Abstract
Human coronavirus (HCoV) causes potentially fatal respiratory disease. Pregnancy is a physiological state that predisposes women to viral infection. In this review, we aim to present advances in the pathogenesis, clinical features, diagnosis, and treatment in HCoV in pregnancy. We retrieved information from the Pubmed database up to June 2020, using various search terms and relevant words, including coronaviruses, severe acute respiratory syndrome coronavirus, Middle East respiratory syndrome coronavirus, 2019 coronavirus disease, and pregnancy. Both basic and clinical studies were selected. We found no evidence that pregnant women are more susceptible to HCoV infection or that those with HCoV infection are more prone to developing severe pneumonia. There is also no confirmed evidence of vertical mother-to-child transmission of HcoV infection during maternal HCoV infection. Those diagnosed with infection should be promptly admitted to a negative-pressure isolation ward, preferably in a designated hospital with adequate facilities and multi-disciplinary expertise to manage critically ill obstetric patients. Antiviral treatment has been routinely used to treat pregnant women with HCoV infection. The timing and mode of delivery should be individualized, depending mainly on the clinical status of the patient, gestational age, and fetal condition. Early cord clamping and temporary separation of the newborn for at least 2 weeks is recommended. All medical staff caring for patients with HCoV infection should use personal protective equipment. This review highlights the advances in pathogenesis, maternal-fetal outcome, maternal-fetal transmission, diagnosis and treatment in HCoV including severe acute respiratory syndrome coronavirus, Middle East respiratory syndrome coronavirus, and coronavirus disease 2019 in pregnancy.
Keywords: Coronavirus, COVID-19, MERS-CoV, Pregnancy, SARS-CoV, SARS-CoV-2
Introduction
Human coronaviruses (HCoV) are a diverse group of viruses capable of infecting humans. HCoV are the second most prevalent cause of the common cold (rhinoviruses are the first). HCoV infections are transmitted through droplets, aerosols, and close contact. Most patients have asymptomatic infection or mild symptoms and good prognosis after infection. Some patients develop severe disease and die from multi-organ failure, including respiratory, gastrointestinal (GI), hepatic, and neurological complications.1–4 Asymptomatic carriers could acquire and transmit coronavirus disease 2019 (COVID-19).5 Some types of HCoV have caused outbreaks of severe acute respiratory syndrome (SARS) caused by SARS-CoV, Middle East respiratory syndrome caused by MERS-CoV and COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6–9
The fatality rates of SARS, MERS, and COVID-19 are 10%, 35%–38%, and 5.6%, respectively.10–13 Poor prognostic factors include advanced age; viral loads; overweight or obese; and comorbidities such as hypertension, diabetes, and coronary heart disease.14,15 HCoV infection in pregnancy may be potentially life-threatening, representing complicated management issues for the mother and fetus.16–18 In the current review, we mainly discuss the three highly lethal and contagious HCoV infections, SARS-CoV, MERS-CoV, and SARS-CoV-2 infection.
Virology
Coronaviruses (CoVs) were so named because, when viewed by electron microscopy, they have club-shaped surface projections that give them a crown-like appearance. The Coronaviridae family includes two subfamilies: Coronavirinae and Torovirinae. HCoV are single-stranded positive-sense RNA viruses with a genome of approximately 30 kb, the largest genome among RNA viruses. HCoV are divided into four genera: alpha, beta, gamma, and delta HCoV, two of which contain viruses infecting humans. The beta genus is further subdivided into four lineages, A-D. Seven HCoV were identified. Four commonly detected HCoV are NL63 and 229E, which belong to the alpha-coronavirus genus, and OC43 and HKU1, which belong to the beta-coronavirus genus. The other three HCoVs, SARS-CoV, MERS-CoV, and SARS-CoV-2, belong to the beta-coronavirus genus and are the major causes of severe pneumonia in humans.8,19,20 The virus contains five structural proteins: the spike or S protein, the haemagglutinin-esterase protein, the M (matrix) protein, the E (envelope) protein, and the N (nucleocapsid) protein. HCoV cause a wide range of human respiratory, GI, neurological, and systemic illnesses.2–4Figure 1 shows the coronavirus taxonomy and related diseases.21
Figure 1.
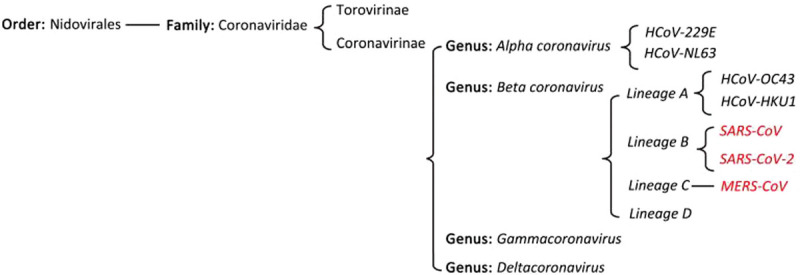
Coronavirinae taxonomy. CoV: Coronavirus; COVID-19: Coronavirus disease 2019; HCoV: Human coronaviruses; SARS: Severe acute respiratory syndrome; MERS: Middle East respiratory syndrome.
