Abstract
COVID-19 has created an enormous health crisis and this spring New York City had a severe outbreak that pushed health and critical care resources to the limit. A lack of adequate space for mechanically ventilated patients induced our hospital to convert operating rooms into critical care areas (OR-ICU). A large number of COVID-19 will develop acute kidney injury that requires renal replacement therapy (RRT). We included 116 patients with COVID-19 who required mechanical ventilation and were cared for in our OR-ICU. At 90 days and at discharge 35 patients died (30.2%). RRT was required by 45 of the 116 patients (38.8%) and 18 of these 45 patients (40%) compared to 17 with no RRT (23.9%, ns) died during hospitalization and after 90 days. Only two of the 27 patients who required RRT and survived required RRT at discharge and 90 days. When defining renal recovery as a discharge serum creatinine within 150% of baseline, 68 of 78 survivors showed renal recovery (87.2%). Survival was similar to previous reports of patients with severe COVID-19 for patients cared for in provisional ICUs compared to standard ICUs. Most patients with severe COVID-19 and AKI are likely to recover full renal function.
Keywords: COVID-19, Provisional ICU, Acute kidney injury, Long-term outcomes
Abbreviations: Corona virus disease 19, (COVID-19); Intensive care unit, (ICU); Acute kidney injury, (AKI); Kidney Disease: Improving Global Outcomes, (KDIGO); End stage renal disease, (ESRD); Chronic kidney disease, (CKD); Columbia University Irving Medical Center, (CUIMC); Operating room ICU, (ORICU); Renal replacement therapy, (RRT); Glomerular filtration rate, (GFR); Modification of Diet in Renal Disease, (MDRD); Continuous renal replacement therapy, (CRRT)
1. Introduction
Corona virus disease 19 (COVID-19) has developed into a catastrophic pandemic with over millions of confirmed cases worldwide and between March and May 2020 New York City became one of the epicenters of the pandemic with some of the largest case numbers in the world. As a result of the unprecedented and rapid spread of the virus, we are left with insufficient knowledge of the ramifications from the sequelae. We assume that about 5% of cases will develop critical COVID-19 defined as COVID-19 with associated organ failure and/or requiring mechanical ventilation. The large number of cases strained resources and particularly intensive care unit (ICU) capacity was scarce and required innovative approaches to increase capacity. One of the many limitations was adequate space to care for mechanically ventilated patients. As a temporary solution to the lack of space our hospital converted a large number of the operating rooms into ICU spaces. While we tried to maintain the best care possible for these patients, outcome of patients in these provisional ICU facilities that were created specifically for the COVID-19 crisis is not well known.
When the pandemic started it became quickly obvious that COVID-19 not affects the lungs but is a systemic disease that can affect all organ systems with a particularly high incidence of acute kidney injury (AKI). In a recent retrospective study of hospitalized COVID-19 patients from the New York metropolitan area, 36.6% developed AKI using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [1]. Other studies have reported incidences of 24–35% of AKI and RRT requirement in approximately 15–35% of all COVID-19 ICU patients [[2], [3], [4]].
Survivors of (non-COVID-19) critical illness with AKI frequently develop chronic kidney disease requiring hemodialysis in the long-term [5,6]. Almost 16% of hospitalized patients who develop severe AKI and need renal replacement therapy will develop end stage renal disease (ESRD) requiring hemodialysis [7]. A large veterans study demonstrated that one year after hospitalization with stage 3 AKI 47% of patients will develop stage 3 or worse chronic kidney disease (CKD) [8]. Considering the high incidence of AKI in the large number of patients with severe COVID-19 the long-term sequelae for patients and society would be tremendous if a similar proportion of recovered COVID-19 patients developed CKD and required chronically hemodialysis. The aim of this study is to assess the mortality and intermediate-term renal outcomes of patients with severe COVID-19 treated in a provisional ICU.
2. Material and methods
In this retrospective cohort study, we aimed to determine the clinical course and renal outcomes of patients treated for severe COVID-19 in a provisional ICU at Columbia University Irving Medical Center (CUIMC). During the height of the COVID-19 crisis in New York City many hospitals experienced a shortage of ICU capacity and as a response CUIMC converted operating rooms into ICU rooms, each with approximately three to six patients similar to what has been previously described for our sister institution New York–Presbyterian Weill Cornell Medical Center [9]. Patients were ventilated either with conventional ventilators or with anesthesiology machine that were present in the operating room. We included any patient who were admitted to the OR-ICU at any time during its existence of this unit during the COVID-19 crisis independent of the duration of stay. We excluded patients with incomplete records and/or follow-up data.
