Highlights
-
•
Currently, unconditional investment is being made into COVID-19 vaccine R&D COVID-19 vaccine R&D investments should also ensure access and affordability.
-
•
The options market for antibiotics, could be adapted for use with COVID-19 vaccines.
-
•
This could help fund R&D, boost manufacturing capacity and secure fair prices.
-
•
Further research on the OMV model in the current COVID-19 crisis is warranted.
Keywords: Covid19, Research and development, Manufacturing capacity, Innovative financing mechanisms, Health policy
There is consensus around the globe that safe and effective vaccines will play a key role in curbing COVID-19 transmission, and thereby in enabling exit strategies. Because of this, there has been tremendous support for vaccine research and development (R&D). At least 137 vaccine candidates are in preclinical phases, 23 candidates are in clinical evaluation stages [1] and hopeful results already have emerged from Phase II of the Oxford-AstraZeneca vaccine – AZD1222 –clinical trials [2]. Ideally, once any safe and effective vaccine gains market approval, it will reach those in need rapidly, equitably and affordably. However, this is not a simple task. Reimbursement models must balance these objectives against the need to encourage expensive R&D. In the below commentary, we discuss existing financing mechanisms that fail to strike this equilibrium; we then propose an innovative model for use in extreme circumstances such as this COVID-19 pandemic that we believe may satisfy stakeholder priorities by addressing market failures – the Options Market for Vaccines (OMV).
Vaccine development is lengthy and costly. The Coalition for Epidemic Preparedness Innovation (CEPI) estimates that bringing one COVID-19 vaccine from basic science research through to clinical trials and then to market approval will take 12–18 months and cost $2 billion [3]. Beyond market approval, manufacturing enough doses for the billions of people who will require them will take many more months, or potentially even years. This has prompted governments and private investors such as the Bill and Melinda Gates Foundation to pre-emptively invest in manufacturing capacity for some of the most promising vaccine candidates [4]. Governments have poured billions of public dollars into COVID-19 vaccine R&D thus far: CEPI has already received $1.4 billion from states around the world [5] and individual countries, such as the US with its $10 billion ‘Operation Warp Speed’, are contributing to pharmaceutical companies’ R&D endeavours [6]. Because of the urgency of the COVID-19 situation, many of these public investments are made quickly with few or no strings attached.
We believe that when there is significant public subsidisation through taxpayer monies into vaccine development, there must also be mechanisms in place to guarantee fair prices. While pharmaceutical companies would receive tremendous political backlash for putting an overly-expensive price tag on a COVID-19 vaccine; there is a risk that without financing mechanisms that guarantee fair prices in place, whichever company reaches the market first with an effective vaccine could capitalise upon its monopoly power and charge exorbitant prices [7]. Given the unprecedented quantities of the vaccine that will need to be distributed, even small differences in prices would have significant financial and equity implications.
One financing mechanism, which has been backed by the WHO and Unitaid, is to set up a global patent pool in which pharmaceutical companies release their patents for COVID-19 medical interventions with no, or with very limited conditions [8]. However, this may have the unintended consequence of disincentivising R&D for other medical developments to address COVID-19 and future pandemics. Moreover, there is a need to pre-emptively invest in manufacturing capacity as a vaccine shifts closer to market approval. An optimal solution would encourage and reward COVID-19 vaccine R&D, generate funds to invest in manufacturing capacity, and ensure that those in need can purchase a successful vaccine affordably.
Another approach to vaccine financing is an Advanced Market Commitment (AMC). Launched in collaboration with CEPI and the WHO in June 2020, Gavi’s COVAX AMC aims to deliver two billion doses of a safe and effective COVID-19 vaccine in an equitable manner to all countries (proportional to their populations) participating in the facility by the end of 2021. As of mid-July, 165 countries – representing more than half of the global population, have expressed interest in joining the COVAX initiative [9]. This is certainly a step in the right direction, but previous experiences show that an AMC alone may not be comprehensive enough to stimulate vaccine R&D for COVID-19. In the early 2000s, Gavi piloted an AMC with $1.5 billion from six donors to pay for pneumococcal vaccines on the basis that once developed, these would be priced affordably for those in need [10]. An independent evaluation concluded that while the AMC helped accelerate the development of two late-stage pneumococcal vaccine candidates, it did little to incentivise early-stage research [11]. As most COVID-19 vaccine candidates are in early phases of research, alternate approaches should also be considered.
