Abstract
Lung ultrasound (LUS) has shown promising diagnostic potential in different pulmonary conditions. We evaluated the diagnostic accuracy of LUS for pulmonary COVID-19. In this prospective cohort study at a Swiss tertiary care center, patients hospitalized with suspected COVID-19 were scanned using a 12-zone protocol. Association of a summation score (0–36 points) with the final diagnosis was tested using the area under the receiver operating characteristic curve and sensitivity and specificity at different cutoff points. Of the 49 participants, 11 (22%) were later diagnosed with COVID-19. LUS score showed excellent diagnostic performance, with an odds ratio of 1.30 per point (95% confidence interval [CI], 1.09–1.54, p = 0.003) and an area under the curve of 0.85 (95% CI, 0.71–0.99). At a cutoff of 8/36 points, 10 of 11 participants later diagnosed with COVID-19 were correctly predicted (sensitivity 91%, 95% CI, 59%–100%), and 29 of the 38 who were not diagnosed with COVID-19 were correctly ruled out (specificity 76%, 95% CI, 60%–89%). LUS demonstrated promising discriminatory potential in people hospitalized with suspected COVID-19.
Key Words: Coronavirus disease, Lung ultrasound, Pleura ultrasound, SARS-CoV-2, COVID-19, Point of care ultrasound, Pneumonia, Interstitial syndrome
Introduction
Early in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, researchers advocated for lung ultrasound (LUS) in the assessment of COVID-19 (Huang et al. 2020; Peng et al. 2020). It is worth noting that “thoracic ultrasound” would be a more exact description because both layers of the pleura are assessed as well. A majority of people with COVID-19 show a peripheral distribution of ground-glass opacities on chest computed tomography (CT) scans, making ultrasound assessment possible. Several studies show a high correlation of LUS and chest CT, which is the current gold standard in imaging of COVID-19 pulmonary disease, with a sensitivity of 95% (Lomoro et al. 2020; Tung-Chen et al. 2020). The sensitivity of chest X-ray has been found to be considerably lower (69%; Wong et al. 2020). While LUS findings in COVID-19 are unspecific, available data suggest a high sensitivity (Mohamed et al. 2020).
Most published LUS protocols for COVID-19 include anterior, lateral and posterior scans examining between 4 and 14 zones (Allinovi et al. 2020; Brahier et al. 2020; Huang et al. 2020; Manivel et al. 2020; Peng et al. 2020; Soldati et al. 2020). Characteristic findings include thickening of the pleural line with irregularities, B-lines in a variety of patterns including focal and multifocal and confluent consolidations with occasional mobile air bronchograms (Peng et al. 2020; Yasukawa and Minami 2020). A shining band-form artifact (“light beam”) spreading down from a large portion of a regular pleural line, often appearing and disappearing with an on–off effect corresponding with ground-glass opacities on chest CT, has also been described (Volpicelli and Gargani 2020). One study found diffuse B-lines and subpleural consolidations in 100% and 27% of COVID-19 patients, respectively (Lomoro et al. 2020). The posterior lower areas seem to be most commonly affected (Brahier et al. 2020; Huang et al. 2020; Lu et al. 2020).
The available evidence suggests a cascade of characteristic changes, starting with single and/or confluent B-lines with a patchy distribution and extending to multiple areas of the lung surface as the disease progresses. Later, small subpleural consolidations appear and increase in size and number (Fiala 2020; Soldati et al. 2020).
The advantages of LUS are the lack of radiation exposure and the possibility of repeated bedside use, thus avoiding patient transport within the hospital and contamination of a radiology suite (Mongodi et al. 2020; Vetrugno et al. 2020). The availability and quality of handheld devices allow its use in remote settings and on house calls (Piscaglia et al. 2020; Shokoohi et al. 2020). Finally, the lower cost makes LUS available to health care systems with limited resources while permitting image transfer and remote interpretation of findings. The use of LUS to monitor individuals recovering from COVID-19 has also been suggested (Pascarella et al. 2020).
The potential for LUS assessment in COVID-19 would be in triage of suspected cases, diagnosis of COVID-19 pneumonia and monitoring of confirmed cases, thereby possibly reducing the need for chest CT.
The goal of this investigation was to assess the performance of LUS in predicting diagnosis of COVID-19 in a pandemic setting.