Pathogenesis
SARS-CoV-2 and SARS-CoV use the angiotensin converting enzyme 2 receptor to facilitate viral entry into target cells.22–24 Dipeptidyl peptidase 4, which is found on the surface of cells in the lungs, kidneys, small intestine, T lymphocytes, and macrophages, is a functional receptor for MERS-CoV.25 All the three highly lethal CoVs (SARS-CoV, MERS-CoV, SARS-CoV-2) induce excessive and aberrant non-effective host immune responses that are associated with severe lung pathology, which can lead to death.26
In the acute phase of HCoV infection, rapid reduction of lymphocytes in peripheral blood, mainly T lymphocytes, is observed, and both CD4+ and CD8+ T lymphocytes are decreased. The loss of lymphocytes even precedes abnormal changes on chest X-ray.
HCoV-specific immunoglobulin G (IgG) antibodies are produced in the late acute stage for approximately 2 weeks and gradually increase with the course of the disease. Recovering patients have high and sustained levels of specific neutralizing antibody responses, which may play an important role in determining disease outcome. In the course of disease progression, patients may have increased pro-inflammatory cytokine and chemokine secretion levels, which peak in the early stage of recovery and gradually decrease with the progression of the disease.27–30
The pathological features of COVID-19 greatly resemble those seen in SARS and Middle Eastern respiratory syndrome (MERS) coronavirus infection. The main pathologic findings from the lungs of fatal cases of COVID-19 pneumonia include hyaline membrane formation, fibrin exudates, epithelial damage, and diffuse type II pneumocyte hyperplasia, which are all features of diffuse alveolar damage and bacterial pneumonia in some patients.31–33
Jiang et al. found SARS-specific immunoglobulin G antibody in the maternal blood, umbilical cord blood, and amniotic fluid of a pregnant SARS patient and suggested potential protection of the fetus from infection. No SARS-CoV genes were detected in the maternal blood, umbilical blood, or amniotic fluid of the patient when using a SARS virus fluorescence quantitative PCR diagnostic kit.34
Maternal-fetal outcome
Pneumonia, an important non-obstetric infectious condition, is an important cause of morbidity and mortality among pregnant women.35,36
Lam et al. compared 10 pregnant and 40 non-pregnant women with SARS. Four out of the 10 pregnant patients required endotracheal intubation, and six were admitted to the intensive care unit (ICU), compared with a 12.5% intubation rate and 17.5% ICU admission rate in the non-pregnant group. More pregnant patients with SARS than non-pregnant patients with SARS developed renal failure and disseminated intravascular coagulopathy. There were three deaths in the pregnant group, whereas there were no deaths in the non-pregnant control group. The authors concluded that pregnant women with SARS experience a worse clinical course and poorer outcomes than non-pregnant women.37 SARS acquired during pregnancy carried a case fatality rate of 25% and was associated with a high incidence of spontaneous miscarriage, preterm delivery, and intrauterine growth retardation.4
The most common adverse obstetrical outcomes associated with maternal pneumonia from all causes include premature rupture of membranes and preterm labor, intrauterine fetal demise, intrauterine growth restriction (IUGR), and neonatal death.34 Pregnancy with SARS is associated with a high incidence of adverse maternal and neonatal complications, such as spontaneous miscarriage, preterm delivery, IUGR, necessity for endotracheal intubation, admission to the ICU, renal failure, and disseminated intravascular coagulopathy.37,38 Assiri et al. reported five pregnant patients with MERS based on a retrospective study, and all experienced adverse outcomes. Ages of patients ranged from 27 to 34 years, with the occurrence of exposure in either the 2nd or 3rd trimester. All five patients received intensive care. Two women died, and there were two cases of perinatal death: one stillbirth and one neonatal death shortly after emergency cesarean section.39 Fassett et al. screened SARS-CoV-2 in 3923 women delivered during the study period and found 17 (0.43%) women positive. None of them were symptomatic on admission.40
Chen reported 118 cases of women with COVID-19 and found 8% (nine cases) was severe disease. Severe disease developed in six of the nine women after delivery.41 COVID-19 seems to be less lethal than SARS or MERS based on the limited number of reported cases. COVID-19 increase low birth weight rate and premature birth rate.42–44 Pregnant women with COVID-19 had fewer adverse maternal and neonatal complications than those with SARS or MERS.45 Knight et al. reported a prospective national population based cohort study using the UK Obstetric Surveillance System. Four hundred and twenty-seven pregnant women admitted to hospital with confirmed SARS-CoV-2 infection. Two hundred and eighty-one (69%) were overweight or obese, 175 (41%) were aged 35 or over, and 145 (34%) had pre-existing comorbidities. Forty-one (10%) women admitted to hospital needed respiratory support, and five (1%) women died. Twelve (5%) of 265 infants tested positive for SARS-CoV-2 RNA, six of them within the first 12 hours after birth.15 Ellington et al. reported 83,205 non-pregnant women of reproductive age (15–44 years) with laboratory-confirmed SARS-CoV-2 infections and 8207 pregnant women of reproductive age (15–44 years) with laboratory-confirmed SARS-CoV-2 infections in USA. The COVID-19-related deaths all were 0.2% (16 pregnant women and 208 non-pregnant women among aged 15–44 years). Hospitalization was reported by a substantially higher percentage of pregnant women (31.5%) than non-pregnant women (5.8%). Pregnant women were admitted more frequently to the ICU (1.5%) than were non-pregnant women (0.9%). Half a percent of pregnant women required mechanical ventilation compared with 0.3% of non-pregnant women.46 We reviewed 235 cases of reported pregnancy with HCoV infection, including SARS (16 cases), MERS (11 cases) and COVID-19 (208 cases including 11 twin cases). Maternal deaths occurring during pregnancy with MERS, SARS, and COVID-19 were 19% (3/16 cases), 27% (3/11 cases), and 4% (8/208 cases), respectively. Table 1 shows the maternal–fetal outcome in pregnancies with SARS, MERS, and COVID-19.47–85Table 2 shows cases of death in women affected by coronavirus during pregnancy.38,39,47,62,86
Table 1.