Instead, an optimal financing mechanism would involve a hybrid of push incentives (such as funding for basic science research and early clinical trials) and pull incentives (such as AMCs and market entry rewards). A pre-existing financing mechanism proposed for antibiotics, the Options Market for Antibiotics (OMA), meets this specification and could be adapted for use with vaccines under pandemic circumstances or for neglected diseases [12]. The OMA, modelled on the principle of financial call options, allows payers investing in R&D to buy the right to purchase antibiotics at discounted prices if and when these products reach market approval. This effectively lets public and private funders share the risk of investing in antibiotics. It also allows governments and non-governmental organisations (NGOs) to fund all stages of vaccine development from pre-clinical phases through to product delivery (including building manufacturing capacity), while also ensuring that successful vaccines can then be purchased at affordable prices, and distributed rapidly and equitably.
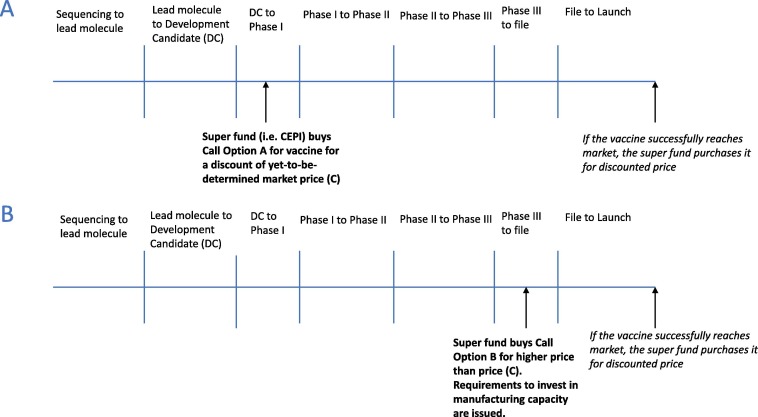
For a proposed options market for vaccines (OMV) model, a public investor (i.e. a government or an international organisation) would purchase options for the COVID-19 vaccine to redeem if and when a vaccine was delivered to market. As co-investors, the options purchasers will be able to co-determine a market price for the vaccine alongside innovators should it prove to be successful. Early investments in Phase 1 trials—which have a higher probability of failure and are therefore riskier—options would be cheaper; while less-risky options purchased towards the end of the vaccine development cycle would be more expensive. As a vaccine shifts closer to market approval, funds generated by options can be used to pre-emptively invest in manufacturing capacity, thereby accelerating access to the vaccines more widely. A requirement to do so may also be included within the terms of investment by the options purchaser. If the vaccine candidate fails, the investor loses their initial investment. However, if the vaccine successfully reaches the market, the investor has the right to purchase the vaccine at a discounted price, potentially making significant savings in the long run (Fig. 1 highlights this process) [13]. The influence on determining the market price of the vaccine and the degree of discount received will be dependent on the timing and size of the investments made by options purchasers. Thus, the option purchaser benefits in a number of ways: firstly, the options holder invests in, and therefore incentivises, the development of the vaccine they desire; secondly, the options holder becomes a co-investor, and therefore will be able to exert influence over price setting if and when that vaccine is delivered successfully; and thirdly, the options holder gets the vaccine at a discounted price [12].
Fig. 1.
COVID-19 Vaccine Approval Timeline with Call Options A and B (Adapted from Brogan & Mossialos, 2013). (A) The price of purchasing Option A is discounted because the vaccine is in early stages of R&D and thus it is a risky investment. If the vaccine successfully develops, the investor will save in the long-term since they will purchase the COVID-19 at a price (C) lower than the market price. (B) The price of purchasing Option B is higher than price (C) because the vaccine is in a late-stage of development and thus the investment has minimal risk. Options purchased at later stages of development, such as Option B, may include requirements for investments in manufacturing capacity to accelerate widespread access to the vaccines once they are successfully developed. The level of discount and the ultimate price level will be dependent on the time and size of investments made by governments/superfunds.
There are potential limitations to the OMV model. First, there is a risk is that that the OMV model may encourage countries that make successful investments to demand priority access to vaccines. However, we argue this does not differ from the current situation, whereby certain governments are actively taking steps to secure this; for example it has been reported Donald Trump tried to purchase exclusive access for the US from a German company developing a vaccine for COVID-19 [14]. Second, it could be argued the OMV model gives an unfair advantage to high-income countries which may have more technical expertise to make strategic investments. While this is plausible, LMICs may stand to benefit from the OMV in several ways. Philanthropic organisations such as Gavi or CEPI could purchase a set of options, or alternatively, high-income countries could purchase large quantities of vaccines and then distribute doses to LMICs. It is important to consider that both the OMA model and the OMV model have not yet been utilised in practice. Therefore, the OMV model and its strengths, limitations, applicability and acceptability in the current crisis warrant further research exploration and discussion.