Materials and Methods
Study site
We conducted a prospective cohort study in the medical isolation ward for suspected and confirmed COVID-19 at the Kantonsspital Aarau, Switzerland, a tertiary care center with around 500,000 outpatient consultations and 30,000 admissions per year.
Study participants
All consecutive adult patients (age ≥18 y) admitted on weekdays to the medical isolation ward with symptoms involving the upper or lower respiratory tract with or without fever from March 30 to April 30, 2020, were eligible. Owing to the unforeseeable dynamic of the pandemic in this early stage, no sample-size calculation was performed. The number of nationwide newly confirmed daily cases declined markedly during the study period from 1093 to 179 (Dong et al. 2020).
During the investigation, all patients remained in individual isolation pending SARS-CoV-2 polymerase chain reaction (PCR) or serology results. Patients were not included if these results were already available or if patients were directly admitted to the intensive care unit (ICU). Assessment included a nasopharyngeal swab for SARS-CoV-2 PCR testing (Allplex 2019-nCoV Assay, Seegene, Seoul, Korea) and serology testing in select cases (SARS-CoV-2 IgM/IgG Antibody Assay Kit by Colloidal Gold Method, Maccura Biotechnology, Chengdu, China). While the electronic medical record contained a precise list of diagnoses and pre-existing conditions, all patients were also assessed using the Charlson Comorbidity Index (Charlson et al. 1994) and the Clinical Frailty Scale (Rockwood et al. 2005) upon admission. Patient consent for participation was obtained through a general consent form. The study protocol was approved by the Ethics Committee for Northwestern and Central Switzerland (EKNZ, Project ID 2020-01295).
Ultrasound equipment
Participants were scanned with a portable ultrasound device (Viamo sv7, 6 DC1 convex probe, Canon Medical Systems Corporation, Otawara, Japan). We programmed a lung preset with a high frame rate and disabled artifact suppression at a frequency of 4 MHz. Personal protective equipment was used as per local COVID-19-specific infection-control protocol, and the equipment was disinfected accordingly.
Ultrasound protocol
Ultrasound assessment of the lungs was performed within 24 h of admission by author V.S. in the medical isolation ward. This ultrasound operator was board-certified in internal and emergency medicine as well as point-of-care ultrasound, with 3 y clinical experience in ultrasound and 1 y in lung ultrasound at the time of the study.
A 12-zone examination protocol was adopted, in which a lung was divided on each side by the anterior and posterior axillary lines into three areas: anterior, lateral and posterior. These areas were further divided into superior and inferior zones, resulting in a total of six zones per side and 12 zones per participant. In order to mark and describe the lesion sites clearly, an R1–6/L1–6 subdivision labeling method was used (Huang et al. 2020; Manivel et al. 2020).
Imaging analysis
Findings were rated during the scans for three categories: B-lines, consolidations and pleural effusions.
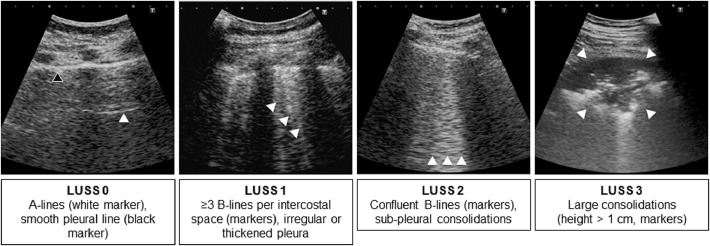
For B-lines, one point was scored for ≥3 B-lines per inter-costal space, two points for ≥7 B-lines or three points for confluent B-lines. For consolidations, one point was scored for a thickened/irregular pleural line with or without thin subpleural consolidations, two points for small consolidations ≤ 1 cm in depth or three points for larger consolidations. These findings were then combined using the lung ultrasound score (LUSS, Fig. 1 ), which combines the assessment of B-lines with pleural irregularities (as described by Bouhemad et al. 2011; Dargent et al. 2020; Manivel et al. 2020; Smith et al. 2020): zero points were scored for a smooth pleural line with ≤2 B-lines per vertical inter-costal space; one point for irregular or thickened pleura and/or ≥3 B-lines; two points for subpleural consolidations (height <1 cm) and/or confluent B-lines; and three points for larger consolidations. The findings of each superior and inferior zone were added up (e.g., equaling one left posterior zone), for a total maximum of six points. “Light beams” were rated the same as confluent B-lines (two points).
