Abstract
Background and aim
Huashi Baidu Decoction (HSBD) is a novel complex prescription which has positive effects on severe COVID-19. This study was aimed to discover key Chinese materia medica, main active compounds, hub therapeutic target proteins and core signal pathways in the potential therapeutic mechanism of HSBD on severe COVID-19 through integrating network pharmacological methods.
Experimental procedure
TCMSP, TCMID and STITCH databases were used to screen out active compounds and target proteins of HSBD. GeneCards database was used to screen out disease genes of severe COVID-19. The potential therapeutic targets of HSBD on severe COVID-19 were used to construct protein-protein interaction network through STRING database and the hub target proteins were discovered. Next, GO and KEGG enrichment analysis were carried out to discover core signal pathways. Finally, the network diagram of “Chinese materia medica-active compounds-therapeutic target proteins” was built, then key Chinese materia medica and main active compounds were selected.
Results and conclusion
HSBD might treat severe COVID-19 through 45 potential target genes, among them, there were 13 hub target genes: RELA, TNF, IL6, IL1B, MAPK14, TP53, CXCL8, MAPK3, MAPK1, IL4, MAPK8, CASP8, STAT1. Meanswhile, GO_BiologicalProcess and KEGG signaling pathways analysis results showed that the core signal pathways were inflammation and immune regulation pathways. Finally, 4 key Chinese materia medica and 11 main active compounds were discovered in the HSBD. In conclusion, the therapeutic mechanism of HSBD on severe COVID-19 might involve its pharmacological effects of anti-inflammation and immune regulation via acting on 45 disease-related proteins of severe COVID-19.
Taxonomy (classification by evise)
Viral Pneumonia, COVID-19, Acute Respiratory Distress Syndrome, Septic Shock, Chinese Herbal Medicine.
Keywords: HSBD, Chinese materia medica, Active compounds, Anti-inflammation, Immune regulation
Graphical abstract
Highlights
-
•
The potential therapeutic mechanisms of HSBD on severe COVID-19 are demonstrated.
-
•
Anti-inflammation and immune regulation are the main therapeutic mechanisms.
-
•
Multi-target therapy is a promising treatment strategy to cure severe COVID-19.
List of abbreviations
- 2019-nCoV
2019 novel coronavirus
- ARDS
acute respiratory distress syndrome
- COVID-19
coronavirus disease 2019
- GO
gene ontology
- HSBD
Huashi Baidu Decoction
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MCODE
Molecular Complex Detection
- PPI
protein-protein interaction
- STITCH
Search Tool for Interacting Chemicals
- STRING
Search Tool for Retrieval of Interacting Genes/Proteins
- TCM
traditional Chinese medicine
- TCMID
Traditional Chinese Medicine Integrative Database
- TCMSP
Traditional Chinese Medicine Systems Pharmacology
1. Introduction
Coronavirus disease 2019 (COVID-19) is the pneumonia caused by 2019 novel coronavirus (2019-nCoV) infection. COVID-19 pandemic has broken out around the world since December 2019. The high infectivity of 2019-nCoV has posed great challenges to the precaution and treatment of COVID-19 worldwide.1 As of 5:04pm CET, January 7, 2021, 85,929,428 confirmed cases have been reported worldwide, and the global death toll has topped 1,876,100. The number of confirmed cases of 2019-nCoV infection have been 97,217 and the death toll has been 4,795 in China.1 At present, the COVID-19 pandemic has been well controlled in China, but it has been still serious in many countries, such as the United States of America, India, Brazil, Russian Federation and so on.
The main clinical symptoms of COVID-19 are fever, cough, anhelation and dyspnea. Severe cases may develop pneumonia, severe acute respiratory failure, renal failure, and even death.2 Many countries are stepping up research and development of 2019-nCoV vaccine. On December 30, 2020, the State Food and Drug Administration of China approved the conditional marketing of Sinopharm Zhongsheng Beijing company’s inactivated 2019-nCoV vaccine.3 The 2019-nCoV vaccine will play a major role in preventing and blocking the spread of COVID-19 in the world. However, there has been no explicit antiviral therapy for COVID-19 in clinic till now.4 Clinical trials have shown that traditional Chinese medicine has a prominent curative effect on viral pneumonia, the symptomatic treatments of combining traditional Chinese and western medicine on COVID-19 has been taken clinically in China that has achieved good therapeutic effect.5 As of March 17, 2020, in the treatment of COVID-19 in China, the participation rate of Traditional Chinese medicine was over 91.05%, that is, more than 91.05% confirmed COVID-19 cases were treated by combination of traditional Chinese medicine and Western medicine. Traditional Chinese medicine has played a very good role in the early intervention and the treatment of severe and critically patients.6
The therapy project of traditional Chinese medicine has been continually made for COVID-19 in the “Guidelines for the diagnosis and treatment of novel coronavirus pneumonia”, which has been updated to the seventh edition and issued by the National Health Commission of the People’s Republic of China.7 In the seventh edition of diagnosis and treatment guidelines, a traditional Chinese medicine (TCM) formula named Huashi Baidu Decoction (化濕敗毒方huà shī bài dú fāng) was recommended for severe COVID-19. Huashi Baidu Decoction is the novel complex prescription based on the theory of prevention and treatment of “epidemic disease” in the clinical diagnosis and treatment of COVID-19, which is specially formulated for the treatment of severe COVID-19 by the Chinese medicine experts from the China Academy of Chinese Medical Sciences and has good therapeutic effect on severe COVID-19.8 Huashi Baidu granules, which is developed based on Huashi Baidu Decoction, has been approved for clinical trials by National Medical Products Administration of the People’s Republic of China on March 18th, 2020.9
Huashi Baidu Decoction consists of 14 Chinese materia medica: Ephedra sinica Stapf, Prunus armeniaca L., Gypsum Fibrosum, Glycyrrhiza uralensis Fisch., Pogostemon cablin (Blanco) Benth., Magnolia officinalis Rehd. et Wils., Atractylodes lancea (Thunb.) DC., Amomum tsao-ko Crevost et Lemaire, Pinellia ternata (Thunb.) Breit., Poria cocos (Schw.) Wolf, Rheum palmatum L., Astragalus membranaceus (Fisch.) Bge., Lepidium apetalum Willd. and Paeonia veitchii Lynch.8 In severe COVID-19 cases, the main clinical symptoms are acute respiratory distress syndrome (ARDS) and septic shock.10 In this study, we attempted to screen out key Chinese materia medica, main active compounds, hub therapeutic target proteins and core signal pathways of Huashi Baidu Decoction in the treatment of severe COVID-19 utilizing integrating network pharmacological methods, furthermore, we tried to reveal its potential therapeutic mechanisms to provide basis for the future drug research and discovery.