To mitigate against the risks of the OMV model, we argue the introduction of the OMV model could be governed by an international panel of experts who can scrutinise validity of vaccine candidates and inform investment decisions. These meetings could be conducted publicly to ensure that transparency around contract and pricing decisions is maintained. Gavi or the WHO would be well positioned to coordinate and host such a panel. The panel could advise high-, middle-, and low-income countries alike and thus enable countries to make smart investment decisions for their particular settings. Additionally, if and when a vaccine comes to market, this panel can recommend purchasing decisions based on the vaccine’s safety, efficacy and strike price and the disease severity and spread in the given country. This would include issuing recommendations on which countries should be prioritised for international financial assistance to purchase vaccines. For countries which wish to combine an OMV with an advanced commitment to purchase vaccines, the expert committee could offer advice on what quality and safety standards could be incorporated with these contracts.
Under the current pandemic circumstances, public investors are having to make large and risky investments in the vaccine development process without any conditions on future access to a successful candidate. This means that much of taxpayer investment is being directed towards development of products which may ultimately fail – a review of vaccine candidates for communicable diseases from 1998 to 2009 found that the average vaccine, taken from the preclinical phase, had a market entry probability of only six percent [15]. And even if a vaccine does make it to market, there is currently no guarantee that people in need will be able to access it affordably; after all, the first developer may choose to charge high prices or to prioritise launching the vaccine in certain countries [16]. Rather than continuing down a path of unconditionally subsidising R&D, super funds such as CEPI or the Gavi Alliance could be ideally placed to purchase a set of options for vaccines. At the early stages of development, this would fund basic science research. At the later stages of development, this would increase funds to invest in manufacturing capacity. Once an effective vaccine is successfully developed, these superfunds could distribute their options according to pre-determined criteria – for example based on need or on country contributions to the fund. We believe that the OMV has the potential to continue to incentivise R&D for a COVID-19 vaccine, to offer the public confidence that their taxpayer money is being spent wisely and will lead to an affordable and accessible vaccine for those in need.
All authors attest they meet the ICMJE criteria for authorship.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization. Draft landscape of COVID-19 candidate vaccines; 2020.
- 2.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet (London, England) 2020 doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CEPI. CEPI gets €140 million funding boost from Germany while expanding coronavirus vaccine search. CEPI 2020. https://cepi.net/news_cepi/cepi-gets-e140-million-funding-boost-from-germany-while-expanding-coronavirus-vaccine-search/ (accessed March 16, 2020).
- 4.Calfas J. Bill Gates to help fund coronavirus-vaccine development. Wall Str J. 2020 [Google Scholar]
- 5.CEPI. Netherlands and Switzerland join the search for COVID-19 vaccines. CEPI 2020. https://cepi.net/news_cepi/netherlands-and-switzerland-join-the-search-for-covid-19-vaccines/ [accessed April 25, 2020].
- 6.Rahn W. Is the US-China rivalry tangling a coronavirus vaccine with geopolitics? Dtsch Welle. 2020 [Google Scholar]
- 7.Togoh I. Health Secretary Alex Azar refuses to guarantee coronavirus vaccine would be affordable for all. Forbes; 2020.
- 8.Mancini D.P. Big drugmakers under pressure to share patents against coronavirus. Financ Times. 2020 [Google Scholar]
- 9.More than 150 countries engaged in COVID-19 vaccine global access facility n.d. https://www.who.int/news-room/detail/15-07-2020-more-than-150-countries-engaged-in-covid-19-vaccine-global-access-facility [accessed July 26, 2020].
- 10.Cernuschi T. Advance Market Commitment: The Pneumococcal Pilot; 2009.
- 11.The Boston Consulting Group. The Advance Market Commitment Pilot for Pneumococcal Vaccines: Outcomes and Impact Evaluation; 2015.
- 12.Brogan D.M., Mossialos E. Incentives for new antibiotics: The Options Market for Antibiotics (OMA) model. Global Health. 2013;9:58. doi: 10.1186/1744-8603-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brogan D.M., Mossialos E. Systems, not pills: the options market for antibiotics seeks to rejuvenate the antibiotic pipeline. Soc Sci Med. 2016;151:167–172. doi: 10.1016/j.socscimed.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Morales A. Germany confirms that Trump tried to buy firm working on coronavirus vaccine. Politico. 2020 [Google Scholar]
- 15.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H.J.H.M., Osterhaus A.D.M.E. Risk in vaccine research and development quantified. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronavirus: Germany tries to stop US luring away firm seeking vaccine. CNBC; 2020.