Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes coronavirus disease 2019 (COVID-19), a severe illness leading to pneumonia, multiorgan failure, and death. With this study, we performed a systematic review of the literature and ongoing clinical trials on convalescent plasma therapy in pediatric patients with COVID-19. The electronic databases Medline PubMed, Scopus, and Web Of Science were searched. Also, clinical trials registries were searched for potentially eligible studies. A total of 90 records were retrieved after duplicate removal. Eight studies were case reports of children treated with convalescent plasma therapy (14 children, age range, 9 weeks to 18 years); 5 children had a chronic disease. During the hospital stay, 5 received drugs (e.g., remdesivir) in addition to convalescent plasma therapy. No convalescent plasma therapy-related adverse events were reported in 5 studies and 3 made no mention of adverse events. Seven studies concluded that convalescent plasma therapy is or could be a useful therapeutic option; one study made no claims. Only 3 of the 13 retrieved trials underway were planned exclusively for children. This is the first systematic review of the literature regarding convalescent plasma therapy for COVID-19 in children. We found insufficient clinical information on the safety and efficacy of convalescent plasma therapy in children. Nevertheless, the positive outcomes of the few case reports published to date suggest that convalescent plasma therapy may be of potential benefit. Further research with well-designed and powered clinical trials is needed.
Abbreviations: CPT, convalescent plasma therapy; RBD, receptor-binding domain; RT-PCR, real-time reverse transcription-polymerase chain reaction
Keywords: Children, COVID-19 pandemic, Plasma, SARS-CoV-2
1. Introduction
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originated in the city of Wuhan in Hubei province, China, in late 2019. This virus is the cause of coronavirus disease 2019 (COVID-19), a severe illness characterized by pneumonia with increased infiltration of inflammatory cells and higher levels of pro-inflammatory cytokines (cytokine storms). Such events may lead to acute pulmonary injury, acute respiratory distress syndrome (ARDS), multiorgan failure, and ultimately death [[1], [2], [3]]. During the first three months of 2020, SARS-CoV-2 infection began to spread worldwide. It was finally classified by the World Health Organization (WHO) as a Public Health Emergency of International Concern. At the time of writing, nearly 50 million people have been infected by the virus and approximately 1,25 million have died (WHO. Coronavirus disease [COVID-19] outbreak, accessed 09/11/2020) [4]. The median age of patients who died due to severe COVID-19 during the first pandemic wave (March-June 2020) was 78 years (interquartile range [IQR], 67–87), according to the U.S. Centers for Disease Control and Prevention (CDC) [5]. Patient age progressively declined over the summer months due to the lack of adoption by younger individuals of minimum preventive measures (use of masks and social distancing) [5]. Even with the decreasing age of symptomatic COVID-19 patients during the second pandemic wave, symptomatic cases among children remain rare for still largely unknown reasons. Nonetheless, cases of multisystem inflammatory and Kawasaki syndrome in children and adolescents have been reported [6].
While there are various treatment options for SARS-CoV-2, no effective vaccine or drug against the virus is currently available [7]. Passive immunotherapy using hyperimmune convalescent plasma (HCP) from SARS-CoV-2 recovered donors has been largely explored [[8], [9], [10]] and reviewed [11]. Previous RCTs in adults with COVID-19 infection did not show any beneficial effects of CP [[12], [13], [14], [15]]. In addition, a Cochrane review supports uncertainty about the safety and efficacy of therapy in COVID-19 adults with CP [11]. Unfortunately, little data for the pediatric population exist. To fill this gap, we conducted a systematic review the literature and ongoing clinical trials on the use of HCP in pediatric patients with COVID-19 infection.