2. Materials and methods
2.1. Constructing database of active compounds and potential target proteins
Using the standard Chinese materia medica name as the search term, all compounds in Huashi Baidu Decoction were retrieved from the online public database Traditional Chinese Medicine Systems Pharmacology (TCMSP).11 The druggability of candidate chemical components and corresponding target proteins were analyzed by oral bioavailability (OB) and drug-likeness (DL). OB ≥ 30% and DL ≥ 0.18 were considered to exhibit good pharmacokinetic properties and were screened out for analysis. Gypsum Fibrosum had not been retrieved from TCMSP. It was known that the ingredients of Gypsum Fibrosum are CaSO4·2H2O and CaSO4 via retrieving TCMID database,12 the target proteins of Gypsum Fibrosum were obtained from STITCH (http://stitch.embl.de/).13 The gene names corresponding to the target proteins were searched through UniProt database.14 All target proteins were standardized as gene names and UniProt IDs utilizing the UniProtKB (https://www.uniprot.org/) database with the “Homo sapiens” species.14
2.2. Predicting disease genes of severe COVID-19
Using “novel coronavirus pneumonia”, “acute respiratory distress syndrome” and “septic shock” as key words, selecting the species as “Homo sapiens”, the corresponding gene sets were retrieved from the GeneCards database, then the intersection of the three gene sets was collected to obtain the disease genes set of severe COVID-19.
2.3. Predicting therapeutic target proteins of Huashi Baidu Decoction on severe COVID-19
The Chinese materia medica target proteins and the disease genes were mapped employing Venny2.1 online tool (https://bioinfogp.cnb.csic.es/tools/venny/) and the potential target proteins of Huashi Baidu Decoction on severe COVID-19 were obtained.
2.4. Constructing protein-protein interaction (PPI) network and screening out the hub target proteins
In order to further clarify the interaction between potential target proteins, all potential therapeutic target proteins of Huashi Baidu Decoction on severe COVID-19 were imported into Cyctoscape-3.8.0 and analyzed by STRING plug-in,15 the protein type was defined as “Homo sapiens”, the relevant information on protein interactions was obtained which was saved as TSV format file. Next, the network topology parameters were analyzed by Cyctoscape-3.8.0 and the hub target proteins were screened out according to the standard that the node Degree value and the Betweenness Centrality value greater than the average value. Furthmore, the main modules of the PPI network were screened out by the Molecular Complex Detection (MCODE) plug-in of Cytoscape-3.8.0 with MCODE scores set as ≥ 3.
2.5. GO_BiologicalProcess and KEGG pathway enrichment analysis
In order to reveal the function and the role of the potential therapeutic target proteins in the signaling pathways by which Huashi Baidu Decoction treated severe COVID-19, the GO_BiologicalProcess and KEGG pathway enrichment analysis of all potential therapeutic target proteins were conducted by the ClueGO + CluePedia16 plug-in of Cytoscape-3.8.0.
2.6. Constructing Chinese materia medica-active compounds-potential therapeutic target proteins networks
Synthesizing the active components of Huashi Baidu Decoction and the potential therapeutic target proteins by which Huashi Baidu Decoction treated severe COVID-19, the “Chinese materia medica-active compounds-potential therapeutic target proteins” network was constructed by Cytoscape 3.8.0 software. Then, key Chinese materia medica and main active compounds were screened out according to the filtering criteria “Degree value > mean value, Betweenness Centrality value > mean value”.
3. Results
3.1. Prediction of the target proteins of Huashi Baidu Decoction
Based on TCMSP, TCMID and STITCH databases, 1615 compounds were discovered in Huashi Baidu Decoction, 204 active compounds were screened out by the filtering criteria “OB ≥ 30%, DL ≥ 0.18″, and 259 potential target proteins were picked out in total (Supplementary Table 1).
3.2. Prediction of disease genes of severe COVID-19
259, 3022 and 1009 disease genes of “novel coronavirus pneumonia”, “acute respiratory syndrome syndrom” and “septic shock” were collected respectively through GeneCards databases, and 120 disease genes related to severe COVID-19 were screened out after intersection.