Fig. 1.
Lung and pleural alterations were documented by semi-quantitative assessment of B-lines and consolidations, adapted from Manivel et al. (2020). A-lines are horizontal linear artifacts mirroring the pleural line; B-lines are vertical comet-tail-like artifacts indicating increased interstitial density that move with the pleural line during respiration; subpleural consolidations have a relatively hypoechoic heterogeneous echotexture with blurred and irregular margins. LUSS = lung ultrasound score.
Pleural effusions were measured in the scapular line of sitting participants, describing the largest vertical distance between the lung and diaphragm.
The ultrasound operator was unaware of all clinical and laboratory data, rating the probability of COVID-19 based on the ultrasound exam alone on a four-point Likert scale (very unlikely, somewhat unlikely, somewhat likely, very likely). He was not involved in clinical decisions regarding individual participants.
Data analysis
The diagnosis in the discharge letter taking into account all available clinical, laboratory and imaging data was considered the gold standard. The primary outcome was defined as diagnosis of COVID-19.
Association of LUS scores with the final diagnosis was assessed by univariate logistic regression analysis providing odds ratios with 95% confidence intervals (95% CIs). Areas under the receiver operating characteristic curves (AUCs) were calculated to illustrate overall score performances. Sensitivity, specificity, positive (LR+) and negative likelihood ratios (LR−) show the individual test performance of scores at different cutoff values. All calculations were done using Stata 15.1 (StataCorp, College Station, Texas, USA); all testing was two-sided, and p < 0.05 was considered statistically significant.
Results
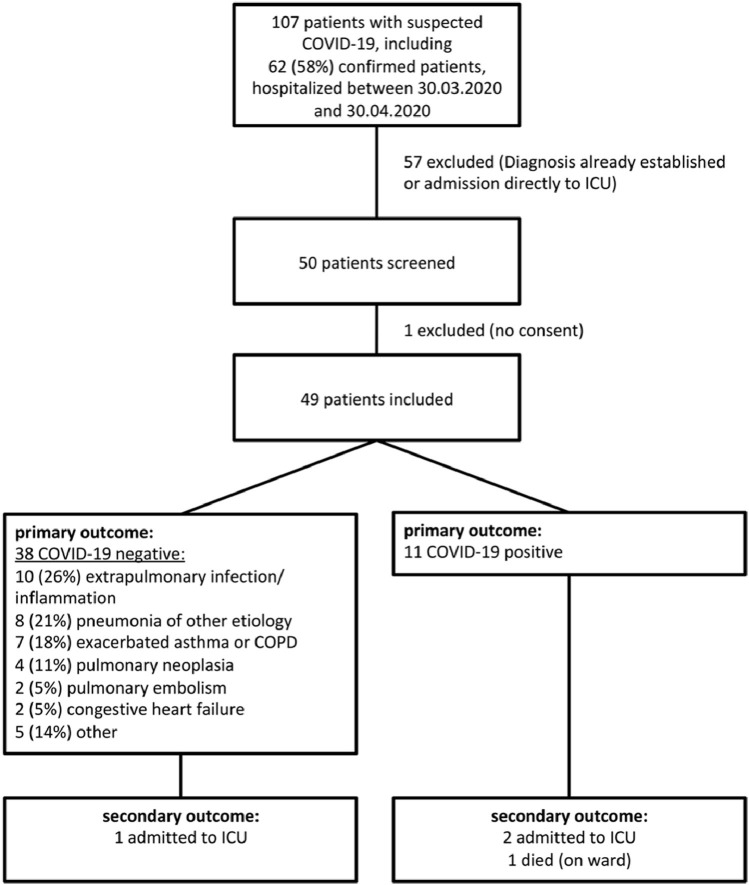
A total of 49 participants hospitalized with clinically suspected SARS-CoV-2 infection (COVID-19) were examined upon hospital admission (Fig. 2 ). Most participants were male (22, 58%) with a median age of 69.5 y (range, 35–89) and different pre-existing medical conditions (median Charlson Comorbidity Index = 2; inter-quartile range [IQR], 1–4; median Clinical Frailty Score = 4; IQR, 2–5). Three participants (6%) were admitted to the ICU and one (other) participant died during hospitalization. Median duration of symptoms before admission was 5 d (IQR, 1–10; range, 0–28). Eleven participants (22%) were diagnosed with COVID-19. Most other participants experienced extrapulmonary inflammatory conditions or pulmonary infections other than COVID-19 (Fig. 2). Participants’ characteristics, vital signs, biomarkers and diagnostic modalities are listed by final diagnosis in Table 1 .