2. Methods
2.1. Search strategy
The electronic databases Medline PubMed Advanced Search Builder, Scopus, and Web Of Science were searched (until November 1, 2020) using medical subject headings (MeSH) terms and text words (their combinations and truncated synonyms) as appropriate: [HYPERIMMUNE PLASMA or CONVALESCENT PLASMA] and [PEDIATRIC OR CHILD] and [COVID-19 or SARS-COV-2]. When available articles were retrieved, the abstracts were screened after removal of duplicate articles. The full text was analyzed and the references were screened for further articles missed in the primary search. This review is not limited to the geographical area or gender. Inclusion criteria were: children with COVID-19 (or SARS-COV-2), in which convalescent plasma (or hyperimmune plasma) was used as treatment. Studies reporting the results of controlled trials, case-control studies, cohort studies, with synthesized data were included. The search was limited to articles published in English. Exclusion criteria were: studies published only as abstracts, letters or conference proceedings, discussion papers, animal studies, or editorials. Initial screening of titles identified potentially relevant studies, followed by screening of abstracts and then full-text review. All titles and abstracts were independently evaluated by two reviewers (MZ, MF), not blinded to authors, journals, results for consistency of inclusion/exclusion and any disagreement was solved by consensus. If the two review authors did not reach an agreement, a third review author was consulted to solve disagreement. No ethical approval was required for this study.
2.2. Study review and data extraction
Two independent reviewers (MZ, MF) evaluated the articles potentially meeting the inclusion criteria and retrieved the full text. Studies that did not fulfil all inclusion criteria were excluded; reasons for exclusion are reported. Table 1 presents the articles excluded from the review because not pertinent to the present study. Full texts were screened, and bibliographic details, as well as data regarding study design, participants, disease severity, intervention, and outcomes were recorded on predefined forms. When data from the same cohort were presented in more than one article, only the reports that most directly evaluated therapy with convalescent plasma (or hyperimmune plasma) and COVID-19 (or SARS-COV-2) in children (age, 0–18 years) [16] were included. All data, numerical calculations, and graphic extrapolations were independently confirmed. We did not deal with missing data. Due to the lack of study homogeneity, a narrative synthesis of the results was conducted.
Table 1.
Characteristics of case reports of children treated with convalescent plasma.
| Author (year) |
Design | Country | Case study | Comorbidity | Clinical condition | Diagnostic approach | Treatment | Reason for CP* treatment | Outcome | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Jin H., et al. (Sep 2020) [20] | Case report | USA | Case 1−10-year-old male; excluded: Case 2−24-year-old man; Case 3−40-year-old man |
Hereditary spherocytosis + X-linked agammaglobulinemia (XLA) | Initial symptoms: 10 dys before hospitalization; chest X-ray: right middle and lower lobe infiltrates | At admission: negative naso-pharyngeal swab RT-PCR;*** day 19: positive bronchoalveolar lavage RT-PCR*** |
10-day course of remdesivir; 2 units 200 mL unmixed ABO-compatible CP* (days 22 and 23) |
Minimal improvement on supportive therapies | Recovered after receiving CP* (6 dys). | CPT may help neutralize virus, shorten duration of illness, also in later stages of COVID-19 |
| Figlerowicz M, et al. (July 2020) [21] | Case report | Poland | 6-year-old girl | Aplastic anemia with severe pancytopenia | Hepatomegaly and bilaterally enlarged kidneys; COVID-19-associated severe aplastic anemia |
RT-PCR*** test on nasopharyngeal swab. | IVIG, lopinavir-ritonavir (10 mg + 2.5 mg twice daily). At 5 wks: CP* with antibodies against IgG titer 1:700 once in a 200 mL/dose |
Poor effect of treatment: IVIG, lopinavir-ritonavir + steroid | Negative SARS-CoV-2 RNA in nasopharyngeal swabs (3 wks); hematologic parameters (pancytopenia) did not improve; no adverse events |
In patients with pancytopenia, transfusion of CP* could be an option |