3.3. Potential therapeutic target proteins of Huashi Baidu Decoction on severe COVID-19
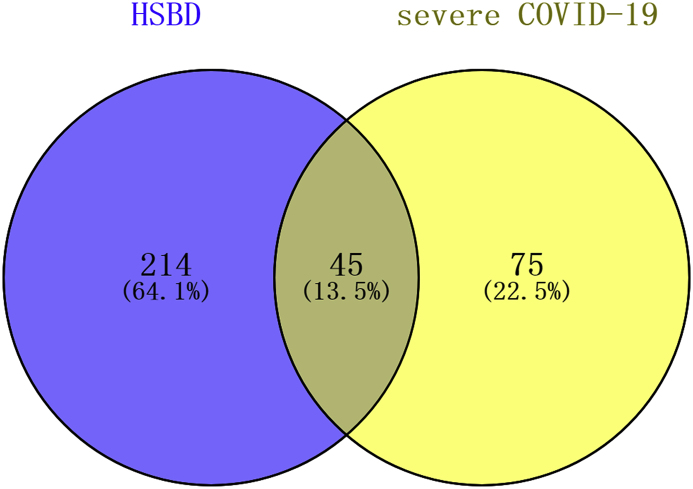
45 potential therapeutic target proteins were obtained by mapping the target proteins set of Huashi Baidu Decoction and the disease genes set of severe COVID-19 (Fig. 1).
Fig. 1.
Venn diagram of the mapping the target proteins set of Huashi Baidu Decoction (HSBD) and the disease genes set of severe COVID-19.
3.4. PPI network construction and the main modules and the hub target proteins analysis in PPI network
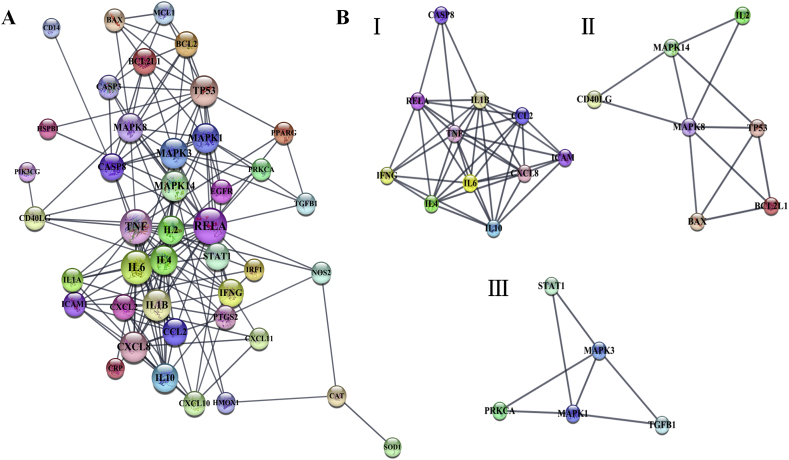
The structure, organization, biological process and function of the cell are mainly achieved through the interaction between proteins in cells. Therefore, it is of great significance to study the protein interactions and the network of protein-protein interaction (PPI) for the understanding of cell structure and function. The PPI analysis of 45 target proteins was carried out by using the STRING plug-in of Cytoscape-3.8.0. The minimum score was set as the highest confidence 0.9 to ensure high cross confidence information. There are 42 nodes and 174 edges in the PPI network (Fig. 2A), 3 disconnected proteins were excluded in the network. The larger the node, the larger the Degree value. The thicker the edge, the higher the score, that is, the closer the relationship between proteins. According to the further analysis of network topology parameters, 13 hub target proteins with node Degree value > mean value (7.73) and Betweenness Centrality value > mean value (0.028) were discovered. They were RELA, TNF, IL6, IL1B, MAPK14, TP53, CXCL8, MAPK3, MAPK1, IL4, MAPK8, CASP8 and STAT1. The target protein with higher Degree value and Betweenness Centrality value plays more important role in the network, which is likely to be the more core therapeutic target protein of Huashi Baidu Decoction on severe COVID-19 (Supplementary Table 2).
Fig. 2.
Protein-protein interaction (PPI) network diagram of potential therapeutic target proteins of Huashi Baidu Decoction on COVID-19. (A) The PPI network of 42 therapeutic target proteins of Huashi Baidu Decoction on severe COVID-19. The nodes indicate proteins, and edges represent protein-protein associations. (B) Clusters of the three main modules resolved from the PPI network by MCODE.
In addition, Molecular Complex Detection (MCODE) plug-in of Cytoscape-3.8.0 was used to get three main modules from the PPI network with the threshold of MCODE score ≥ 3.0 (Fig. 2B). Module Ⅰ included CASP8, CCL2, CXCL8, ICAM1, IFNG, IL10, IL1B, IL4, IL6, RELA and TNF genes. Module II included BAX, BCL2L1, CD40LG, IL2, MAPK14, MAPK8 and TP53 genes. Module III included MAPK1, MAPK3, PRKCA, STAT1 and TGFB1 genes. All 13 hub target proteins in PPI network appeared in three main modules respectively. The results further illustrated the key role of 13 hub target proteins in the treatment of severe COVID-19 by Huashi Baidu Decoction.