Maternal-fetal characteristics and outcome in pregnancies with SARS, MERS and COVID-19.
| Items | SARS (n = 16) no./total no. (%) | ERS (n = 11) no./total no. (%) | COVID-19 (n = 208) no./total no. (%) |
|---|---|---|---|
| Age (years) < 35 | 12/16 (75) | 8/11 (73) | 154/208 (74) |
| Age (years) ≥ 35 | 3/16 (19) | 3/11 (27) | 54/208 (26) |
| No pregnancy age reported | 1/16 (6) | 0/11 (0) | 0/208 (0) |
| Nulliparous | 6/16 (38) | 3/11 (27) | 79/208 (38) |
| Parous | 8/16 (50) | 7/11 (64) | 83/208 (40) |
| No pregnancy history reported | 2/16 (13) | 1/11 (9) | 46/208 (22) |
| Singleton pregancy | 16/16 (100) | 11/11 (100) | 197/208 (95) |
| Twin pregnancy | 0/16 (100) | 0/11 (0) | 11/208 (5) |
| First trimester | 8/16 (50) | 1/11 (9) | 46/208 (22) |
| Second trimester | 5/16 (31) | 4/11 (36) | 34/208 (16) |
| Third trimester | 3/16 (19) | 6/11 (55) | 151/208 (73) |
| Laboratory-diagnosed | 16/16 (100) | 11/11 (100) | 174/208 (84) |
| Clinically-diagnosed | 0/16 (0) | 0/11 (0) | 34/208 (16) |
| Asymptomatic | 0/16 (0) | 1/11 (9) | 23/208 (11) |
| Symptomatic | 16/16 (100) | 9/11 (81) | 185/208 (89) |
| No report | 0/16 (0) | 1/11 (9) | 0/208 (0) |
| Mechanical ventilation | 5/16 (31) | 5/11 (45) | 40/208 (19) |
| Extracorporeal membrane oxygenation | 0/16 (0) | 0/11 (0) | 3/208 (1) |
| Pulmonary embolism | 0/16 (0) | 0/11 (0) | 2/199 (1)§ |
| Disseminated intravascular coagulopathy | 3/16 (19) | 0/11 (0) | 4/199 (2)§ |
| Kidney injury/renal failure | 3/16 (19) | 2/11 (18)† | 6/199 (3)§ |
| Secondary bacterial pneumonia | 1/16 (6) | 0/11 (0) | 5/199 (3)§ |
| Sepsis | 2/16 (13) | 0/11 (0) | 7/199 (4)§ |
| Respiratory failure/ARDS | 5/16 (31) | 5/11 (45) | 34/199 (17)§ |
| Cardiovascular collapse/shock | 2/16 (13) | 1/11 (9) | 13/199 (7)§ |
| Cardiac arrest | 0/16 (0) | 1/11 (9) | 2/199 (1)§ |
| Cardiomyopathy | 0/16 (0) | 0/11 (0) | 2/199 (1)§ |
| Acute hepatic failure | 0/16 (0) | 0/11 (0) | 1/199 (1)§ |
| Overlapping HELLP manifestation | 0/16 (0) | 0/11 (0) | 2/199 (1)§ |
| Cerebral infarction | 0/16 (0) | 0/11 (0) | 2/199 (1)§ |
| Acute severe ulcerative colitis | 0/16 (0) | 0/11 (0) | 1/199 (1)§ |
| Pancreatitis | 0/16 (0) | 0/11 (0) | 1/199 (1)§ |
| Multiple organ dysfunction syndrome | 3/16 (19) | 2/11 (18) | 15/199 (8)§ |
| Maternal mortality | 3/16 (19) | 3/11 (25) | 8/208 (4) |
| Pregnancy ongoing | 5/16 (31) | 4/11 (27) | 53/208 (25) |
| Delivery after recovery | 5/16 (31) | 4/11 (27) | 0/208 (0) |
| Delivery when infection | 3/16 (19) | 6/11 (55) | 144/208 (69)|| |
| Vaginal delivery when infection | 0/16 (0) | 2/11 (18) | 19/144 (13) |
| Cesarean section when infection | 3/16 (19) | 4/11 (36) | 125/144 (87) |
| Preterm birth | 5/16 (31)∗ | 6/11 (55)‡ | 43/155 (28)¶ |
| Perinatal death | 0/16 (0) | 3/11 (27) | 11/155 (7)¶ |
| Neonatal infection | 0/16 (0) | 0/11 (0) | 5/155 (3)¶ |
| Intrauterine growth restriction | 2/16 (13) | 0/11 (0) | 8/155 (5)¶ |
| Abortion | 8/16 (50) | 0/11 (0) | 11/208 (5) |
| Spontaneous abortion | 4/8 (50) | 0/11 (0) | 5/11 (45) |
| Induced abortion | 4/8 (50) | 0/11 (0) | 4/11 (36) |
| Ectopic pregnancy | 0/16 (0) | 0/11 (0) | 2/11 (18) |
SARS: Severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; COVID-19: Coronavirus disease 2019; HELLP: Hemolysis, elevated liver enzymes, and low platelets; ARDS: Acute respiratory distress syndrome.