Our main endpoints were mortality as well as renal recovery rate of hospitalized, intubated patients with AKI (as defined by KDIGO) and those who required RRT secondary to COVID-19.
AKI was defined and graded by the KDIGO criteria stage 1, 2 and 3 within 7 days after admission as well as patients requiring RRT during their hospitalization (continuous or intermittent hemodialysis or peritoneal dialysis). Baseline serum creatinine, when not available was estimated by reverse use of the Modification of Diet in Renal Disease Study Group (MDRD) [10] formula based on a GFR of 75 mL/min/m2 of body surface area (BSA) as suggested by KDIGO.
We defined recovery of renal function by change of serum creatinine of less than 50% at discharge and 90 days after admission when compared to baseline serum creatinine as has been suggested by other studies [11,12].
We further assessed the number of patients who required RRT during the hospitalization who still required hemodialysis at 30 days, 90 days and at discharge.
2.1. Statistical methods
An unpaired t-test or Pearson's test was used for comparing values with Gaussian distribution, and Mann-Whitney (Wilcoxon rank) test or Spearman's test was used for continuous variables without normal distribution. Gaussian distribution was determined using the Kolmogorov-Smirnov test. Categorical data were compared using Chi-square. P values were 2 tailed and p < 0.05 was considered significant. SAS 9.1 (SAS Inc., Cary, NC, USA), PASW 18.0 (SPSS Inc., Chicago, IL, USA) and Graphpad Prism 6.0 (San Diego, CA, USA) software was used for the statistical analysis.
3. Results
One hundred sixteen subjects with severe COVID-19 who were admitted to the OR-ICU at Columbia University Irving Medical Center in New York between March 24 and May 7, 2020 were included. 60 patients were admitted to the ORICU within 24 h after intubation and the remaining subjects were cared for at other ICUs or the emergency department first before being transferred to the ORICU (mean 8.5 +/− 8.5 days after intubation). The mean +/− SD duration of ICU stay was 28.0 +/− 14.0 days. Demographics are listed in Table 1 . Thirty-five of 116 (30.2%) patients died during their hospitalization; 23 of these died after undergoing palliative extubation. Seventy patients underwent tracheostomy and of those 8 patients died (11.4%).
Table 1.
Demographics of patients with severe COVID-19 who did and did not require continuous renal replacement therapy (CRRRT) during hospitalization (p-values comparing patients who did and did not require CRRT.
| All n = 116 |
AKI Stage 1 n = 24 (20.9%) |
AKI Stage 2 n = 15 (12.9%) |
AKI Stage 3 n = 37 (31.9%) |
CRRT n = 45 (38.8%) |
No CRRT n = 71 (61.2%) |
P value | |
|---|---|---|---|---|---|---|---|
| Female Sex (%) | 41 (35.3%) | 25 (32.9%) | 19 (36.5%) | 12 (32.4%) | 14 (31.1%) | 27 (38%) | ns |
| Age (years) | 61.5 +/− 12.1 | 62 +/− 12.1 | 68.4 +/− 10.0 | 61.7+/− 10.3 | 63.3 +/− 10.1 | 60.3 +/− 13.2 | ns |
| Height (cm) | 168.3 +/− 9.3 | 171.0 ±10.3 | 167.9 +/− 7.7 | 169.4 +/− 9.1 | 169.9 +/− 8.7 | 167.3 +/− 9.5 | ns |
