Coronavirus disease 2019 (Covid-19) is characterized by fever and respiratory symptoms. Typical presentation is pneumonia resulting in acute respiratory distress syndrome which may be fatal. The mortality rate of Covid-19 is 2.7–3.4% (San-Juan et al., 2020) but many patients require short- or long-term intensive care unit (ICU) therapy. Neurological complications of COVID-19 are not well-described but there is an increasing number of reports of patients with complications in the central and peripheral nervous system (San-Juan et al., 2020). Here, we describe a patient who developed neurological complications as a consequence of Covid-19.
A 68-year old man with no past medical history was admitted on the 22nd March, 2020 to a local hospital with an 8-day history of fever, cough and dyspnea. He had not recently travelled. His body temperature exceeded 40 °C, respiratory rate was 24 breaths/min and the oxygen saturation was 95% on ambient air. A chest X-ray was compatible with pneumonia. He received treatment with piperacillin/tazobactam. Real-time polymerase-chain-reaction assay (RT-PCR) of nasal swab returned positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). He deteriorated and was intubated two days after hospitalization, and treated in the ICU for 11 days. He failed a spontaneous-breathing trial and needed re-intubation within 48 h following extubation. He was moved to the ICU at Aarhus University Hospital because of weaning failure from mechanical ventilation, muscle weakness and multiple organ failure. Neuroimaging of the central nervous system and cerebrospinal fluid examinations excluded neuroinfection or structural lesion. On clinical examination he had severe symmetrical proximal and distal weakness (Medical Research Council-score, 2/5), diffuse muscle wasting, and absent deep tendon reflexes.
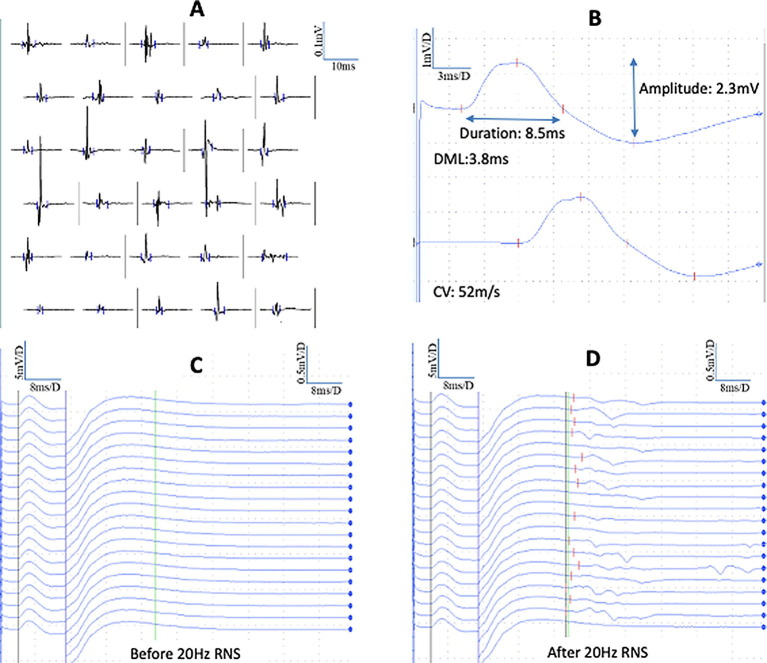
On day 65 of hospitalization, nerve conduction studies (NCS) and quantitative electromyography were performed using conventional methods described elsewhere (Tankisi et al., 2019) with the suspicion of Guillain-Barré-Syndrome (GBS) or critical illness polyneuropathy (CIP). Abnormal findings on needle EMG were shortened motor unit potentials (MUPs) consistent with myopathy in biceps brachii and vastus medialis muscles with passive movements of the joints (Fig. 1 a) but there was no fibrillations or positive-sharp-waves whereas in the anterior tibial muscles bilaterally, profuse denervation activity was found. Left peroneal motor conduction velocity (CV) was decreased across the knee (27.8 m/s) and normal in lower leg segment (42.2 m/s), right motor response was absent as well as bilateral superficial peroneal sensory action potentials suggesting bilateral peroneal compression neuropathy. There were normal sensory NCS of median (CV:53.7 m/s, amplitude:10.2 µV), ulnar (CV:51.2 m/s, amplitude:6.9 µV) and right sural (CV:51.6 m/s, amplitude:2.7 µV) and left sural (CV:49.2 m/s, amplitude:2.8 µV) nerves. Furthermore, there were reduced compound muscle action potential (CMAP) amplitudes in median (2.3 mV), ulnar (1.6 mV) and tibial (right:0.8 mV, left:0.9 mV) nerves. CMAP duration was only increased in median nerve (8.5 ms) (Fig. 1B) and normal in ulnar (6.9 ms) and tibial (right:7.2 ms, left:7.3 ms) nerves. F-waves were absent in all motor nerves. In the right median nerve, a 1-second burst of 20 Hz repetitive nerve stimulation (RNS) was performed and immediately after RNS, F-waves were easily obtained with normal minimum F-wave latencies and frequencies (Fig. 1c and d). There was no decrement with 3 Hz repetitive stimulation. In conclusion, the findings indicated critical illness myopathy (CIM) but not GBS or CIP.