Two cases preterm birth after recovery.
One case with history of end stage renal disease (ESRD) and hypertension on hemodialysis.
Two cases preterm birth after recovery.
Nine severe disease cases’ details no report.
One case delivery way unknown.
Eleven cases of twins.
Table 2.
Characteristics of maternal death cases from women with coronavirus infection.
| Case No. | HCoV infection | Age (years) | G/P | GA (weeks) | CoV PCR | CXR or CT | Comorbidities | Delivery | Neonatal outcome | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SARS | 44 | 4/3 | 5 | + | + | None | SM | – | Die from progressive respiratory failure |
| 2 | SARS | 34 | 2/1 | 32 | + | + | None | CS | Preterm labor; fetal distress | Die from progressive respiratory failure |
| 3 | SARS | 34 | 2/1 | 27 | + | + | None | CS | Preterm labor; RDS;NEC;PDA; Fetal distress | Die from methicillin-resistant Staphylococcus aureus pneumonia associated with shock |
| 4 | MERS | 32 | 2/1 | 38 | + | + | None | VD | Survived | Die from multiple organ failure |
| 5 | MERS | 31 | 1/0 | 24 | + | + | Asthma, Pulmonary fibrosis, Recurrent spontaneous pneumothoraces | CS | Death after CS 4 hours | Die from severe refractory hypoxia and cardiac arrest |
| 6 | MERS | 32 | 3/2 | 32 | + | + | None | CS | Health | Die from septic shock |
| 7 | COVID-19 | 25–29∗ | 2/1 | 303/7 | + | + | None | VD | Death | Die from multiple organ failure |
| 8 | COVID-19 | 25–29∗ | 1/0 | 383/7 | + | + | Obesity | CS | Health | Die from cardiopulmonary collapse |
| 9 | COVID-19 | 40–44∗ | 2/1 | 305/7 | + | + | Subclinical hypothyroid | CS | Preterm labor; negative† | Die from end organ failure |
| 10 | COVID-19 | 30–34∗ | 3/0 | 240/7 | + | + | None | Undelivered | Stillborn | Die from multiple organ failure |
| 11 | COVID-19 | 30–34∗ | 2/1 | 360/7 | + | + | Type A2 gestational diabetes | CS | Health | Die from cardiopulmonary collapse |
| 12 | COVID-19 | 35–39∗ | 2/0 | 240/7 | + | + | None | Undelivered | Dichorionic, diamniotic twin gestation in utero at the time of maternal death, undelivered | Die from multiple organ failure |
| 13 | COVID-19 | 45–49∗ | 2/1 | 280/7 | + | + | Underweight | CS | Experienced complications of premature birth and both died on day-of-life 3 | Die from a failed cardiopulmonary resuscitation |
| 14 | COVID-19 | 29 | 2/1 | 29 | + | + | Type 2 diabetes mellitus (T2DM) on metformin and insulin, renal tubular acidosis, asthma and vitamin D deficiency | CS | Preterm labor | Die from progressive respiratory failure and basilar artery thrombosis |
COVID-19: Coronavirus disease 2019; CS: Cesarean section; CXR or CT: Chest X-ray or computed tomograph; G/P: Gravida and parity; GA: Gestational age; HCoV: Human coronaviruses; MERS: Middle East respiratory syndrome; NEC: Necrotizing enterocolitis; PCR: Polymerase chain reaction; PDA: Patent ductus arteriosus; RDS: Respiratory distress syndrome; SARS: Severe acute respiratory syndrome; SM: Spontaneous miscarriage; VD: Vaginal delivery; –: not applicable.
For protection of patient identification, maternal age was gated in inclusive 5-year blocks in original report.
As detailed in the case description, case 3 was negative on day of life 1, but converted to positive on day of life 7.
Placental pathology
Ng et al. reported the placental pathology of seven mothers with SARS, of whom six were SARS-CoV RNA positive and one was seroconverted. The gestation ages at delivery were 15–38 weeks. Two placentas from women who were convalescing from SARS-CoV infection during the first trimester of pregnancy were normal. Three placentas that were delivered from pregnancies with acute SARS-CoV infection were abnormal and demonstrated increased subchorionic and intervillous fibrin, a finding that can be associated with abnormal maternal blood flow to the placenta. Two placentas from women who were convalescing from SARS-CoV infection in the 3rd trimester of pregnancy were highly abnormal. The placentas showed extensive fetal thrombotic vasculopathy with areas of avascular chorionic villi-chronic findings of fetal vascular malperfusion. Two women also had oligohydramnios complications and had poor obstetrical outcomes. The infants had developed IUGR. No villitis was identified in any of the placentas.87 Shanes et al. studied sixteen placentas from patients with SARS-CoV-2 (15 with live birth in the third trimester, one delivered in the second trimester after intrauterine fetal demise). They found the third trimester placentas have more feature of maternal vascular malperfusion, particularly abnormal or injured maternal vessels, and intervillous thrombi. The placenta from the patient with intrauterine fetal demise showed villous edema and a retroplacental hematoma. Rates of acute and chronic inflammation were not increased.88 Baergen et al. studied 20 placentas whose mother tested positive for the SARS-CoV-2 cases. Ten of the 20 cases showed some evidence of fetal vascular malperfusion or fetal vascular thrombosis.89 The placental pathology may explain the adverse fetal and neonatal outcomes in women with HCoV infection. The placental changes may reflect a systemic inflammatory or hypercoagulable state influencing placental physiology.