| Shankar AU, et al. (2020) [22] | Case report | India | 4-year-old girl | Acute lymphoblastic leukemia | Chest X-ray: bilateral fluffy opacities; hypoxia with increasing oxygen requirement to 7 L/min with face mask | RT-PCR*** for SARS-COV-2 RNA from nasopharyngeal swab | CP* 15 mL/kg on day 8 and 9. Lopinavir-ritonavir and remdesivir dexamethasone (0.2 mg/kg) and IVIG (1 g/kg) |
Children with cancer (high-risk population); severe COVID-19 associated pneumonia |
Remarkable improvement with reduction in respiratory rate, work of breathing and oxygen requirement (10 dys) No transfusion reaction |
Positive outcome following use of IVIG, steroids and CP* alone |
| Schwartz SP, et al. (Oct 2020) [17] | Case report (n = 4) | USA | 1) 15-year-old obese Hispanic male; 2) 16-year-old obese Asian male; 3) 5-year-old Hispanic female; 4) 12-year-old obese Hispanic female |
None | Acute respiratory failure requiring high-flow nasal cannula (HFNC) at admission | Anti-SARS-CoV-2 antibodies targeted to RBD** of SARS-CoV-2 spike protein | CP* units transfused: Case 1) no. 2 (RBD** binding titer 1:160; same donor); Remdesivir. IV anakinra. Case 2) no. 2, 10 mL/kg (titer unknown). remdesivir. Case 3) no. 2 (separate donors; titer 1:1,280). remdesivir Case 4) no. 2 (titer: Unit 1 = 1: 2,560, Unit 2 = 1:640). remdesivir. IV methylprednisolone |
CPT* as a treatment strategy for severe disease | Discharged home after CP*: 7 dys; 5 dys; 23 dys; 10 dys, respectively. Off oxygen support. 4) binding titer: unit 1 = 1:2,560, unit 2 = 1:640 No adverse events |
CPT* is feasible therapy for critically ill pediatric patients |
| Rodriguez Z, et al. (Sep 2020) [23] | Case report | USA | 9-week-old female | Trisomy 21; congenital heart disease | Cardiopulmonary failure secondary to unrepaired congenital heart disease exacerbated by COVID-19 | SARS-CoV-2 nucleic acid testing of nasopharyngeal swab | Remdesivir (5 mg/kg) 2 aliquots of CP* from 2 donors (10 mL/kg per aliquot; donor no. 1 had IgG titer 1:12724 and neutralizing titer 1:126; donor no. 2 had IgG titer 1:816 and neutralizing titer 1:50) from 2 COVID-19 recovered donors |
Deteriorating clinical status because lack of response to remdesivir (5 mg/kg per day) on hospital day 15 and 2.5 mg/kg per day on hospital days 16−25). | Uneventful complete recovery (47 dys) | CP* may be safe and effective treatment option in SARS-CoV-2 infection refractory to remdesivir. |
| Diorio C, et al (Sep 2020) [18] | Case report | USA | N = 4 patients, 14–18 years old; CD4, CD15, CD17, CD25# | None | Intubation and ventilation; two required extracorporeal membrane oxygenation |
RT-PCR*** testing of respiratory tract mucosa | Patient CD4 received CP* 2 mL/kg Patients CD15, CD17, CD25 received CP* 4 mL/kg (RBD**-specific antibody titer levels <1:160) |
Life-threatening COVID-19-associated respiratory disease | Donor for patient CD25# had higher SARS-CoV-2 RBD** antibody titers (>1:6000) than donor for other patients; no adverse event | CP* may be of greatest benefit early in illness |
| Greene AG, et al (Jun 2020) [19] | Case report | USA | 11-year-old female | None | Toxic shock-like syndrome; LV systolic function mildly decreased based on decreased shortening fraction | RT-PCR*** positive for SARS-CoV-2 | Furosemide, enoxaparin, tocilizumab, CP*, remdesivir, steroids, IVIG | Signs of distributive shock, multi-organ injury, systemic inflammation associated with COVID-19 | Improved dramatically (24 h) | Close follow-up for children presenting with fever lasting 3 dys |
| Balashov D, et al. (Nov 2020) [24] | Case report | Russia | 9-month-old girl | Juvenile myelomonocytic leukemia; hematopoietic stem cell transplantation | Polysegmental bilateral viral pneumonia with 60 % damage of lung tissue | RT-PCR***, throat swab positive for SARS-CoV-2 on day 99 after hematopoietic stem cell transplantation | Tocilizumab (10 mg/kg), CP* (10 mL/kg; 3 doses; titers 1:160, 1:160 and 1:80) | Secondary immunodeficiency | Full resolution of lung lesions; complete elimination SARS-CoV-2 4 mths after first detection CT* well tolerated |
SARS-CoV-2 CP* in combination with other therapeutic approaches possible curative options |
Legend: *CP denotes convalescent plasma; **RBD receptor-binding domain; ***RT-PCR real-time reverse transcription-polymerase chain reaction; #antibody titers expressed as reciprocal serum dilution against SARS-CoV-2 antigens in four children.