Fig. 2.
Flowchart of participants included for LUS examination, with primary (confirmed COVID-19) and secondary outcomes (ICU transfer and/or in-hospital death). COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; LUS = lung ultrasound; LUSS = lung ultrasound score.
Table 1.
Characteristics of patients with and without COVID-19. Alternative diagnoses are shown in Figure 1.
| Baseline characteristic | COVID-19 (n = 11) | Other (n = 38) | p |
|---|---|---|---|
| Age (y) | 76 (54–82) | 69.5 (59–81) | 0.81 |
| Sex (male/female) | 8/3 (73/27) | 18/20 (47/53) | 0.14 |
| Days since onset | 6 (3–8) | 2 (0–7) | 0.18 |
| Diabetes mellitus | 2 (18) | 8 (21) | 0.84 |
| Arterial hypertension | 5 (45) | 25 (66) | 0.22 |
| Cardiomyopathy | 6 (55) | 20 (53) | 0.91 |
| Pneumopathy | 4 (36) | 18 (47) | 0.52 |
| Immunosuppression | 0 | 9 (24) | 0.07 |
| Chronic renal insufficiency | 2 (18) | 10 (26) | 0.58 |
| Charlson Comorbidity Index | 1 (0–4) | 2.5 (1–4) | 0.17 |
| Clinical Frailty Scale | 4 (2–7) | 4 (2–5) | 0.65 |
| Vital statistics and laboratory values | |||
| PaO2 (mmHg, ambient air) | 64 (59–67) | 66 (55–83) | 0.37 |
| SpO2 (%) | 92 (89–94) | 94 (92–95) | 0.05 |
| Oxygen flow (L/min) | 2 (0–4) | 0.5 (0–2) | 0.26 |
| CRP (mg/L) | 116 (54–145) | 61.5 (9–103) | 0.03 |
| LDH (IU/L) | 345.5 (268–398) | 199 (171–244) | <0.01 |
| Neutrophil granulocytes (g/L) | 4.23 (3.14–6.83) | 9.78 (5.28–11.79) | 0.01 |
| Neutrophil/lymphocyte ratio | 5.79 (4.01–12.83) | 6.68 (3.73–12.39) | 0.82 |
| Diagnostic results | |||
| Pathology on X-ray | 9 (90) | 7 (23) | <0.01 |
| Pathology on CT scan | 1 (100) | 3 (27) | 0.14 |
| Positive SARS-CoV-2 PCR | 8 (73) | 0 (0) | <0.01 |
| Positive SARS-CoV-2 serology | 4 (100) | 0 (0) | <0.01 |
| Combined adverse outcomes (ICU and death) | 3 (27) | 1 (3) | 0.01 |
| ICU admission | 2 (18) | 1 (3) | 0.06 |
| Death in hospital | 1 (9) | 0 (0) | 0.06 |
COVID-19 = coronavirus disease 2019; CRP =C-reactive protein, norm. < 3 mg/L; CT = computed tomography; ICU = intensive care unit; LDH = lactate dehydrogenase, norm. < 250 U/L; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
All values are given as number (percentage) or median (inter-quartile range).
