1. Introduction
Covid-19 has significant implications of hematologic systems, including lymphocytopenia, thrombocytopenia, ischemic or hemorrhagic stroke, pulmonary thromboembolism, and myocardial infarction [1,2]. Iwasaki et al. reported that the pathogen of Covid-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can induce immune dysfunction, inflammation, and antibody-dependent enhancement by activating host cells via the FcγIIa receptor in the same way as SARS-CoV-1 [3].
Other diseases related to the hemostasis function mediated by the FcγIIa receptor, are the antiphospholipid syndrome, immune thrombocytopenic purpura, and heparin-induced thrombocytopenia and thrombosis (HITT) [1,2]. HITT results from platelet activation and consumption caused by the interaction between the FcγIIa receptor and the heparin, platelet factor (PF) 4, and anti-PF4-heparin antibody complex [4,5]. HITT has rarely been reported in Covid-19 patients [1,2,6]. We reported a Covid-19 patient developed acute myocardial infarction and was retrospectively diagnosed with a HITT.
2. Case presentation
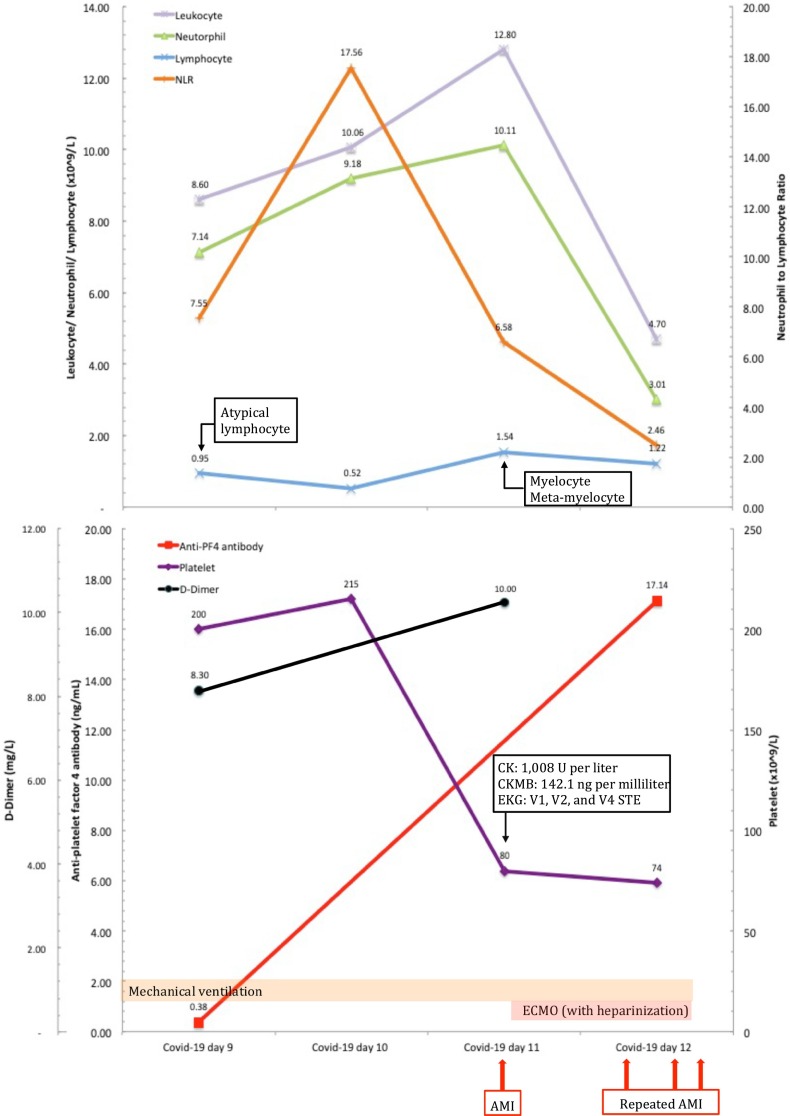
A 44-year-old male weighed 100 kg and 175 cm tall with thalassemia who suffered from fever and cough for three days was diagnosed with Covid-19. He developed dyspnea a few days later and was transferred to our hospital with an oxygen saturation of 90% on room air and a respiratory rate of 26 breaths/min on Covid-19 Day 9 (March 26th, 2020). Laboratory results showed a normal white blood cell count (8.60 × 109/L), relatively low hemoglobin level (11.8 g/dL), and platelet count (200 × 109/L) (Table 1 ). The prothrombin time and renal function were normal range, with creatinine clearance calculated according to the Cockroft-Gault equation of 152 ml/min. The D-dimer was significantly elevated (8.30 mg/L) (Fig. 1 ). The chest X-ray showed bilateral moderate to severe infiltration with ill-defined patchy opacities. All the bacteria and virus tests other than SARS-CoV-2 were negative.