Materno-fetal transmission
In a prospective pilot study, Gagneur et al. studied 159 samples from mother–child couples: maternal vaginal and respiratory samples taken during labor and newborn gastric samples were tested for HCoV (229E, OC-43, NL-63, HKU1) by using real-time PCR (RT-PCR). HCoV were detected in 12 samples (229E: 11; HKU1: 1) from seven mother–child couples. All three samples (maternal vaginal, maternal respiratory, and newborn gastric) tested positive in two couples. The authors concluded that vertical transmission may be possible in HCoV infection.90 Dong et al. reported one newborn with elevated immunoglobulin M (IgM) antibodies to SARS-CoV-2 who was born from a mother with COVID-19. Five RT-PCR tests of nasopharyngeal (NP) swab samples taken from 2 hours to 16 days of age were negative.91 Zeng et al. detected SARS-CoV-2 IgG and IgM antibodies at birth in the blood of six infants born to mothers with COVID-19 pneumonia and found two infants’ blood was IgM positive. Five of six infants’ blood was IgG positive. Neonatal throat swab and blood samples all demonstrated SARS-CoV-2-negative RT-PCR test results. IgM, which was detected in two infants, is not usually transferred from mother to fetus because of its larger macromolecular structure. IgM could have been produced by the fetus if the virus crossed the placenta.92 The two reports suggested the possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn in utero. High-quality research is needed to elucidate whether SARS-CoV-2 can be transmitted in utero from the mother to the fetus.93
By using electron microscopy, Algarroba et al. were able to identify virions invading syncytiotrophoblasts in placental villi of a pregnant woman SARS-CoV-2 infection.49 Zhang et al. reported four newborn infected with COVID-19. The ages ranged from 30 hours to 17 days old. All four mothers were infected by SARS-CoV-2, three showing symptoms before and one after delivery. Cesarean section was used for all four mothers.94 Kamali Aghdam reported one cases of neonatal COVID-19 who was confirmed at 15 days after CS birth. Alzamora reported neonate SARS-CoV-2 infection confirmed at 16 hours after CS delivery. The six cases of neonate SARS-CoV-2 infection all were born from CS.95 Chen et al. tested eight throat swabs from newborns whose mothers infected with SARS-CoV-2 and three breast-milk samples of the mothers and did not find positive SARS-CoV-2.41
Ferrazzi et al. reported 42 women with COVID-19 and 24 women delivered vaginally. One newborn infected with COVID-19, who developed GI symptoms within a few hours after vaginal delivery. After 3 days of birth, the newborn developed respiratory symptoms and was transferred to the neonatal ICU, where he recovered after 1 day of mechanical ventilation. The mother did not breastfeed. In this case series, 11 newborns received breastfeed. No newborn infected COVID-19.96
In current case review, none of the 20 neonates experienced HCoV infection when they were born through vaginal delivery. Only a newborn was asymptomatic but a nasopharyngeal swab was positive tested for SARS-CoV-2. A further RT-PCR was performed on the same neonatal nasopharyngeal swab 37 hours later and tested negative for SARS-CoV-2.
We summary that neonatal HCoV infection may have the possibility of occurring in utero, during delivery through the birth canal, or through postpartum contact. HCoV-specific immunoglobulin G passively transfers across the placenta from mother to fetus at the end of the second trimester and reaches high levels at the time of birth, which may decrease the neonatal infection rate.34,97Figure 2 shown the systemic and respiratory disorders caused by HCoV infection and vertical transmission.
Figure 2.