3.5. GO_BiologicalProcess enrichment analysis
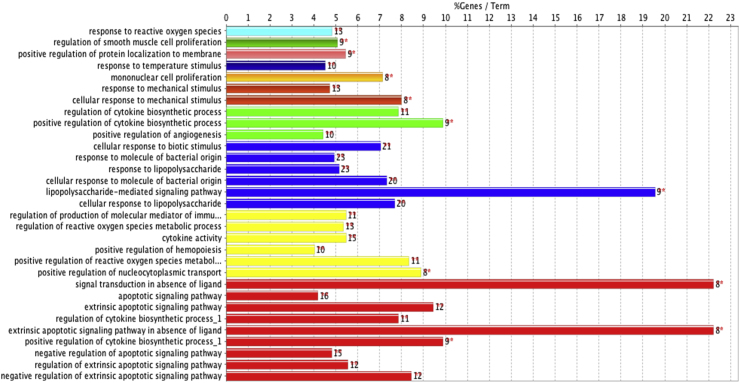
In order to have a more comprehensive understanding of the therapeutic mechanism of Huashi Baidu Decoction on severe COVID-19, 45 potential therapeutic target proteins were analyzed by GO_BiologicalProcess enrichment, using ClueGO + CluePedia plug-in of Cytoscape-3.8.0 based on GO_BiologicalProcess-EBI-UniProt-GOA_February 17, 2020_00h00 database. 399 terms were obtained with p value ≤ 0.05 as the threshold. The following figures and table showed 29 terms with p value ≤ 1.00E-7 as the threshold (Fig. 3, Supplementary Fig.1, Supplementary Table 3). The main terms were related to cellular response to biotic stimulus, response to lipopolysaccharide, cytokine activity, extrinsic apoptotic signaling pathway and so on.
Fig. 3.
GO_BiologicalProcess enrichment analysis of 45 potential therapeutic target proteins of Huashi Baidu Decoction on severe COVID-19. The bars show the percentage of genes in GO_BiologicalProcess terms.
3.6. KEGG pathway enrichment analysis
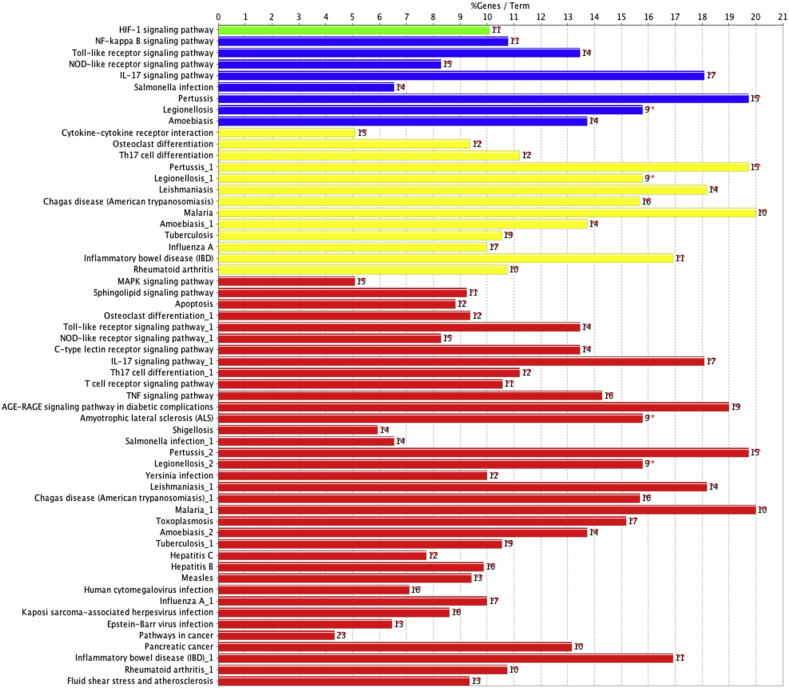
In order to further comprehensively understand the therapeutic mechanism of Huashi Baidu Decoction on severe COVID-19, 45 potential target proteins were analyzed by KEGG pathway enrichment, using ClueGO + CluePedia plug-in of Cytoscape-3.8.0 based on KEGG_February 17, 2020 database.
115 terms were obtained with the threshold of p value ≤ 0.05, and 39 terms were obtained with the threshold of p value ≤ 1.00E-9 (Fig. 4, Supplementary Fig.2, Supplementary Table 4). The main terms were AGE-RAGE signaling pathway in diabetic complications, IL-17 signaling pathway, TNF signaling pathway, Toll-like receptor signaling pathway, C-type lectin receptor signaling pathway, NOD-like receptor signaling pathway, NF-kappa B signaling pathway, T cell receptor signaling pathway, HIF-1 signaling pathway, MAPK signaling pathway and so on.
Fig. 4.
KEGG pathway enrichment analysis of 45 potential therapeutic target proteins of Huashi Baidu Decoction on severe COVID-19. The bars show the percentage of genes in KEGG pathway terms.
3.7. Analysis of the “Chinese materia medica-active compounds-potential therapeutic target proteins” network
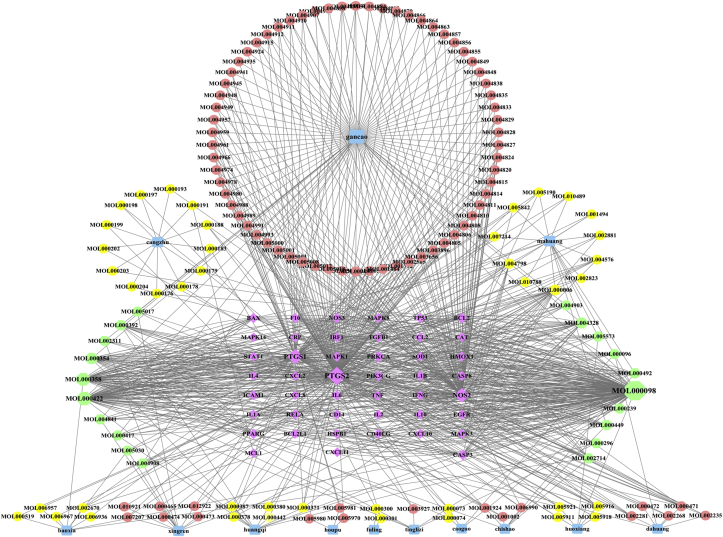
The 45 potential therapeutic target proteins and related Chinese materia medica and their active compounds in Huashi Baidu Decoction were used to construct the “Chinese materia medica-active compounds-therapeutic target proteins” network by Cytoscape-3.8.0 (Fig. 5). 4 key Chinese materia medica which were Glycyrrhiza uralensis Fisch, Ephedra sinica Stapf, Atractylodes lancea (Thunb.) DC and Astragalus membranaceus (Fisch.) Bge., and 11 main active compounds which were Quercetin, Kaempferol, β-sitosterol, Isorhamnetin, Naringenin, Luteolin, (+)-catechin, Delphinidin, aloe-Emodin, Baicalein and Irisolidone, were screened out according to filtering criteria “Degree value > mean value (8.81), Betweenness Centrality value > mean value (0.0083)” (Supplementary Fig. 3A and 3B, Supplementary Table 5).