2.3. Ongoing trials involving pediatric patients
We searched the clinical trials registries (censored 5th November 2020) for eligible studies under way or planned to investigate the use of CPT for COVID-19 infection in children. The six online databases used for this research were https://clinicaltrials.gov/; https://eudract.ema.europa.eu/; https://www.clinicaltrialsregister.eu/; https://www.who.int/ictrp/network/en/; http://www.chictr.org.cn/abouten.aspx; https://www.irct.ir/.
3. Results
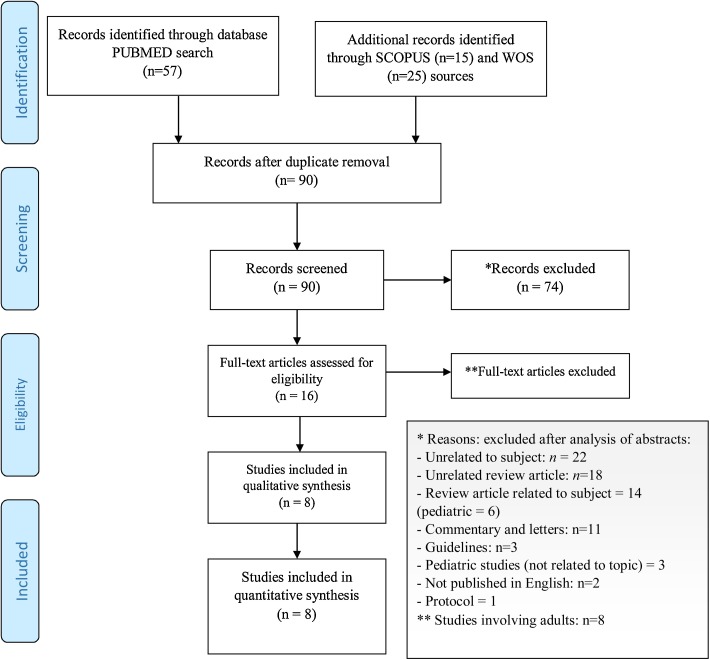
The initial search yielded 90 records as detailed in the PRISMA flow diagram (Fig. 1 ). Further screening of abstracts excluded 74 records unrelated to the topic (n = 22), unrelated reviews (n = 18), related reviews (n = 14; in childhood n = 6), commentaries or letters (n = 11), guidelines (n = 3), unrelated studies involving children, and studies not published in English (n = 2), and protocol (n = 1). Eight of the remaining 16 full-text records were excluded because the study population was adults (Supplementary Table 1). The 8 other records were case reports involving 14 children. The study population ranged in size from 1 to 4 children (age, 9 weeks to 18 years). Table 1 presents the characteristics of the studies included in the final review. Three case reports included patients (n = 9) without comorbidity [[17], [18], [19]]; 5 reported on patients (n = 5) with a comorbid condition: one each with agammaglobulinemia [20], aplastic anemia and severe pancytopenia [21], acute lymphoblastic leukemia [22], trisomy 21 with congenital heart disease [23], and juvenile myelomonocytic leukemia [24].
Fig. 1.
PRISMA 2009 Flow Diagram.
