. Neurol. Neurosurg. Psychiatry. 2020:1–6. doi: 10.1136/jnnp-2020-324837. [DOI] [PubMed] [Google Scholar]
- 64.Koralnik I.J., Tyler K.L. COVID-19: a global threat to the nervous system. Ann. Neurol. 2020;88(1):1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romero-Sanchez C.M., Diaz-Maroto I., Fernandez-Diaz E., Sanchez-Larsen A., Layos-Romero A., Garcia-Garcia J., Gonzalez E., Redondo-Penas I., Perona-Moratalla A.B., Del Valle-Perez J.A., Gracia-Gil J., Rojas-Bartolome L., Feria-Vilar I., Monteagudo M., Palao M., Palazon-Garcia E., Alcahut-Rodriguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 23, 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyle P.A., Malloy P.F., Salloway S., Cahn-Weiner D.A., Cohen R., Cummings J.L. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am. J. Geriatr. Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 69.McIntosh R.C., Rosselli M., Uddin L.Q., Antoni M. Neuropathological sequelae of human immunodeficiency virus and apathy: a review of neuropsychological and neuroimaging studies. Neurosci. Biobehav. Rev. 2015;55:147–164. doi: 10.1016/j.neubiorev.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Boksem M.A., Meijman T.F., Lorist M.M. Mental fatigue, motivation and action monitoring. Biol. Psychol. 2006;72(2):123–132. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Lorist M.M., Boksem M.A., Ridderinkhof K.R. Impaired cognitive control and reduced cingulate activity during mental fatigue. Brain Res. Cogn. Brain Res. 2005;24(2):199–205. doi: 10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 72.Klaasen N.G., Kos C., Aleman A., Opmeer E.M. Apathy is related to reduced activation in cognitive control regions during set-shifting. Hum. Brain Mapp. 2017;38(5):2722–2733. doi: 10.1002/hbm.23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Z., Chen R., Liu X., Zhao G., Zheng Y., Gong M., Zhang J. The impairing effects of mental fatigue on response inhibition: an ERP study. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorstensen J.R., Taylor J.L., Tucker N.G., Kavanahj J.J. Enhanced serotonin availability amplifies fatigue perception and modulates the TMS-induced silent period during sustained low-intensity elbow flexions. J. Physiol. 2020;598(13):2685–2701. doi: 10.1113/JP279347. [DOI] [PubMed] [Google Scholar]
- 75.Taylor J.L., Butler J.E., Allen G.M., Gandevia S.C. Changes in motor cortex excitability during human muscle fatigue. J. Physiol. 1996;490(2):519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sacco P., Thickbroom G.W., Thompson M.L., Mastaglia F.L. Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve. 1997;20(9):1158–1166. doi: 10.1002/(sici)1097-4598(199709)20:9<1158::aid-mus11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 77.Taylor J.L., Allen G.M., Butler J.E., Gandevia S.C. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J. Appl. Physiol. 2000;89(1):305–313. doi: 10.1152/jappl.2000.89.1.305. 1985. [DOI] [PubMed] [Google Scholar]
- 78.Mileva K.N., Sumners D.P., Bowtell J.L. Decline in voluntary activation contributes to reduced maximal performance of fatigued human lower limb muscles. Eur. J. Appl. Physiol. 2012;112(12):3959–3970. doi: 10.1007/s00421-012-2381-1. [DOI] [PubMed] [Google Scholar]
- 79.Taylor J.L., Butler J.E.., Gandevia S.C. Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. Eur. J. Apll. Physiol. 2000;83:106–115. doi: 10.1007/s004210000269. [DOI] [PubMed] [Google Scholar]
- 80.Stetkarova I., Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin. Neurophysiol. 2013;124(2):339–345. doi: 10.1016/j.clinph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R., Muller-Dahlhaus F. TMS and drugs revisited 2014. Clin. Neurophysiol. 10, 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 82.Miyaguchi S., Kojima S., Sasaki R., Kotan S., Kirimoto H., Tamaki H., Onishi H. Decrease in short-latency afferent inhibition during corticomotor postexercise depression following repetitive finger movement. Brain Behav. 2017;7(7) doi: 10.1002/brb3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonnì S., Ponzo V., Di Lorenzo F., Caltagirone C., Koch G. Real-time activation of central cholinergic circuits during recognition memory. Eur. J. Neurosci. 11, 2017;45:1485–1489. doi: 10.1111/ejn.13588. [DOI] [PubMed] [Google Scholar]
- 84.Garcia-Oscos F., Salgado H., Hall S., Thomas F., Farmer G.E., Bermeo J., Galindo L.C., Ramirez R.D., D’Mello S., Rose-John S., Atzori M. The stress-induced cytokine interleukin-6 decreases the inhibition/excitation ratio in the rat temporal cortex via trans-signaling. Biol. Psychiatry. 2012;71(7):574–582. doi: 10.1016/j.biopsych.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McKlveen J.M., Morano R.L., Fitzgerald M., Zoubovsky S., Cassella S.N., Scheimann J.R., Ghosal S., Mahbod P., Packard B.A., Myers B., Baccei M.L., Herman J.P. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry. 10, 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Northoff G., Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol. Psychiatry. 2014;19(9):966–977. doi: 10.1038/mp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fogaca M.V., Duman R.S. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front. Cell. Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krabbe D., Ellbin S., Nilsson M., Jonsdottir I.H., Samuelsson H. Executive function and attention in patients with stress-related exhaustion: perceived fatigue and effect of distraction. Stress. 2017;20(4):333–340. doi: 10.1080/10253890.2017.1336533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neurophysiological data in COVID-19 patients and healthy controls.
Data Availability Statement
The authors confirm that the data supporting the results of this study are saved at the Department of Neurorehabilitation, Hospital of Vipiteno (SABES-ASDAA), Vipiteno-Sterzing, Italy. They are available upon request from the corresponding author.