Fig. 5.
The “Chinese materia medica-active compounds-therapeutic target proteins” network of Huashi Baidu Decoction on severe COVID-19. Diamond represents the potential therapeutic targets, circle represents the active compounds and square represents the Chinese materia medica. The larger the node, the more important it is. The edges represent the relationship between Chinese materia medica, active compounds and the potential therapeutic target proteins.
4. Discussion
The common clinical symptoms of COVID-19 included fever (88.7%), cough (67.8%), fatigue (38.1%), expectoration (33.4%), shortness of breath (18.6%), sore throat (13.9%), headache (13.6%), diarrhea (3.8%) and vomiting (5%).17 The old people and the people with basic diseases (i.e. hypertension, chronic obstructive pulmonary disease, diabetes, cardiovascular disease) would rapidly develop into acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, coagulation dysfunction and other severe symptoms, even death.2,17 Huashi Baidu Decoction (HSBD) was mainly used in clinical treatment of severe COVID-19 of which the main symptoms are acute respiratory distress syndrome (ARDS) and septic shock.10 However, the underlying therapeutic molecular mechanisms of HSBD on severe COVID-19 remain unclear.
Acute respiratory distress syndrome (ARDS) and septic shock might cause the death of patients, in which ARDS was the main cause of death in severe COVID-19 patients.2 ARDS is an unexpected inflammatory disease, characterized by dyspnea, refractory hypoxia, diffuse destruction of alveoli and high mortality.18 Sepsis and pneumonia often play a major role in its etiology.18 In the pathogenesis of ARDS, inflammatory cytokines TNF, IL-1, IL-6 and IL-8 play a key role.19,20 In the treatment of severe COVID-19, anti-cytokine therapy, especially anti-IL-6 and IL-1 antagonists, has been proposed to reduce hyperinflammation that may develop with 2019-nCoV induced-ARDS due to the lack of vaccines or proven antiviral therapy.21
Septic shock is newly defined as sepsis with fluid-unresponsive hypotension. It can be caused by any microbial infection (bacteria, viruses, fungi, parasites, etc.). Lipopolysaccharide (LPS) of Gram-negative bacteria and lipoteichoic acid and peptidoglycan of Gram-positive bacteria are the most common sepsis media.22 Similar to acute respiratory distress syndrome (ARDS), cytokine storm plays an important role in the pathological process of septic shock. The key inflammatory cytokines are TNF-α and IL-1, which can lead to hypotension and multiple organ failure (MOF).22 IL-6 and procalcitonin (PCT) are also important inflammatory biomarkers of sepsis.23
According to the PPI network topology parameters, 13 hub target proteins were screened out from 45 potential therapeutic target proteins by which Huashi Baidu Decoction (HSBD) treated severe COVID-19. They were RELA > TNF > IL6 > IL1B > MAPK14 > TP53 > CXCL8 > MAPK3 >MAPK1 > IL4 > MAPK8 > CASP8 > STAT1 based on the order of Degree value (Fig. 2). RELA is the first one, which is a subunit of transcription factor NF-κB, indicating that NF-κB signaling pathway is the key therapeutic pathway of HSBD in the treatment of severe COVID-19. NF-κB signaling pathway is an important pathway to regulate the expression of inflammatory factors. It is one of the main pathways involved in immune and inflammatory response. Blocking NF-κB pathway could correct the abnormal sepsis, restore systemic hypotension and inhibit the expression of many pro-inflammatory genes, so as to prevent multiple organ damage and improve the survival rate of septic shock rats.24 NF-κB signaling pathway was also involved in LPS-stimulated coagulation and fibrinolysis factors expression in alveolar epithelial cells of type II (AECII), thus correcting the abnormality of coagulation and fibrinolysis in alveoli of ARDS patients.25 Therefore, these results suggested that HSBD might treat severe COVID-19 by inhibiting inflammation progress. TNF, IL-6, IL-1 are the most important cytokines in the course of acute respiratory distress syndrome (ARDS) or septic shock. In the PPI network of therapeutic target proteins of HSBD on severe COVID-19, TNF, IL-6, IL-1 were respectively ranked at the second, third and fourth positions in the hub therapeutic target proteins. These results suggested that HSBD might block the progression of ARDS or septic shock through inhibiting the expression of the core inflammatory cytokines.