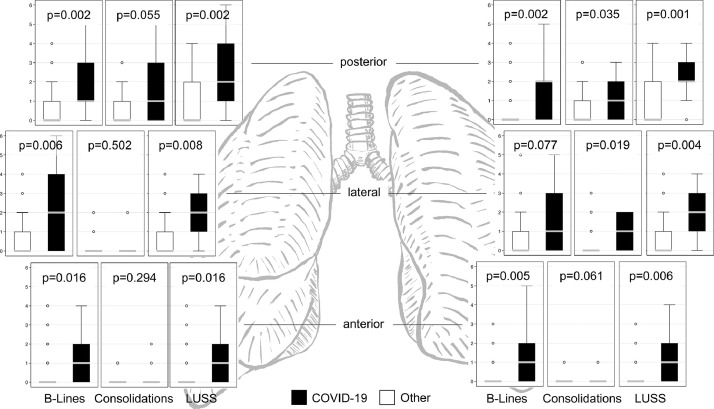
All participants were scanned according to the protocol. B-lines (≥3 per inter-costal space) were found in 24% (left anterior zone) to 45% (both lateral zones) of zones. B-line density, as well as the number of affected zones, correlated well with COVID-19 (Fig. 3 ). On the other hand, an irregular pleural line with or without subpleural consolidations was found in 6% (left anterior zone) to 33% and 39% (right and left posterior zones), only correlating at the left anterior and posterior zones (Fig. 3); pleural effusions showed no correlation (p = 0.371, not shown). Combined findings according to the LUSS protocol showed excellent correlation in all examination zones (Fig. 3), and the summarized score showed excellent predictive ability, with an AUC of 0.85 (95% CI, 0.71–0.99) and an increasing odds ratio of 1.30 per point (95% CI, 1.09–1.54, p = 0.003). A LUSS of ≥8 correctly predicted 10 of 11 (sensitivity 91%, 95% CI, 59%–100%) cases of COVID-19 in this cohort while correctly ruling out 29 of 38 (specificity 76%, 95% CI, 60%–89%). The predictive characteristics at different cutoffs in this setting are presented in Table 2 .
Fig. 3.
Ultrasound presentation of B-lines, consolidations and lung ultrasound scores at different locations in patients with and without COVID-19. Boxplots around median and inter-quartile ranges, with adjacent lines for the most extreme values within 1.5 IQR of the nearer quartile. IQR = inter-quartile range; LUSS = lung ultrasound score.
Table 2.
Test performance of LUSS at different cutoffs points.
| LUSS cutoff point | Sensitivity (%) | Specificity (%) | LR+ | LR− |
|---|---|---|---|---|
| ≥1 | 100 | 18 | 1.23 | 0.00 |
| ≥2 | 91 | 34 | 1.38 | 0.27 |
| ≥3 | 91 | 50 | 1.82 | 0.18 |
| ≥4 | 91 | 61 | 2.30 | 0.15 |
| ≥5 | 91 | 68 | 2.88 | 0.13 |
| ≥6 | 91 | 71 | 3.14 | 0.13 |
| ≥7 | 91 | 74 | 3.45 | 0.12 |
| ≥8 | 91 | 76 | 3.84 | 0.12 |
| ≥9 | 73 | 82 | 3.95 | 0.33 |
| ≥10 | 55 | 92 | 6.91 | 0.49 |
| ≥11 | 45 | 92 | 5.76 | 0.59 |
| ≥12 | 36 | 95 | 6.91 | 0.67 |
| ≥14 | 27 | 95 | 5.18 | 0.77 |
| ≥15 | 27 | 97 | 10.36 | 0.75 |
| ≥17 | 18 | 97 | 6.91 | 0.84 |
| ≥19 | 9 | 97 | 3.45 | 0.93 |
| ≥24 | 9 | 100 | — | 0.91 |
LR+ = positive likelihood ratio; LR− = negative likelihood radio; LUSS = lung ultrasound score.
While sensitivity was highest in both posterior scan zones, an accumulated LUSS of ≥2 showed equal sensitivity (91%, 95% CI, 59%–100%) but slightly lower specificity (58%, 95% CI, 41%–74%). Correlation was similar (p = 0.408) in upper (AUC = 0.85, 95% CI, 0.74–0.97) and lower zones (AUC = 0.80, 0.64–0.96).
The examiner's personal estimate (scale 0–3) predicted COVID-19 with an AUC of 0.82 (95% CI, 0.67–0.98) and an increase in odds ratio of 3.38 per point (95% CI, 1.66–6.90, p = 0.001), sensitivity of 82% (95% CI, 48%–98%) and specificity of 68% (95% CI, 51%–83%) with ≥1 point.