Clinical conditions at admission before treatment were severe, including pneumonia [20,22,24], respiratory failure [17,18], and (multi)organ failure [21,23,19]. Among the additional drug treatments administered during hospital stay, the most frequent was remdesivir (n = 5) [20,22,17,23,19]. Five case reports described the use of CP antibodies against SARS-CoV-2 IgG [21,17,23,18,24], whereas 3 did not [20,22,19]. No CPT-related adverse events were reported in 5 studies [21,17,18,24,22], and 3 studies made no mention of adverse events [20,23,19]. Patient outcomes were reported as recovery and/or discharge from hospital (n = 6) [20,22,17,23,19,24] or as amelioration of markers of SARS-CoV-2 infection [21,18]. Based on clinical observations, 2 case reports concluded that CPT is a useful option [22,17], 5 concluded that it could be a useful choice [20,21,23,18,24], and 1 case report made no statement [19].
Table 2 presents the ongoing and planned clinical trials (n = 13). Most are registered in the United States (n = 8), followed by the UK (n = 2), Canada, Pakistan, and Brazil. Ten involve both pediatric and adult populations [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. One trial did not state the upper limit of age at enrollment [29]. Finally, 3 trials are planned specifically to involve children (total of 160 participants), 2 in the United States [35,36] and 1 in Canada [37]. Two trials are currently in Phase 1 [35,36] and 1 trial is in Phase 2 [37] (total of 160 participants). The unit of measure of CP infusion is defined as “Unit” (200−250 ml) in 1 trial and as dosage per kg of body weight (5–10 ml/kg) in 2 trials.
Table 2.
Characteristics of ongoing clinical and preclinical trials of convalescent/hyperimmune plasma treatment against COVID-19 (updated on November 05, 2020).
| Trial no. | Country | Objective | Design | Phase(s) | Last update | Indication | Age Eligible for Study | Study population | Schedule | Donor titer |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT04377672 [35] | USA | Safety of CP* administration; prevent or lessen disease severity | Interventional (clinical trial) | Phase 1 | June 2, 2020 | High risk of developing COVID-19 due to recent exposure | 1 mth - 18 yrs | 30 | 1−2 unit (200−250 mL per unit) of CP* | >1:320 |
| NCT04377568 [37] | Canada | CP* for hospitalized children | Multicenter, open-label, randomized controlled trial | Phase 2 | October 8, 2020 | Hospitalized with COVID-19 illness | < 18 yrs | 100 | One infusion of CP* 10 mL/kg, up to a maximum of 500 m L | – |
| NCT04462848 [36] | USA | Safety and pharmacokinetics | Interventional (clinical trial); single group assignment) | Phase 1 | July 8, 2020 | Cardiovascular disease, lung disease, immunosuppression | 1 mth - 17 yrs | 30 | CP* 5 mL/kg. Maximum volume 500 m L | |
| NCT04352751 [34] | Pakistan | Real-life setting clinical data in local population; evidence-based management of disease condition | Interventional (clinical trial) | Not Applicable | September 29, 2020 | Severe or critical illness | 18−55 yrs (adults) | 2000 | Children: CP* 15 ml/kg if <35 kg body weight. Adults: CP* max 450 - 500 ml once in all adults. |
NA |
| NCT04360486 [25] | USA | Treatment option for patients with severe COVID-19 infection | Expanded access open-label, single-arm, multi-site protocol | – | April 27, 2020 | Severe or life-threatening | Child, adult, older adult | – | – | – |
| NCT04374370 [26] | USA | Expanded access to CP* | – | – | May 5, 2020 | Severe Acute Respiratory Syndrome | 6−99 yrs | – | – | – |
| NCT04458363 [27] | USA | Safety of CP* for children | Interventional (clinical trial); randomized | Early Phase 1 | July 7, 2020 | Severe COVID-19 disease | <22 Yrs (child, adult) | 50 | 10 mL/kg/dose (up to 2 units per dose); two doses per patient for a total dose of 20 mL/kg | – |
| NCT04528368 [28] | Brazil | Efficacy and safety of CP* | Interventional (clinical trial) | Phase 2 | August 27, 2020 | No indication of ventilatory support | Child, adult, older adult | 60 | 400 mL of CP* | ≥ 1: 320 |