The top three terms in GO_BiologicalProcess enrichment results were “cell response to biological stimulus”, “response to lipolysaccharide”, “cell response to lipolysaccharide” (Fig. 3, Supplementary Table 3). Septic shock is caused by infection of extrinsic microorganism. Lipopolysaccharide is the most common endotoxin secreted by gram-negative bacteria, and it is also the main inflammatory mediator that leads to septic shock. In the course of acute respiratory distress syndrome (ARDS), sepsis and pneumonia caused by exogenous microorganism infection are the most important inducements. The GO_BiologicalProcess enrichment results showed that Huashi Baidu Decoction (HSBD) could reverse the progression of severe COVID-19 by regulating the body response to exogenous biological stimulation and inhibiting LPS induced-inflammatory response. At the same time, HSBD might also reduce the apoptosis of lung tissue caused by 2019-nCoV by regulating the biological processes such as extrinsic apoptotic signaling pathway in absence of ligand, negative regulation of apoptotic process, finally inhibit the progress of ARDS or septic shock. In addition, HSBD might also regulate the lung tissue peroxidation damage caused by 2019-nCoV infection through the regulation of reactive oxygen species metabolic process.
According to the results of KEGG enrichment analysis, the core pathways involved in the treatment of Huashi Baidu Decoction (HSBD) on severe COVID-19 were AGE-RAGE signaling pathway, IL-17 signaling pathway, TNF signaling pathway, Toll-like receptor signaling pathway, C-type lectin receptor signaling pathway and NOD-like receptor signaling pathway, etc (Fig. 4, Supplementary Table 4). AGE-RAGE signaling pathway is an oxidative stress-induced pathway. The increase of oxidative stress in cells leads to the translocation and activation of NF-κB, and then up-regulates the expression of NF-κB dependent genes, and finally exerts harmful effects on cells. Inhibition of RAGE-mediated MAPK/NF-κB signaling pathway and NLRP3 inflammatory body activation could repress the gene expression of proinflammatory cytokines and chemokines such as IL-1 β, IL-6, TNF-α, MCP-1.26 The interleukin 17 (IL-17) family plays crucial roles in both acute and chronic inflammatory responses. The IL-17 family signals activates downstream pathways that include NF-κB and MAPKs to induce the expression of antimicrobial peptides, cytokines and chemokines via their correspondent receptors.27 IL-17 level was elevated in sepsis induced-ARDS patients and IL-17 antibody could reduce the symptoms of acute lung injury (ALI) by regulating RORγt level and PI3K pathway.28 Microbial infection of cells activates the inflammatory response. The initial sensing of infection is mediated by innate pattern recognition receptors (PRRs), including Toll-like receptors, NOD-like receptors, C-type lectin receptors and RIG-I-like receptors. The intracellular signaling cascade triggered by these PRRs leads to the transcription and expression of inflammatory mediators, coordinating the clearance of pathogens and infected cells. However, septic shock will be induced by abnormal activation of this system.29 In addition, T cell receptor signaling pathway is involved in septic shock, septic shock patients have T-cell dysfunction which leads to T-cell failure and increases the risk of death. T cell receptor activation would affect the occurrence of T-cell population and the development of T-cell alterations in septic shock.30 Therefore, it can be concluded that HSBD might exert its therapeutic effects on severe COVID-19 by inhibiting the pathogens-induced inflammatory response and regulating the immune function of T cells. This conclusion was consistent with the results of GO_BiologicalProcess enrichment.
4 key Chinese materia medica: Glycyrrhiza uralensis Fisch, Ephedra sinica Stapf, Atractylodes lancea (Thunb.) DC and Astragalus membranaceus (Fisch.) Bge. have 78, 18, 13 and 12 active compounds and 41, 42, 14 and 38 potential therapeutic target proteins involved in the treatment of severe COVID-19, respectively (Fig. 5, Supplementary Fig.3A, Supplementary Table 5). There were total 45 potential therapeutic target proteins of Huashi Baidu Decoction (HSBD) on severe COVID-19, among which Glycyrrhiza uralensis Fisch, Ephedra sinica Stapf and Astragalus membranaceus (Fisch.) Bge. covered respectively 93.3%, 91.1% and 84.4% total potential therapeutic target proteins, indicating that they played a key role in the therapeutic process of HSBD on severe COVID-19. In the future clinical practice, Glycyrrhiza uralensis Fisch, Ephedra sinica Stapf and Astragalus membranaceus (Fisch.) Bge. may be the first choice for the treatment of severe COVID-19.
Among the 11 main active compounds of Huashi Baidu Decoction (HSBD), many active compounds are common to several Chinese materia medica (Fig. 5, Supplementary Fig.3B, Supplementary Table 5). For example, quercetin is a common component of Glycyrrhiza uralensis Fisch, Ephedra sinica Stapf, Astragalus membranaceus (Fisch.) Bge., Amomum tsao-ko Crevost et Lemaire and Pogostemon cablin (Blanco) Benth. Kaempferol is a common component of Glycyrrhiza uralensis Fisch, Ephedra sinica Stapf, Astragalus membranaceus (Fisch.) Bge. and Lepidium apetalum Willd. β-sitosterol is a common component of Ephedra sinica Stapf, Pinellia ternata (Thunb.) Breit., Paeonia veitchii Lynch, Rheum palmatum L and Lepidium apetalum Willd. The analysis results showed that the compatibility of different Chinese materia medica might increase the content of many active compounds in the whole prescription and enhance the therapeutic effects of these active compounds.