Fig. 1.
(A) Polyphasic and short duration myopathic motor unit potentials (MUPs) with quantitative needle electromyography of the biceps brachii muscle. Normal mean duration of all potentials (11.7 ms) (27% polyphasia) but shortened duration of simple potentials (9.4 ms, lower limit of normal: 9.8 ms), (B) synchronous and smooth long duration compound muscle action potentials (CMAPs) in median nerve with normal distal motor latency (DML) and conduction velocity (CV), (C) right median motor F-waves: 20 trials of supramaximal stimulation delivered at 1/s, before a 20 Hz repetitive nerve stimulation (RNS) showing absent F-waves, (D) normal latency and frequency of F-waves after 20 Hz RNS.
While there is agreement in the literature that CIM is more common than CIP, the incidence of CIP is controversial (ŹGraggen and Tankisi, 2020), probably because of the difficulty of performing sensory NCS in ICU (Tankisi et al., 2020). Additionally, reduced CMAP amplitudes and absent F-waves may cause misinterpretation in favor of CIP. We found normal latency and frequency of F-waves in the median nerve after 20 Hz RNS as suggested recently (Zimnowodzki et al., 2020). This method may contribute to the diagnosis of CIM as opposed to demyelinating polyneuropathies since absent F-waves may reflect abnormalities of excitability in the proximal nerve segments or proximal conduction block. The initial lack of F-waves in CIM that can be reverted after 20 Hz RNS suggests decreased motor neuron excitability, possibly due to a prolonged period of reduced mobility, which is proposed as a pathophysiological mechanism of CIM in addition to other risk factors including sepsis and multiorgan failure (ŹGraggen and Tankisi, 2020).
To the best of our knowledge, this is the first case of CIM in a patient surviving from severe Covid-19. While clinical and electrophysiological findings resembled CIM of other causes, we think that our case deserves special attention in the context of a growing awareness of long-term complications of Covid-19. At present, health care systems worldwide are overwhelmed by the striking speed of the Covid-19 pandemic. As a consequence of the distinctive feature of Covid-19 requiring long-term ventilatory therapy, the next several months the Covid-19 pandemic probably will cause more cases of CIM or CIP resulting in long-term physical, cognitive and emotional complications and growing health care costs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- San-Juan D., Jiménez C.R., Camilli C.X., de la Cruz Reyes L.A., Galindo E., Burbano G. Guidance for clinical neurophysiology examination throughout the COVID-19 pandemic. Latin American chapter of the IFCN task force-COVID-19. Clin Neurophysiol. 2020;131:1589–1598. doi: 10.1016/j.clinph.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankisi H., Pugdahl K., Beniczky S., Andersen H., Fuglsang-Frederiksen A. Evidence-based recommendations for examination and diagnostic strategies of polyneuropathy electrodiagnosis. Clin Neurophysiol Pract. 2019;4:214–222. doi: 10.1016/j.cnp.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankisi H., Burke D., Cui L., de Carvalho M., Kuwabara S., Nandedkar S.D. Standards of instrumentation of EMG. Clin Neurophysiol. 2020;131:243–258. doi: 10.1016/j.clinph.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Z’Graggen W.J., Tankisi H. Critical illness myopathy. J Clin Neurophysiol. 2020;37:200–204. doi: 10.1097/WNP.0000000000000652. [DOI] [PubMed] [Google Scholar]
- Zimnowodzki S., Butrum M., Kimura J., Stålberg E., Mahajan S., Gao L. Emergence of F-waves after repetitive nerve stimulation. Clin Neurophysiol Pract. 2020;5:100–103. doi: 10.1016/j.cnp.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]