Discussion
While LUS has recently aroused great interest because of broad availability and ease of application, there is still no commonly accepted examination protocol nor scoring system. Comparing different approaches (Brahier et al. 2020; Huang et al. 2020; Lomoro et al. 2020; Manivel et al. 2020; Soldati et al. 2020), we documented lung alterations in a 6 × 2-zone semi-quantitative assessment of B-lines and consolidations as well as the aggregated LUSS (as illustrated and referenced in Fig. 1). B-lines in all scanning zones—especially the bilateral posterior zones—LUSS and the subjective estimation score all showed excellent predictive potential for COVID-19. Using a cutoff of ≥8 points, the LUSS showed a sensitivity of 91% (95% CI, 59%–100%) in our cohort. The limited number of subpleural consolidations in our sample may be an expression of an early disease stage, as scanning occurred just 6 d (IQR, 3–8) after symptom onset. Participants directly transferred to the ICU for respiratory failure—and therefore not included in this study—might have had more extensive consolidations (Brahier et al. 2020). The results may differ for patients in the ICU or outpatients.
Importantly, the diagnostic yield strongly depends on the epidemiologic situation: With the current prevalence (11/49 = 22%), the cutoff of ≥8 resulted in an excellent negative predictive value of 97% (95% CI, 83%–100%); LUS could virtually exclude COVID-19 in settings with lower pre-test probability, demonstrating potential utility as a rule-out test. This may be particularly helpful in individuals with clinically suspected COVID-19 infection but a negative SARS-CoV-2 PCR from nasopharyngeal swab, which was positive in only 8/11 (73%) COVID-19 infections. In this situation, a negative LUS finding might be a powerful argument to abstain from further investigations such as serology, chest CT scans (with a reported sensitivity of 95%; Lomoro et al. 2020) or chest X-ray (with a reported sensitivity of 69%; Brahier et al. 2020). On the other hand, the positive predictive value of 53% (95% CI, 29%–76%) in our setting does not confirm the diagnosis, but supports further investigations in the context of clinically suspected COVID-19 infection with negative PCR testing.
Participants did not report discomfort caused by LUS, nor were other adverse events observed, and the expense of scanning was minimal (examination time between 5 and 10 min per person). Using a highly portable ultrasound device, disinfection procedures were simple and efficient, as opposed to the expenses of performing a chest CT scan.
This study has several limitations. Because there was no temporal overlap between seasonal influenza and the SARS-CoV-2 pandemic, we cannot estimate the discriminatory power of LUS between these two entities of viral pneumonia. Neither did we assess its specific discriminatory potential between COVID-19 and chronic heart failure (often accompanied by pleural effusion), pulmonary embolism or pneumothorax, lacking appropriate numbers of respective controls. Acquisition and interpretation of ultrasound images remains somewhat subjective despite efforts to standardize assessment with protocols. While a single-operator study increased internal validity, external validity might be limited. Available studies on LUS in COVID-19, however, show good inter-observer agreement among even examiners with different experience (Trauer et al. 2020). We therefore advocate for a unified protocol for LUS scanning and reporting as our results show encouraging diagnostic potential for COVID-19 in people hospitalized with moderate to severe symptoms.
Conclusion
In conclusion, LUS demonstrated excellent predictive characteristics in a high-prevalence setting of individuals hospitalized with suspected COVID-19 and moderate to severe symptoms. More studies are needed to characterize test performance in different epidemiologic and case-mix scenarios.
Conflict of interest disclosure
The authors declare no competing interests.