| Weight (kg) | 84.7 +/− 20.5 | 83.7 +/− 18.2 | 79.9 +/− 17.2 | 169.4 +/− 9.1 | 84.8 +/− 18.1 | 84.6 +/− 22.1 | ns |
| Body-mass index (kg/m2) | 30.2 +/− 8.5 | 29.1 +/− 8.7 | 28.7 +/− 7.8 | 30.1 +/− 8.0 | 29.7 +/− 7.7 | 30.6 +/− 9.0 | ns |
| Baseline SCreat (mg/dL) | 1.03±0.21 | 1.08±0.14 | 1.05±0.26 | 1.02±0.19 | 1.06±0.24 | 1.01±0.19 | ns |
| Admission SCreat (mg/dL) | 1.67 +/− 1.73 | 1.40 +/− 0.958 | 2.20 +/− 1.81 | 2.45 +/− 2.475 | 2.34 +/− 2.35 | 1.24 +/− 0.99 | <0.001 |
| Mortality (%) | 35 (30.2%) | 9 (37.5%) | 6 (40.0%) | 13 (35.1%) | 18 (40.0%) | 17 (23.9%) | ns |
| Obesity (BMI > 30) | 51 (44%) | 10 (41.7%) | 7 (46.7%) | 15 (40.5%) | 19 (42.2%) | 32 (45.1%) | ns |
| Diabetes mellitus | 50 (43.1%) | 9 (37.5%) | 6 (40.0%) | 17 (45.9%) | 16 (35.6%) | 34 (47.6%) | ns |
| Hypertension | 62 (53.4%) | 10 (41.7%) | 8 (53.3%) | 19 (51.4%) | 20 (44.4%) | 42 (59.2%) | ns |
| s/p Transplant | 13 (11.2%) | 1 (4.2%) | 1 (6.7%) | 5 (13.5%) | 6 (13.3%) | 7 (9.9%) | ns |
For 98 patients a reliable baseline serum creatinine was not available and we therefore used the MDRD formula to estimate the baseline serum creatinine based on a GRF of 75 mL/min/1.75 m2 BSA. Twenty four of 116 subjects (20.9%) developed AKI stage 1, 15 (14.8%) AKI stage 2 and 37 (30.4%) AKI stage 3. Table 2 depicts the incidence of different stages of AKI and their mortalities and renal outcomes. Of note there was no significant difference in mortality between patients with different stages of AKI and no AKI, however patients with stage 3 AKI were more likely to require renal replacement therapy (RRT) (p < 0.001) and to die during hospitalization (p < 0.001).
Table 2.
Renal outcome and mortality 30 days, at discharge (after 58.3 +/− 21.8 days) and 90 days after admission N (%) or mean +/− SD; SCreat – serum creatinine, RRT - renal replacement therapy.
| All n = 116 |
Stage 1 AKI n = 24 (20.9%) |
Stage 2 AKI n = 15 (12.9%) |
Stage 3 AKI n = 37 (31.9%) |
||
|---|---|---|---|---|---|
| Baseline SCreat (mg/dL) | 1.03 +/− 0.21 | 1.08 ±0.41 | 1.05 +/− 0.26 | 1.02 +/− 0.19 | |
| 30 days | RRT | 22 (19.0%) | 0 (0%) | 3 (20.0%) | 17 (45.9%) |
| Dead | 19 (16.4%) | 4 (16.7%) | 2 (13.3%) | 8 (21.6%) | |
| SCreat (mg/dL) only alive and no RRT |
1.17 +/− 0.99 | 0.80 +/− 0.48 | 1.26 +/− 0.74 | 2.54 +/− 1.33 | |
| # of patients with worse SCreat (>150% baseline) | 14 of 68 (20.6%) | 1 of 19 (5.3%) | 3 of 10 (30%) | 8 of 11 (72.7%) | |
| Discharge | RRT | 2 (1.7%) | 0 (0%) | 0 (0%) | 2 (5.4%) |
| Dead | 35 (30.2%) | 9 (37.5%) | 6 (40.0%) | 13 (35.1%) | |
| SCreat (mg/dL) only alive and no RRT |
0.89 +/− 0.63 | 0.64 +/− 0.17 | 0.71 +/− 0.21 | 1.50 +/− 0.78 | |
| # of patients with worse SCreat (>150% baseline) | 10 of 78 (12.8%) | 0 of 4 (0%) | 0 of 2 (0%) | 9 of 22 (40.9%) | |
| 90 days | RRT | 2 (1.7%) | 0 (0%) | 0 (0%) | 2 (5.4%) |
| Dead | 35 (30.2%) | 9 (37.5%) | 6 (40.0%) | 13 (35.1%) | |
| SCreat (mg/dL) only alive and no RRT |
1.22 +/− 0.70 | 0.7+/− 0.0.35 | 0.63 +/− 0.07 | 1.50 +/− 0.66 | |