Table 1.
4T's score of heparin-induced thrombocytopenia and thrombosis with laboratory findings and anti-platelet factor 4 antibody.
| Covid-19 Day 9 | Covid-19 Day 10 | Covid-19 Day 11a | Covid-19 Day 12 | |
|---|---|---|---|---|
| Thrombocytopenia: percentage of platelet count fall | 0 (fall in platelet count < 30%) | 0 (fall in platelet count < 30%) | 2 (fall in platelet count > 50%) | 2 (fall in platelet count > 50%) |
| Timing of platelet count fall | 0 (no fall of platelet count) | 0 (no fall of platelet count) | 0 (platelet fall without exposure to heparin in the 100 previous days) | 0 (within 24 h after exposure of heparin) |
| Thrombosis or other clinical events | 0 (no event) | 0 (no event) | 2 (new confirmed acute myocardial infarction) | 2 (repeated myocardial infarctions) |
| Other possible causes of thrombocytopenia | 0 (no fall of platelet count) | 0 (no fall of platelet count) | 2 (no evident explanation for platelet count fall) | 2 (no evident explanation for platelet count fall) |
| 4T's score | 2 | 2 | 6 | 6 |
| Anti-PF4-heparin antibody (ng/mL) | 0.38 | 17.14 | ||
| Platelet count (×109/L) | 200 | 215 | 80 | 74 |
| D-Dimer (ng/mL) | 8.3 | N/A | >10.0 | >10.0 |
| Prothrombin time (s) | 11.8 | N/A | 40.4 | 36.0 |
| Activated partial thromboplastin time (s) | 40.4 | N/A | >100 | >100 |
| International normalized ratio | 1.1 | N/A | 4.0 | 3.6 |
The item of “timing of platelet count fall” was scored 0, and the 4T's score was 6 at both Covid-19 Days 11 and 12 using the diagnosis guideline of HITT by Gruel Y et al. in 2020.
The blood sample on Covid-19 Day 11 was collected before heparin therapy for extracorporeal membrane oxygenation.
Fig. 1.
Clinical characteristics, laboratory findings, and treatment of a Covid-19 patient complicated with acute myocardial infarction with heparin-induced thrombocytopenia and thrombosis. Footnotes: AMI: acute myocardial infarction, ECMO: extracorporeal membrane oxygenation, NLR: neutrophil-to-lymphocyte ratio, CK: creatine kinase, CKMB: creatine kinase-myoglobin, EKG: electrocardiogram, STE: S-T segment elevation, PF4: platelet factor 4.