| NCT04361253 [29] | USA | Early addition of CP* to standard treatment improves clinical outcome | Prospective randomized, double-masked, placebo-controlled trial | Phase 3 | May 18, 2020 | Active COVID-19 infection in hospitalized patients | Age >1 yr | 220 | 250 mL, max500 mL | – |
| NCT04376034 [30] | USA | Help fight infection in patients with COVID-19 | Interventional (clinical trial), non-randomized, prospective | Phase 3 | May 6, 2020 | Mild, moderate and severe/critical severity | 31 dys and older | 240 | 10 mg/kg up to 2 units of CP* | |
| NCT04381936 [31] | UK | Prevention of death in patients with COVID-19 | Randomized trial | Phase 3 | September 29, 2020 | Patients with COVID-19 in hospital care | Child, adult, older adult | 15,000 | 275 ml ± 75 ml per day on study days 1 and 2 (minimum 12-h interval) | |
| NCT04349410 [32] | USA | Fleming method for tissue and vascular differentiation and metabolism | Randomized trial | Phase 3 | October 29, 2020 | Patients with COVID-19 | Child, adult, older adult | 1800 | CP* 2-units infused over 4-h | 1:320 |
| ISRCTN50189673 [33] | UK | To compare several different treatments potentially useful for patients with COVID-19 | Interventional, randomized adaptive trial | Recruiting | October 06, 2020 | COVID-19 (clinically suspected or laboratory-confirmed), and in hospital | Child, adult | – | – | – |
Legend: * CP denotes convalescent plasma.
4. Discussion
This is the first systematic review of the literature investigating CPT for COVID-19 in children. All children had serious COVID-19, some with severe concomitant conditions and treated with various drugs. Most studies reported no CPT-related adverse events. We found insufficient information to compare the evidence for the efficacy of CPT. Among the registered clinical trials (mainly with clinicaltrials.gov), very few have been designed exclusively for children. The broad clinical interest in this vulnerable patient subpopulation is scarce.
The prevalence of COVID‐19 in children and adolescents is relatively low, accounting for about 2.4 % of all reported cases [38]. Although most children rarely progress to severe disease, there is concern for an inflammatory cascade [39]. Between January and June 2020, 55,270 children/adolescents diagnosed with and 3,693 hospitalized for COVID-19 were included in a large-scale multinational cohort study. While the mortality rate due to COVID-19 is negligible in this age group, complications including pneumonia, ARDS, and multisystem inflammatory syndrome should not be underestimated [40]. Early identification of COVID‐19 and prompt treatment are essential, especially in children with underlying/comorbid disease(s) [38].
Our review revealed a wide range of medications for the inpatient management of pediatric COVID-19. An international network cohort study, performed in children/adolescents diagnosed with and/or hospitalized for COVID-19 at age <18 years, reported a variety of adjunct therapies: systemic corticosteroids (6.8 %), famotidine (9.0 %), antithrombotic therapy, antibiotics, and immunoglobulins [40].
The U.S. Food and Drug Administration (FDA) approved remdesivir for emergency use in children hospitalized with severe suspected or laboratory-confirmed COVID-19 [41]. Parenteral remdesivir has been approved by the European Medicines Agency for pediatric and adolescent patients (≥12 years, body weight ≥40 kg), and has shown potential benefits [42]. Some limitations may regard neonatal intensive care unit (NICU) and pediatric intensive care unit (PICU) patients with severe disease (mechanical ventilation or extracorporeal membrane oxygenation [ECMO]).
CPT can be administered in children with rapid exacerbation of conditions and those with severe and critical diseases [43]. However, the currently available scientific literature is limited to case reports. Research providing higher quality evidence for the efficacy and safety of CPT in the treatment of pediatric COVID-19 infection has been planned [44] or is still in the protocol phase.
The main limitations and biases of the present study are that it includes only clinical case reports. Another bias (bias of reporting) is that only cases with a positive outcome have been reported, precluding representativeness of the whole pediatric population treated with CP.