| # of patients with worse SCreat (>150% baseline) | 8 of 32 (25%) | 0 of 4 (0%) | 0 of 2 (0%) | 7 of 18 (38.9%) | |
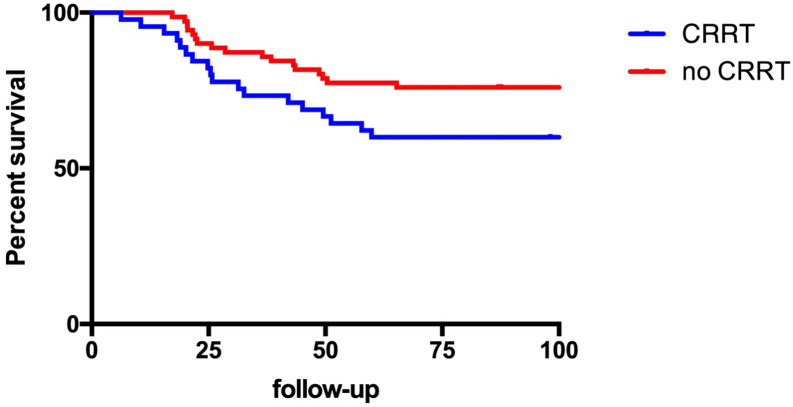
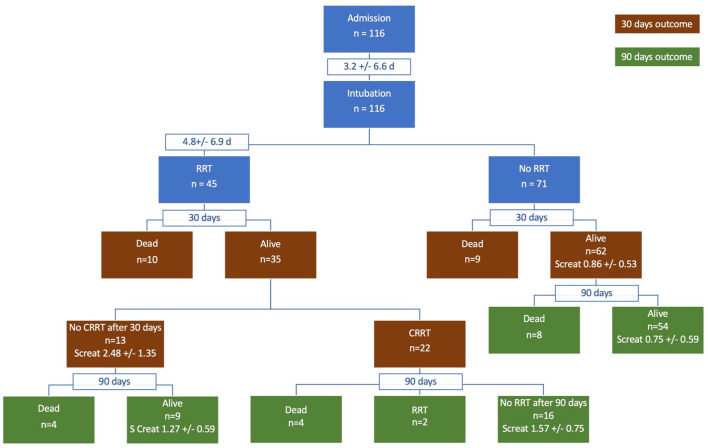
Forty-five patients subsequently required RRT; almost all continuous RRT but four subjects underwent transiently peritoneal dialysis due to a shortage of continuous renal replacement therapy (CRRT) machines and circuits during the COVID-19 crisis. Of these 45 patients 18 (40.0%) subsequently died compared to 17 of 71 (23.9%) patients who did not require CRRT (ns). Kaplan-Meier Survival curves are depicted in Fig. 1 and Fig. 2 depicts a flow diagram of all patients. The mean length of stay was 53.8 +/− 31.2 days for patients with CRRT compared to 51.5 +/− 24.6 days without CRRT. However, when only including patients who survived, the mean length of stay was 69.1 +/− 29.5 days for patients with CRRT compared to 57.2 +/− 24.6 days without CRRT.
Fig. 1.
Survival of patients with severe COVID-19 treated in the OR-ICU who did and did not require renal replacement therapy (CRRT) during hospitalization.
Fig. 2.
Flowchart of patients with severe COVID-19 requiring mechanical ventilation cared for in our provisional ICU (OR-ICU). CRRT – continuous renal replacement therapy, SCreat – serum creatinine.
The 27 patients who survived required CRRT for 20.3 +/− 15.1 days; 22 of these patients (81.5%) subsequently required intermittent hemodialysis for 14.6 +/− 12.2 days and 2 patients required intermittent hemodialysis at the time of discharge and at 90 days. While most patients who survived had good recovery at 30 and 90 days and at discharge, ten out of 78 patients (12.8%) had a worsening of serum creatinine by more than 50% when comparing baseline to discharge serum creatinine.
4. Discussion
Our study demonstrates that the mortality of patients with severe COVID-19 cared for in a provisional ICU was similar to the larger cohort of critically-ill COVID-19 patients at our institution [13]. As previously reported in other cohorts a large number of patients with severe COVID-19 requiring mechanical ventilation also developed AKI and needed CRRT. However, of those patients who survived only two patients required dialysis at discharge and the majority of patients saw recovery of renal function by the time of discharge and at 90 days.
These results have important implications. Cummings et al. previously reported a mortality of 39% in the larger, 1150 critically-ill patients with COVID-19 that were cared for at our institution (the present cohort was included in that study). Initial concerns that patients who were treated in the provisional OR-ICU fared much worse than those in conventional ICUs fortunately did not materialize. This is particularly encouraging as the present study has long enough follow-up for all survivors to be discharged from the hospital. Other publications have assessed the incidence of AKI with COVID-19 [1,3] but did not include longer-term outcomes and recovery rates.