Among the 11 main active compounds of Huashi Baidu Decoction (HSBD), quercetin has the largest Degree value and the largest number of potential therapeutic target proteins, it can act on 60% of the total potential therapeutic target proteins including IL6, IL1B, TP53, MAPK1 and CASP8 hub target proteins that are involved in the core pathways such as AGE-RAGE, IL-17, TNF, Toll-like receptor, C-type lectin receptor and NOD-like receptor signaling pathways by which HSBD treats severe COVID-19, indicating that it may be the most important therapeutic active compound of HSBD on severe COVID-19. Luteolin has the second largest number of potential therapeutic target proteins, which covers 46.7% total potential therapeutic target proteins including RELA, TNF, MAPK1, MAPK3, TP53, IL-4 and IL-6 hub target proteins that are also involved in the same core pathways of HSBD’s pharmacological effects. Kaempferol has the third largest number of potential therapeutic target proteins, which covers 35.6% total potential therapeutic target proteins including RELA, TNF, STAT1 and MAPK8 hub target proteins that are also involved in the same core pathways of HSBD’s pharmacological effects. In addition, naringenin, β-sitosterol, delphinidin, isorhamnetin, aloe-emodin, irisolidone, baicalein and (+)-catechin act on 26.7%, 20%, 22.2%, 20%, 17.8%, 17.8%, 8.9% and 6.7% total potential therapeutic target proteins, respectively. Among them, naringenin and delphinidin act on hub target proteins MAPK1, MAPK3 and RELA; β-sitosterol acts on hub target protein CASP8; isorhamnetin acts on hub target proteins MAPK14 and RELA; aloe-emodin acts on hub target proteins IL-1B and TP53; irisolidone acts on hub target proteins MAPK1, MAPK3, RELA IL-1B and TNF; baicalein acts on hub target protein TP53. Except baicalein and (+)-catechin, the other 6 main active compounds are involved in the same core pathways of HSBD’s pharmacological effects. These data indicated that the anti-inflammatory pharmacological effects of HSBD were exerted through these main active compounds.
Literature search results also showed that, all 11 main active compounds of Huashi Baidu Decoction (HSBD): quercetin,31 luteolin,32 kaempferol,33 naringenin,34 β-sitosterol,35 delphinidin,36 isorhamnetin,37 aloe-emodin,38 irisolidone,39 baicalein40 and (+)-catechin41 had anti-inflammatory and anti-acute lung injury effects. These literature data proved the results of our network pharmacology analysis. Further literature study suggested that, among the 11 main active compounds of HSBD, naringenin, beta-sitosterol, aloe-emodin, luteolin, and quercetin were the potent inhibitors of SARS-CoV 3CLPRO and kaempferol could inhibit SARS-CoV 3a protein. The antiviral property of these active compounds could also contribute to therapeutic effect of HSBD on severe COVID-19.42 In summary, the anti-inflammatory effect, anti-acute lung injury effect and antiviral property of 11 main active compounds should be the pharmacological basis of HSBD on the treatment of severe COVID-19.
5. Conclusions
Huashi Baidu Decoction (HSBD) can treat severe COVID-19 by acting on 45 potential therapeutic targets including 13 hub target proteins. In the therapeutic mechanism of HSBD on severe COVID-19, 4 key Chinese materia medica, 11 main active compounds, multiple anti-inflammatory and immunomodulatory pathways, and the antiviral property are involved. It seems that the compatibility of Chinese materia medica or active compounds, based on the theory of multi-target therapy, may be a very promising treatment strategy to cure severe COVID-19. Our work also provided a basis for the new drug development from the effective monomer compounds of Huashi Paidu Decoction for the treatment of severe COVID-19.
Funding
This work was supported by the Teachers’ Academic Community Project of Shanghai University of Traditional Chinese Medicine (Grant No. P304030105), the National Natural Science Foundation of China (Grant No. 81703832), Key Projects “Clinical evaluation research of Chinese medicine intervention during the recovery period of new coronary pneumonia (COVID-19)” (Grant No. 2020YFC0845000) of the National Key Research and Development Plan “Prevention, Control and Emergency Technical Equipment of Public Safety Risk”.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.01.004.
Contributor Information
Cheng-Hai Liu, Email: 0000001034@shutcm.edu.cn.
Xu-Dong Hu, Email: huxudongsh@shutcm.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/table, 2021-01-07..
- 2.Huang C.L., Wang Y.M., Li X.W. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.State Council, PRC Transcript of press conference on December 31. 2020. http://www.gov.cn/fuwu/2020-12/31/content_5575768.htm, 2020-12-31
- 4.Panyod S., Ho C.T., Sheen L.Y. Dietary therapy and herbal medicine for COVID-19 prevention: a review and perspective. J Tradit Complement Med. 2020;10(4):420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q.Y., Wang X.L. Strategies for the development of drugs targeting novel coronavirus 2019-nCov. Acta Pharm Sin. 2020;2:181–188. [Google Scholar]
- 6.National Health Commission, PRC Transcript of press conference on March 17. 2020. http://www.nhc.gov.cn/xcs/s3574/202003/01426fc0590249ecac89a2874214e523.shtml, 2020-03-17
- 7.National Health Commission, PRC. Guidelines for the diagnosis and treatment of novel coronavirus pneumonia (Trial Version 7). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml, 2020-03-04.