The treatments started with oral administration of hydroxychloroquine 200 mg thrice per day and invasive mechanical ventilation. The patient developed sudden onset ventricular arrhythmia, cardiac arrest, and received emergent cardiopulmonary resuscitation on Day 11. The electrocardiogram showed S-T segment elevation on the V1, V2, and V4 leads. Laboratory tests showed a significant elevation of creatine kinase (1008 U/L) and creatine kinase-myoglobin (142.1 ng/mL). D-dimer was over 10.00 mg/L (Fig. 1). Acute anteroseptal myocardial infarction was diagnosed. Extracorporeal membrane oxygenation (ECMO) was immediately applied due to persistent cardiogenic shock, with heparinization including bolus dose of 20 IU/kg and continuous infusion to keep activated partial thromboplastin time ratio between 1.5 and 2.0. Before the myocardial infarction attacked, the patient didn't receive any heparin therapy. As the disease progressed and the dose of heparin accumulated, the patient developed severe upper gastroenteric tract bleeding, episodes of ventricular arrhythmia, and repeated myocardial infarctions with significantly elevated creatine kinase and creatine kinase-myoglobin levels. The patient died of cardiac failure 23 h after applying of ECMO with heparinization (Fig. 1).
Since the HITT was suspected on this patient one month after hospitalization, the 4T's score (four items of Thrombocytopenia, Timing of platelet fall, Thrombosis or other clinical episode and Other causes of thrombocytopenia, range from 0 to 2 for each item) was retrieved of 6 with a platelet count decreased of 62.7% and 65.6% (from 215 × 109/L to 80 × 109/L and 74 × 109/L) on both Days 11 and 12, respectively, using the diagnosis guideline of HITT by Gruel Y et al. (Table 1, Fig. 1) [4,5]. Because the serotonin releasing assay (SRA) was not available at our hospital [4,5], the patient's serum samples collected on Days 9 and 12 were retrospectively sent for enzyme-linked immunosorbent assay (ELISA) of anti-PF4-heparin antibody, which revealed 0.38 ng/mL on Day 9, and 17.14 ng/mL on Day 12 with an optical density > 2.0 units, verifying the clinical diagnosis of HITT by a high probability of HITT with 4T's score over 6 and strong positive on immunoassay (Fig. 1) [4,5].
3. Discussion
In addition to heparin exposure, bacterial and viral infections, and autoimmune reaction can also induce anti-PF4-heparin antibody generation [7]. Anti-PF4-heparin antibody carriers may stay in hypercoagulation status as the platelet was partially activated but not strong enough to cause thrombosis [5,7]. When the antigen increases, numerous antibodies could trigger a clinical cascade of thromboembolism [5,7]. The thrombosis in HITT has two unique differences from other thrombotic diseases: 1. Thrombosis and thrombocytopenia occur simultaneously; 2. Heparin will aggravate the thrombosis [4,5]. Hypercoagulability and acute myocardial dysfunction in Covid-19 have been reported, but its etiology has not been well investigated [8,9]. Some of the Covid-19 cases with hypercoagulability and thrombotic events with thrombocytopenia showed similar presentations as HITT [8,9]. The platelet count at the time of and after acute myocardial infarction without HITT usually remained normal or decreased by only about 6%.
On the contrary, the platelet count in acute myocardial infection with HITT often reduced by more than 50% or lower than 150 × 109/L [4,5,9]. Creel-Bulos et al. addressed 80% of those Covid-19 critical patients who underwent heparin therapy but still had a significant vessel thrombosis observed with thrombocytopenia [8]. In our opinion, the unusually high incidence of acute pulmonary embolism with thrombocytopenia highly suggests the underline etiology of the Creel-Bulos's Covid-19 series might be the HITT [4,5,8,9].
One of the limitations of this study was the absence of a definitive diagnosis of the HITT using SRA, which was not available at our hospital. In 2020, Gruel Y et al. suggested the diagnosis of HITT with a high probability of HITT of 4T's score of 6–8 and significantly positive immunoassay, but a functional test of SRA is not mandatory [4]. Cuker also suggested, “A functional assay may not be necessary for patients with a high-probability 4T's score and very strongly positive immunoassay” (e.g., an ELISA value of >2.0 OD units) [5].