References
- Allinovi M, Parise A, Giacalone M, Amerio A, Delsante M, Odone A, Franci A, Gigliotti F, Amadasi S, Delmonte D, Parri N, Mangia A. Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med Biol. 2020;46:2908–2917. doi: 10.1016/j.ultrasmedbio.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure–induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- Brahier T, Meuwly JY, Pantet O, Brochu Vez MJ, Gerhard Donnet H, Hartley MA, Hügli O, Boillat Blanco N. Lung ultrasonography features and risk stratification in 80 patients with COVID-19: A prospective observational cohort study [e-pub ahead of print] Clin Infect Dis. 2020 doi: 10.5281/zenodo.3763421. Accessed June 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Dargent A, Chatelain E, Kreitmann L, Quenot JP, Cour M, Argaud L, COVID-LUS Study Group Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala MJ. Ultrasound in COVID-19: A timeline of ultrasound findings in relation to CT. Clin Radiol. 2020;75:553–554. doi: 10.1016/j.crad.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang S, Liu Y, Zhang Y, Zheng C, Zheng Y, Zhang C, Min W, Zhou H, Yu M, Hu M. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). SSRN 2020, 10.2139/ssrn.3544750. [DOI]
- Lomoro P, Verde F, Zerboni F, Simonetti I, Borghi C, Fachinetti C, Natalizi A, Martegani A. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: Single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7 doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Zhang S, Chen B, Chen J, Xian J, Lin Y, Shan H, Su ZZ. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 2020;41:300–307. doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- Manivel V, Lesnewski A, Shamim S, Carbonatto G, Govindan T. CLUE: COVID-19 lung ultrasound in emergency department. Emerg Med Australas. 2020;32:694–696. doi: 10.1111/1742-6723.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MFH, Al-Shokri S, Yousaf Z, Danjuma M, Parambil J, Mohamed S, Mubasher M, Dauleh MM, Hasanain B, AlKahlout MA, Abubeker IY. Frequency of abnormalities detected by point-of-care lung ultrasound in symptomatic COVID-19 patients: Systematic review and meta-analysis. Am J Trop Med Hyg. 2020;103:815–821. doi: 10.4269/ajtmh.20-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongodi S, Orlando A, Arisi E, Tavazzi G, Santangelo E, Caneva L, Pozzi M, Pariani E, Bettini G, Maggio G, Perlini S, Preda L, Iotti GA, Mojoli F. Lung ultrasound in patients with acute respiratory failure reduces conventional imaging and health care provider exposure to COVID-19. Ultrasound Med Biol. 2020;46:2090–2093. doi: 10.1016/j.ultrasmedbio.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascarella G, Strumia A, Stone MB, Piliego C. The evolution of ultrasound role in COVID-19 pandemic: From triage to screening. Anesth Analg [forthcoming] 2020 doi: 10.1213/ANE.0000000000004920. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7389741 Available at: Accessed September 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study Group Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscaglia F, Stefanini F, Cantisani V, Sidhu PS, Barr R, Berzigotti A, Chammas MC, Correas JM, Dietrich CF, Feinstein S, Huang P, Jenssen C, Kono Y, Kudo M, Liang P, Lyshchik A, Nolsøe C, Xie X, Tovoli F. Benefits, open questions and challenges of the use of ultrasound in the COVID-19 pandemic era: The views of a panel of worldwide international experts. Ultraschall Med. 2020;41:228–236. doi: 10.1055/a-1149-9872. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokoohi H, Duggan NM, García-de-Casasola Sánchez G, Torres-Arrese M, Tung-Chen Y. Lung ultrasound monitoring in patients with COVID-19 on home isolation. Am J Emerg Med. 2020;38 doi: 10.1016/j.ajem.2020.05.079. 2759.E5–2759.E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Hayward SA, Innes SM, Miller ASC. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia. 2020;75:1096–1104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, Perlini S, Torri E, Mariani A, Mossolani EE, Tursi F, Mento F, Demi L. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020;39:1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauer MM, Matthies A, Mani N, McDermott C, Jarman R. The utility of lung ultrasound in COVID-19: A systematic scoping review. Ultrasound. 2020;28:208–222. doi: 10.1177/1742271X20950779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung-Chen Y, de Gracia MM, Díez-Tascón A, Alonso-González R, Agudo-Fernández S, Parra-Gordo ML, Ossaba-Vélez S, Rodríguez-Fuertes P, Llamas-Fuentes R. Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease 2019 (COVID-19) Ultrasound Med Biol. 2020;46:2918–2926. doi: 10.1016/j.ultrasmedbio.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrugno L, Bove T, Orso D, Barbariol F, Bassi F, Boero E, Ferrari G, Kong R. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli G, Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020;12:22. doi: 10.1186/s13089-020-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HYF, Lam HYS, Fong AHT, Leung ST, Chin TWY, Lo CSY, Lui MMS, Lee JCY, Chiu KH, Chung T, Lee EYP, Wan EYF, Hung FNI, Lam TPW, Kuo M, Ng MY. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa K, Minami T. Point-of-care lung ultrasound findings in patients with novel coronavirus disease (COVID-19) pneumonia. Am J Trop Med Hyg. 2020;102:1198–1202. doi: 10.4269/ajtmh.20-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]