AKI is frequent in patients with severe COVID-19 [1] and as a consequence, CRRT machines and circuits were in shortage during the COVID-19 crisis in New York City. While our results only reflect intermediate-term outcomes it is likely that only few patients are going to require dialysis (and possible kidney transplants in the future); less than other groups of critically ill patients [5,8]. This is reassuring as we initially did not know if the large proportion of patients with severe COVID-19 who required RRT would result in a vast number of patients with chronic kidney disease requiring chronic dialysis and/or renal transplants. At least for the intermediate follow-up there seem to be full recovery of renal function in most patients, however it is too early to comment on long-term renal sequalae. It is worth mentioning that due to the high rate of mortality, follow-up data could not be gathered for subjects at discharge, specifically of patients with stage 1 and 2 AKI.
A limitation of our study is the assessment of baseline serum creatinine. Many of our patients did not have a reliable baseline serum creatinine available and admission serum creatinine likely does not reflect baseline renal function. We found that mean admission creatinine was more than 60% higher than baseline estimated serum creatinine (assuming a GFR of 75 mL/min/m2 BSA) and we must assume that many patients already had (early stages of) AKI on admission and therefore admission serum creatinine did not reflect true baseline renal function.
Prowle et al. further observed in a general ICU population that serum creatinine significant decreases in critically ill (non-COVID-19) patient from baseline to discharge from hospital, except for those who sustained severe AKI [14]. This may be explained by a decrease in muscle mass after critical illness resulting in lower serum creatinine and is not necessarily a reflection of improved renal function. Similarly, most of our patients may have had no worsening of serum creatinine because they were deconditioned and lost muscle mass during a prolonged hospitalization.
The mechanism of AKI in COVID-19 is poorly understood and the degree and severity of AKI is beyond what can be explained with hypoperfusion and renal ischemia-reperfusion injury seen with other forms of critical illness and hypoxemic respiratory failure. Sepsis, hypotension, exposure to nephrotoxic drugs and hypovolemia are frequent with severe COVID-19 [15] but recent studies also hinted at renal endothelium is affected by SARS-CoV-2 similar to involvement of lung endothelium [16].
It can be further hypothesized that SARS-CoV-2 by entering cells through the angiotensing-converting enzyme −2 (ACE-2) receptor downregulates this enzyme and lowers levels of its product angiotensin (1–7), an anti-inflammatory vasodilator. As a consequence, angiotensin II may increase and cause (renal) vasoconstriction, aldosterone synthesis and secretion and increased vasopressin secretion through angiotensin II receptors 1 and 2 (ATR-1 and ATR-2). This could result in a profound renal vasoconstriction. This would explain why patients even with severe oliguric renal failure and COVID-19 fully recover renal function. COVID-19 remains a dreadful disease with a high mortality but it is encouraging that the majority of patients who survived regained renal function.
Authorship
Gebhard Wagener MD helped with conception, coordination of study groups, analysis of existing literature, writing and submission of manuscript.
Funding
Support was provided solely from institutional and/or departmental sources.
Declaration of Competing Interest
None.
References
- 1.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argenziano M.G., Bruce S.L., Slater C.L., et al. medRxiv. 2020. Characterization and clinical course of 1000 patients with COVID-19 in New York: Retrospective case series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S., Coca S.G., Chan L., et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2020 doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Corte W., Dhondt A., Vanholder R., et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. 2016;20:256. doi: 10.1186/s13054-016-1409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.F., Wu V.C., Huang T.M., et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16:R123. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo L.J., Go A.S., Chertow G.M., et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heung M., Steffick D.E., Zivin K., et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67:742–752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters A.W., Chawla K.S., Turnbull Z.A. Transforming ORs into ICUs. N Engl J Med. 2020;382:e52. doi: 10.1056/NEJMc2010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N. Roth D. a more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kellum J.A., Sileanu F.E., Bihorac A., Hoste E.A., Chawla L.S. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195:784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korenkevych D., Ozrazgat-Baslanti T., Thottakkara P., et al. The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Ann Surg. 2016;263:1219–1227. doi: 10.1097/SLA.0000000000001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prowle J.R., Kolic I., Purdell-Lewis J., Taylor R., Pearse R.M., Kirwan C.J. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9:1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]