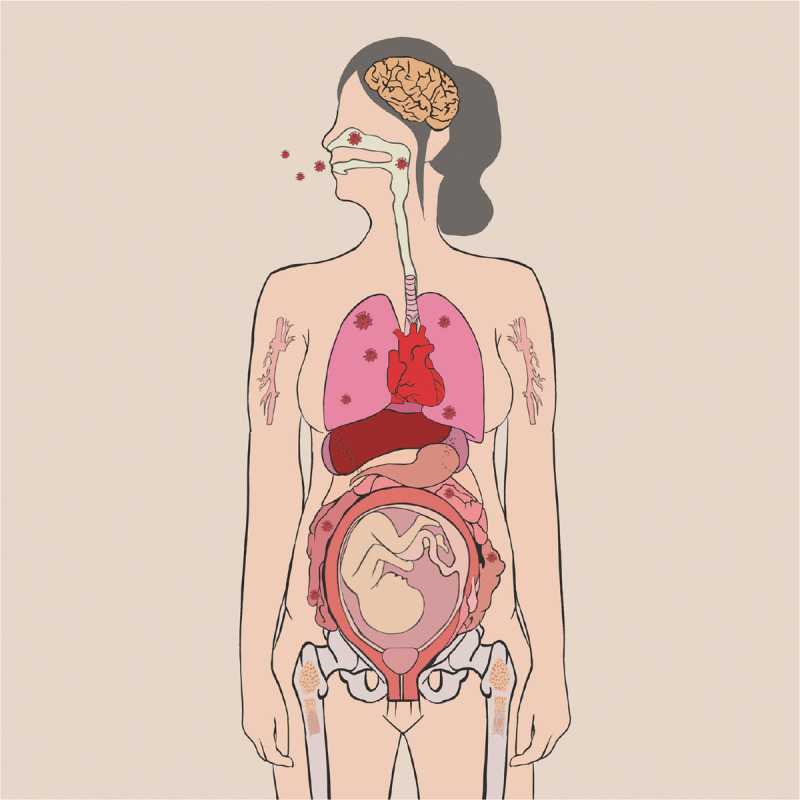
Systemic and respiratory disorders caused by HCoV infection and vertical transmission. One of 24 infant appeared SARS-CoV-2 infection when it was born through vaginal delivery suggests the possible vertical transmission of SARS-CoV-2 through birth canal.96 That three newborns with elevated IgM antibodies to SARS-CoV-2 who born from mothers with COVID-19 suggests the possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn in uterine.91,92 That two neonates SARS-CoV-2 infection 16 hours93 and 36 hours94 after birth (through cesarean section) and one 15-day-old neonate95 (birth through cesarean section) SARS-CoV-2 infection suggests their infection may be from postpartum contact. COVID-19: Coronavirus disease 2019; HCoV: Human coronavirus; IgM: Immunoglobulin M; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Diagnosis
Clinical features
Most infected patients have symptoms such as fever, fatigue, dry cough, and dyspnoea. Patients with mild symptoms may not present positive signs. Patients in a severe condition may have shortness of breath, moist rales in lungs, and weakened breath sounds. Patients with an asymptomatic carrier state may also be contagious and exist during pregnancy.51
Laboratory features
The diagnosis of SARS, MERS, and COVID-19 mainly requires the demonstration of virus. RT-PCR methods may generate false-positive or false-negative results. The first generation of RT-PCR assays were more sensitive at the end of the first week of illness, with only 35%–65% of specimens testing positive in the first few days of SARS-CoV infection.98 The positive test results from sputum (n = 104), nasal swab (n = 8), and pharyngeal swab specimens (n = 398) were 75 (72%), 5 (63%), and 126 (32%), respectively.99 False-negative result ranges from 17%–63% for NP swabs RT-PCR for SARS-CoV-2 have been reported in 12 studies in non-pregnant patients.100
CoV IgG and IgM could be used in the diagnosis of CoV infection. Serological tests using enzyme linked immune sorbent assay and indirect immunofluorescence assays have been used to monitor convalescent patients and perform serosurveys. The majority of infected patients will seroconvert: IgM values peak between weeks 2 and 3 after the onset of symptoms while remaining detectable up to 12 weeks post-infection; IgG values reach peak titres more slowly and may persist for a long time.101,102 Other laboratory tests include analyses of blood gases, liver and kidney function, myocardial enzymes, myoglobin, erythrocyte sedimentation rate, C-reactive protein, procalcitonin, lactate, D-dimer, coagulation factors, routine urine parameters, and inflammatory factors (interleukin-6, interleukin-10, tumor necrosis factor-α). Lymphopenia, disseminated intravascular coagulation, elevated lactate dehydrogenase, and creatinine kinase were the common laboratory features of HCoV infection.4,103
Imaging features
Computed tomography and X-ray imaging may be used in the diagnosis of CoV infection. In the pandemic area, Computed tomography and X-ray imaging may be the main diagnostic tool. Common radiographic features of HCoV infection include the predominant involvement of the lung periphery and the lower zone in addition to the absence of cavitation, hilar lymphadenopathy or pleural effusion. Radiographic progression from unilateral focal air-space opacity to either multifocal or bilateral involvement occurred during the second phase of the disease, followed by radiographic improvement with treatment.4,102
Typical ultrasound features include diffuse hyperechoic vertical artifacts with thickened pleural line and “white lung” with patchy distribution.104,105
Differential diagnosis
HCoV infection should mainly be distinguished from other known viral causes of pneumonia, such as influenza viruses, parainfluenza virus, adenovirus, respiratory syncytial virus, and rhinovirus, and from Mycoplasma pneumonia, Chlamydia pneumonia, and bacterial pneumonia. In addition, HCoV infection should be distinguished from non-infectious diseases, such as vasculitis, dermatomyositis, and organizing pneumonia.12,106
Management
Preparation
When managing patients with some kinds of HCoV infection during pregnancy, healthcare workers are particularly at risk and must take appropriate infection control precautions. One or two designated hospitals should be prepared for the management of patients with confirmed HCoV infection, including pregnant women. A group of tertiary hospitals should be prepared for screening pregnant women with possible HCoV infection. These hospitals should also manage the pregnant women who are persons under investigation (PUIs) and patients with confirmed cases in emergency.107–124
For hospital
A labor room/delivery room/operation room (OR) complex comprising several separate rooms, two with a negative-pressure environment, which is separate from the main labor room/delivery room/OR, should be designated for delivery and CS of patients with suspected or confirmed cases of HCoV infection. The labor room/delivery room/OR should have its own ventilation system with an integrated high-efficiency particulate air filter. The hospital that is designated to care for patients with HCoV infection, including infected pregnant women or PUIs, must ensure that their personnel are correctly trained and capable of implementing recommended infection control interventions. Hospital should have recommended infection control practices for hospitalized pregnant patients who are confirmed to have HCoV infection or are PUIs. Hospital should follow the infection control guidance on managing visitor access, including essential support persons for women in labor. Hospital should have a policy of limiting visitors, with the exception of a healthy parent or caregiver. Hospital should have a policy of instructing visitors to wear appropriate personal protective equipment (PPE), including gowns, gloves, face masks, and eye protection. In the United Kingdom (UK), there already exist significant protections in law for pregnant workers. In light of the limited evidence, pregnant women of any gestation should be offered the choice of whether to work in direct patient-facing roles during the COVID-19 pandemic.107 The ideal OR/labor room/delivery room, which is a complex and two with negative pressure environment and the isolation wards with a negative pressure environment are shown on Figures 3–4 (The figures were draw based on Third People's Hospital of Shenzhen).110–113
Figure 3.