- 8.Zou B.L., Li M., Fan T.B. Experience summary and the diagnosis and treatment program of TCM on severe COVID-19. J Tradit Chin Med. 2020;61(15):1289–1293. [Google Scholar]
- 9.Luo N.Y. Huashi Baidu granule was approved by the state Food and drug administration of China. J Trad Chin Med Manag. 2020;28:142. 06. [Google Scholar]
- 10.Jin Y.H., Cai L., Cheng Z.S. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ru J.L., Li P., Wang J.A. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue R.C., Fang Z., Zhang M.X., Yi Z.H., Wen C.P., Shi T.L. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 2013;41(D1):D1089–D1095. doi: 10.1093/nar/gks1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szklarczyk D., Santos A., von Mering C., Jensen L.J., Bork P., Kuhn M. Stitch 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016;44(D1):D380–D384. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UniProt Consortium T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45(D1):D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szklarczyk D., Gable A.L., Lyon D. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bindea G., Mlecnik B., Hackl H. GlueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal N.R., King L.S., D’Alessio F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306(8):L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araz O. Current pharmacological approach to ARDS: the place of bosentan. Eurasian J Med. 2020;52(1):81–85. doi: 10.5152/eurasianjmed.2020.19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlach H. vol. 5. 2016. p. 2909. (Agents to Reduce Cytokine Storm). F1000Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honore P.M., Hoste E., Molnár Z. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9(1):56. doi: 10.1186/s13613-019-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S.F., Malik A.B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 25.Liu B., Wu Y., Wang Y. NF-κB p65 Knock-down inhibits TF, PAI-1 and promotes activated protein C production in lipopolysaccharide-stimulated alveolar epithelial cells type II. Exp Lung Res. 2018;44(4-5):241–251. doi: 10.1080/01902148.2018.1505975. [DOI] [PubMed] [Google Scholar]
- 26.Yu W., Tao M., Zhao Y., Hu X., Wang M. 4’-Methoxyresveratrol alleviated AGE-induced inflammation via RAGE-mediated NF-κB and NLRP3 inflammasome pathway. Molecules. 2018;23(6):1447. doi: 10.3390/molecules23061447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amatya N., Garg A.V., Gaffen S.L. IL-17 signaling: the yin and the yang. Trends Immunol. 2017;38(5):310–322. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Q., Liu G.Q., Zeng Y.Y. Role of IL-17 in LPS-induced acute lung injury: an in vivo study. Oncotarget. 2017;8(55):93704–93711. doi: 10.18632/oncotarget.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Mouillaux J., Allam C., Gossez M. TCR activation mimics CD127lowPD-1high phenotype and functional alterations of T lymphocytes from septic shock patients. Crit Care. 2019;23(1):131. doi: 10.1186/s13054-018-2305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerin F., Sener U., Erman H. The effects of quercetin on acute lung injury and biomarkers of inflammation and oxidative stress in the rat model of sepsis. Inflammation. 2016;39(2):700–705. doi: 10.1007/s10753-015-0296-9. [DOI] [PubMed] [Google Scholar]
- 32.Park E.J., Kim Y.M., Kim H.J., Chang K.C. Luteolin activates ERK1/2- and Ca2+-dependent HO-1 induction that reduces LPS-induced HMGB1, iNOS/NO, and COX-2 expression in RAW264.7 cells and mitigates acute lung injury of endotoxin mice. Inflamm Res. 2018;67(5):445–453. doi: 10.1007/s00011-018-1137-8. [DOI] [PubMed] [Google Scholar]
- 33.Qian J., Chen X., Chen X. Kaempferol reduces K63-linked polyubiquitination to inhibit nuclear factor-κB and inflammatory responses in acute lung injury in mice. Toxicol Lett. 2019;306:53–60. doi: 10.1016/j.toxlet.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Wang N., Fan S. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci Rep. 2016;6:39735. doi: 10.1038/srep39735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampronti I., Dechecchi M.C., Rimessi A. β-Sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front Pharmacol. 2017;8:236. doi: 10.3389/fphar.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z., Zhang R., Shi W. The multifunctional benefits of naturally occurring delphinidin and its glycosides. J Agric Food Chem. 2019;67(41):11288–11306. doi: 10.1021/acs.jafc.9b05079. [DOI] [PubMed] [Google Scholar]
- 37.Yang B., Li X.P., Ni Y.F. Protective effect of isorhamnetin on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2016;39(1):129–137. doi: 10.1007/s10753-015-0231-0. [DOI] [PubMed] [Google Scholar]
- 38.Li X., Shan C., Wu Z., Yu H., Yang A., Tan B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm Res. 2020;69(4):365–373. doi: 10.1007/s00011-020-01331-3. [DOI] [PubMed] [Google Scholar]
- 39.Jang H.M., Park K.T., Noh H.D., Lee S.H., Kim D.H. Kakkalide and irisolidone alleviate 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by inhibiting lipopolysaccharide binding to toll-like receptor-4 and proteobacteria population. Int Immunopharm. 2019;73:246–253. doi: 10.1016/j.intimp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Guo Q.H., Liu G.Y., Liu X.B., Huang Q.S. Baicalein exerts a protective role in pneumonia caused by Streptococcus pneumoniae. Front Biosci (Landmark. Ed) 2019;24:849–858. doi: 10.2741/4755. [DOI] [PubMed] [Google Scholar]
- 41.Liang O.D., Kleibrink B.E., Schuette-Nuetgen K., Khatwa U.U., Mfarrej B., Subramaniam M. Green tea epigallo-catechin-galleate ameliorates the development of obliterative airway disease. Exp Lung Res. 2011;37(7):435–444. doi: 10.3109/01902148.2011.584359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuzimoto A.D., Isidoro C. The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds - additional weapons in the fight against the COVID-19 pandemic? J Tradit Complement Med. 2020;10(4):405–419. doi: 10.1016/j.jtcme.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.