5. Conclusions
Although COVID-19 is rare in childhood, children with chronic illness are vulnerable and may require treatment. We found no high quality studies investigating the efficacy and safety of CPT for COVID-19 in children and adolescents. Although available reports in pediatric age are case reports and case series (reporting bias), they have the potential to stimulate future research based on well-designed and powerful studies.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Marco Zaffanello: Conceptualization, Data curation, Writing - original draft. Giorgio Piacentini: Supervision, Validation, Writing - review & editing. Luana Nosetti: Data curation, Investigation, Writing - original draft. Massimo Franchini: Writing - original draft, Methodology, Writing - review & editing.
Declaration of Competing Interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.transci.2020.103043.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2020. Coronavirus disease (COVID-19)https://www.who.int/emergencies/diseases/novel-coronavirus-2019 n.d. (accessed November 9, 2020) [Google Scholar]
- 5.Wortham J.M., Lee J.T., Althomsons S., Latash J., Davidson A., Guerra K., et al. Characteristics of persons who died with COVID-19 — United States, February 12–May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:923–929. doi: 10.15585/mmwr.mm6928e1. [DOI] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr Allergy Immunol. 2020 doi: 10.1111/pai.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavriatopoulou M., Ntanasis-Stathopoulos I., Korompoki E., Fotiou D., Migkou M., Tzanninis I.G., et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2020 doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Psaltopoulou T., Sergentanis T., Pappa V., Politou M., Terpos E., Tsiodras S., et al. The emerging role of convalescent plasma in the treatment of COVID-19. HemaSphere. 2020;4 doi: 10.1097/HS9.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perotti C., Baldanti F., Bruno R., Del Fante C., Seminari E., Casari S., et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020 doi: 10.3324/haematol.2020.261784. haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371 doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piechotta V., Chai K.L., Valk S.J., Doree C., Monsef I., Wood E.M., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;2020 doi: 10.1002/14651858.CD013600.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA J Am Med Assoc. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharbharan A., Jordans C.C.E., Geurtsvankessel C., Den Hollander J.G., Karim F., Mollema F.P.N., et al. Convalescent plasma for COVID-19. A randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.07.01.20139857. 2020.07.01.20139857. [DOI] [Google Scholar]
- 14.Avendaño-Solà C., Ramos-Martínez A., Muñez-Rubio E., Ruiz-Antorán B., Malo De Molina R., Torres F., et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.08.26.20182444. 2020.08.26.20182444. [DOI] [Google Scholar]
- 15.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/nejmoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawyer S.M., McNeil R., Francis K.L., Matskarofski J.Z., Patton G.C., Bhutta Z.A., et al. The age of paediatrics. Lancet Child Adolesc Heal. 2019;3:822–830. doi: 10.1016/S2352-4642(19)30266-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz S.P., Thompson P., Smith M., Lercher D.M., Rimland C.A., Bartelt L., et al. Convalescent plasma therapy in four critically ill pediatric patients with coronavirus disease 2019: a case series. Crit Care Explor. 2020;2 doi: 10.1097/cce.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diorio C., Anderson E.M., McNerney K.O., Goodwin E.C., Chase J.C., Bolton M.J., et al. Convalescent plasma for pediatric patients with SARS-CoV-2-associated acute respiratory distress syndrome. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin H., Reed J.C., Liu S.T.H., Ho H., Lopes J.P., Ramsey N.B., et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figlerowicz M., Mania A., Lubarski K., Lewandowska Z., Służewski W., Derwich K., et al. First case of convalescent plasma transfusion in a child with COVID-19-associated severe aplastic anemia. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar R., Radhakrishnan N., Dua S., Arora S., Rana M., Sahu D.K., et al. Convalescent plasma to aid in recovery of COVID-19 pneumonia in a child with acute lymphoblastic leukemia. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez Z., Shane A.L., Verkerke H., Lough C., Zimmerman M.G., Suthar M., et al. COVID-19 convalescent plasma clears SARS-CoV-2 refractory to remdesivir in an infant with congenital heart disease. Blood Adv. 2020;4:4278–4281. doi: 10.1182/BLOODADVANCES.2020002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balashov D., Trakhtman P., Livshits A., Kovalenko I., Tereshenko G., Solopova G., et al. SARS-CoV-2 convalescent plasma therapy in pediatric patient after hematopoietic stem cell transplantation. Transfus Apher Sci. 2020:102983. doi: 10.1016/j.transci.2020.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.2020. Treatment of CORONAVIRUS DISEASE 2019 (COVID-19) with Anti-Sars-CoV-2 convalescent plasma (ASCoV2CP) - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04360486 (accessed November 1, 2020) [Google Scholar]