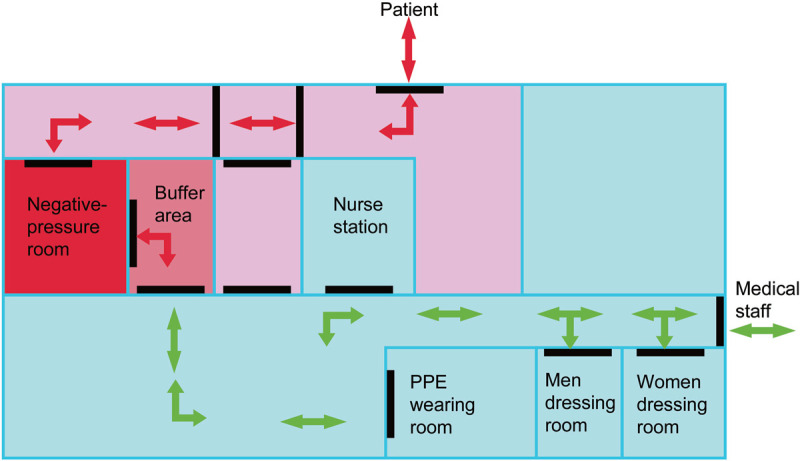
Operation room complex with a negative pressure environment. PPE: Personal protective equipment.
Figure 4.
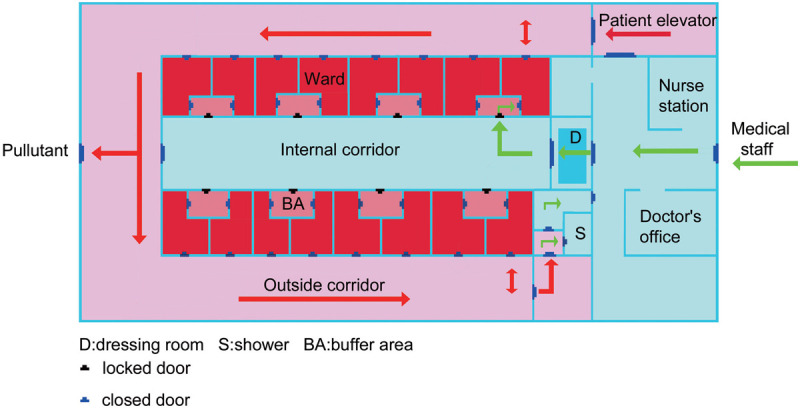
Isolation ward with a negative pressure environment.