This patient showed rapid progressive simultaneous thrombosis and severe bleeding with thrombocytopenia, elevated D-dimer, elevated anti-PF4 antibody during heparin therapy for the ECMO. In this case, HITT was triggered within 12 h after heparin therapy on Day 12, but the anti-PF4-heparin antibody may be generated during the previous ten days of Covid-19 infection. These presentations suggested that HITT was the possible etiology of hypercoagulability and acute myocardial infarction in this Covid-19 patient.
Due to the hypercoagulability and high incidence of thrombosis episodes, prophylactic anticoagulation agents, majorly heparin, or low-molecular-weight heparin were suggested [8,10]. However, prophylactic heparin is not practical in cases in Creel-Bulos's series. [8] SARS-CoV-2 might induce anti-PF4-heparin antibody generation [3,7], Bulos's Covid-19 cases might be anti-PF4-antibody carriers and resulted in non-effective to heparin on thrombosis prevention or even aggravated [4,5,8]. Tang et al. indicated that low-molecular-weight heparin decreased mortality rate in critical Covid-19 cases [10]. Comparing with unfractionated heparin, low-molecular-weight heparin has a ten times lower risk of HITT, which is compatible with Tang's result that low-molecular-weight heparin is more effective in preventing thrombosis in Covid-19 patients [4,5,10].
Cautiously applying heparin therapy with early identification and treatment of HITT may further reduce the mortality rate of Covid-19 patients. Riker et al. addressed three Covid-19 patients with HITT who had been successfully treated with bivalirudin [6], but did not discuss the emergent event as myocardial infarction as our case. In Covid-19 patients with thrombocytopenia and high 4T's score (greater than 6), heparin or low-molecular-weight heparin should be stopped or replaced by other non-heparinoid anticoagulants, including direct thrombin inhibitors (argatroban, bivalirudin) and factor X inhibitors (fondaparinux, rivaroxaban) as the standard treatment of HITT [4,5]. Non-heparinoid anticoagulation agents can be considered as a first-line prophylactic anticoagulation agent for Covid-19 patients but require further study.
Funding
No specific funding received.
Declaration of competing interest
None.
Acknowledgment
The authors thank Dr. Kuang-Tso Lee, Miffy Chia-Yu Lin, M. Sc., Hsing-Yu Lin, M. Sc., and Chin-Yu Yang M. Sc. for their great support in the preparation of this manuscript.
Footnotes
The English grammar of the original manuscript was polished by the American Journal of Experts with a Certificate Verification Key of D897-7A81-9016-AA67-961E, and the revised manuscript was corrected using the software of Grammarly Premium (Grammarly, San Francisco, CA).
References
- 1.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zulfiqar A.-A., Lorenzo-Villalba N., Hassler P., Andrès E. Immune thrombocytopenic purpura in a patient with Covid-19. N. Engl. J. Med. 2020:e43–2. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020;35:179-3. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruel Y., De Maistre E., Pouplard C., Mullier F., Susen S., Roullet S. Diagnosis and management of heparin-induced thrombocytopenia. Anaesth. Crit. Care Pain Med. 2020;39:291–310. doi: 10.1016/j.accpm.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Cuker A., Arepally G.M., Chong B.H., Cines D.B., Greinacher A., Gruel Y. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–3392. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riker R.R., May T.L., Fraser G.L., Gagnon D.J., Bandara M., Zemrak W.R. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res. Pract. Thromb. Haemost. 2020;18:1023. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauel K., Pötschke C., Weber C., Kessler W., Fürll B., Ittermann T. Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370–1378. doi: 10.1182/blood-2010-08-301424. [DOI] [PubMed] [Google Scholar]
- 8.Creel-Bulos C., Hockstein M., Amin N., Melhem S., Truong A., Sharifpour M. Acute cor pulmonale in critically ill patients with Covid-19. N. Engl. J. Med. 2020:e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B. ST-segment elevation in patients with Covid-19 — a case series. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]