- 26.2020. SARSCoV2 (COVID-19) convalescent plasma (CP) expanded access protocol (EAP) - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04374370 (accessed November 1, 2020) [Google Scholar]
- 27.2020. Convalescent plasma in pediatric COVID-19 - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04458363 (accessed November 1, 2020) [Google Scholar]
- 28.2020. Convalescent plasma for treating patients with COVID-19 pneumonia without indication of ventilatory support - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04528368 (accessed November 1, 2020) [Google Scholar]
- 29.2020. Evaluation of SARS-CoV-2 (COVID-19) antibody-containing plasma thErapy - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04361253 (accessed November 1, 2020) [Google Scholar]
- 30.2020. Convalescent plasma collection and treatment in pediatrics and adults - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04376034 (accessed November 1, 2020) [Google Scholar]
- 31.2020. Randomised evaluation of COVID-19 therapy - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04381936 (accessed November 1, 2020) [Google Scholar]
- 32.2020. The fleming [FMTVDM] directed CoVid-19 treatment protocol - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04349410 (accessed November 1, 2020) [Google Scholar]
- 33.ISRCTN - ISRCTN50189673 . 2020. A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus) n.d.http://www.isrctn.com/ISRCTN50189673 (accessed November 1, 2020) [Google Scholar]
- 34.2020. Experimental use of convalescent plasma for passive immunization in current COVID-19 pandemic in Pakistan in 2020 - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04352751 (accessed November 1, 2020) [Google Scholar]
- 35.2020. Human convalescent plasma for high risk children exposed or infected with SARS-CoV-2 (COVID-19) - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04377672 (accessed November 1, 2020) [Google Scholar]
- 36.2020. Covid-19 convalescent plasma as prevention and treatment for children with underlying medical conditions - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04462848 (accessed November 1, 2020) [Google Scholar]
- 37.2020. Efficacy of human coronavirus-immune convalescent plasma for the treatment of COVID-19 disease in hospitalized children - full text view - ClinicalTrials.gOv n.d.https://clinicaltrials.gov/ct2/show/NCT04377568 (accessed November 1, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.She J., Liu L., Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol. 2020;92:747–754. doi: 10.1002/jmv.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.T D-S, D V, A P, P C, AG S, LYH L, et al. Baseline characteristics, management, and outcomes of 55,270 children and adolescents diagnosed with COVID-19 and 1,952,693 with influenza in France, Germany, Spain, South Korea and the United States: an international network cohort study. MedRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.10.29.20222083. [DOI] [Google Scholar]
- 41.Zhand S., Jazi M.S., Mohammadi S., Rasekhi R.T., Rostamian G., Kalani M.R., et al. Covid-19: the immune responses and clinical therapy candidates. Int J Mol Sci. 2020;21:1–34. doi: 10.3390/ijms21155559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frauenfelder C., Brierley J., Whittaker E., Perucca G., Bamford A. Infant with SARS-CoV-2 infection causing severe lung disease treated with remdesivir. Pediatrics. 2020;146 doi: 10.1542/PEDS.2020-1701. [DOI] [PubMed] [Google Scholar]
- 43.Shen K.L., Yang Y.H., Jiang R.M., Wang T.Y., Zhao D.C., Jiang Y., et al. Updated diagnosis, treatment and prevention of COVID-19 in children: experts’ consensus statement (condensed version of the second edition) World J Pediatr. 2020;16:232–239. doi: 10.1007/s12519-020-00362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai H., Ji Y., Wang J., Zhang X. Efficacy of human coronavirus immune convalescent plasma for the treatment of corona virus disease -19 disease in hospitalized children. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000022017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.