For staff
A team of dedicated doctors and nurses should be designated to handle potential HCoV-infected patients. The team caring for pregnant women with confirmed or suspected HCoV infection should not care for other low-risk patients at the same time. All healthcare workers should be trained and fitted appropriately for N95 masks and powered air-purifying respirator. Individual healthcare personnel should ensure that they understand and can adhere to infection control requirements. The healthcare workers working in the triage areas should wear protective N95 masks and be strictly compliant with hand hygiene requirements. All healthcare workers should wear a powered air-purifying respirator when collecting NP swab specimens. The team caring for pregnant women with HCoV infection or PUI should use PPE, including a disposable gown, N95 mask, gloves, and eye protection, before they enter the isolation rooms. The team caring for patients with suspected or confirmed cases of HCoV infection should be closely monitored for fever or other signs of infection and should not be working in the presence of any individuals with HCoV infection symptoms.107–110
General management
All pregnant women presenting to the hospital should be screened using a standard questionnaire. On presentation to the triage areas, pregnant patients who meet the screening criteria should be placed in a negative-pressure isolation room, if available, to complete the screening procedure. The screening criteria are as follows: (1) travel to or residence in an outbreak area within the last 14 days; (2) a history of contact with a healthcare facility in an outbreak area; (3) a history of close contact with an individual with a confirmed case of HCoV infection within 14 days before the onset of illness; (4) person accompanying patient has been to an outbreak area within the last 14 days; (5) frequent or close contact with recent travellers from outbreak areas (travel history in the last 14 days); and (6) full pneumonia criteria regardless of travel/contact history. All pregnant women should be screened and assessed for symptoms and risk factors for HCoV infection.107,108,114–121
If a pregnant patient who has confirmed HCoV infection or is a PUI is arriving via transport by emergency medical services, the driver should contact the receiving emergency department or healthcare facility and follow previously agreed-upon local or regional transport protocols. Healthcare providers should promptly notify infection control personnel at their facility of the anticipated arrival of a pregnant patient who has confirmed HCoV infection or is a PUI. If negative-pressure isolation rooms are not available, patients should be isolated in single rooms or grouped together once HCoV infection has been confirmed. Patients should be categorized based on clinical evaluation, into mild or critical.18,107
The general management include oxygen therapy, antiviral drugs, antibacterial drugs, glucocorticoids, and symptomatic treatment based on doctor recommendations. Antiviral treatment may reduce the incidence of or mortality from acute respiratory distress syndrome.102,122–125 Antiviral therapy may have some benefits for parents with HCoV infection. Well designed therapy clinical trials in pregnant women with HCoV infection is still lack. Only one national guideline has the recommendation of antiviral therapy for pregnant women with COVID-19.126 There were some reported pregnant women with HCoV infection who had been cured and discharged from hospital.39,41 Antibacterial treatment is indicated only if there is evidence of secondary bacterial infection or if bacterial sepsis is suspected. Intravenous third-generation cephalosporins can be administered initially while awaiting culture and sensitivity results.
Maternal-fetal management
Antepartum
Pregnant women with confirmed HCoV infection who are asymptomatic may select self-monitoring for clinical features of HCoV at home or at designated hospitals for at least 14 days based on the local policy. Pregnant women with any of the three high fatal and contagious HCoV infections who are asymptomatic or are recovering from mild illness should be monitored with 2–4 weekly ultrasound assessments of fetal growth and amniotic fluid volume, with umbilical artery Doppler if necessary.
Pregnant women with confirmed HCoV infection should be managed by a multi-disciplinary team of midwives, obstetricians, and specialists in intensive care medicine, microbiologists, anesthetists, and neonatologists at a designated tertiary care center. Close monitoring and timely interventions should be performed to minimize maternal hypoxia. Supplemental oxygen should be administered to maintain oxygen saturation values above 95%; if requiring mechanical ventilation, pregnant patients should be maintained in the left lateral position to maximize uterine blood flow. Delivery of the fetus should be considered to improve maternal oxygenation. For preterm cases requiring delivery, the use of antenatal steroids for fetal lung maturation in a critically ill patient can potentially worsen the clinical condition, and the administration of antenatal steroids would delay the delivery that is necessary for the management of the patient. The use of antenatal steroids should be considered in discussion with infectious disease specialists, maternal–fetal medicine subspecialists, and neonatologists. In the case of an infected woman presenting with spontaneous preterm labor, tocolytics should not be used in an attempt to delay delivery to administer antenatal steroids.106,107
Labor and delivery
The timing of delivery should be individualized based on disease severity; existing comorbidities such as preeclampsia, diabetes, and cardiac disease; obstetric history; and gestational age and fetal condition. In mild and stable patients responding to treatment and in the absence of fetal compromise, pregnancy may be continued to term under close surveillance. The termination of pregnancy should be considered as an option before fetal viability is reached to save the pregnant woman's life after careful consultation with the patient, her family and an ethics board. In critical patients, continuing pregnancy may endanger the safety of the mother and her fetus. Criteria that have been considered for early delivery include: (1) rapid maternal deterioration; (2) failure to maintain adequate blood oxygenation; (3) difficulty with mechanical ventilation due to the gravid uterus; (4) multi-organ failure; (5) fetal compromise; and (6) other obstetric indications.123 The mode of delivery is mainly determined by obstetric indications. Solid evidence for vaginal shedding of virus and vertical transmission is lacking, and vaginal delivery may be considered in stable patients. In the event that an infected woman has spontaneous onset of labor with optimal progress, provided that appropriate preventative measures are in place, patients with HCoV infection can be allowed to deliver vaginally, but with a shortened second stage.106,107
Operation management
If the pregnant woman is very sick and/or far from term, cesarean section is likely to be chose. Careful consideration should be given regarding the choice of anesthesia when delivery by cesarean section is required. Those who have severe respiratory involvement without being ventilated would be better managed with elective general anesthesia. Women who are already being ventilated will be delivered with general anesthesia. A designated anesthesiologist should be called in to care for HCoV-infected patients. An OR with a negative-pressure environment is ideal to reduce the dissemination of the virus from CS.106,107,112
Postpartum
Workflow needs to be established to coordinate care among obstetricians, neonatologists, midwives, and nurses to ensure the safety of the mother and baby. Routine postoperative visits from anesthesiologists should be suspended and replaced by phone calls when applicable to reduce the movement of staff around the hospital. If possible, a dedicated breast pump should be provided. Before expressing breast milk, mothers should practice hand hygiene.114
During temporary separation, mothers who intend to breastfeed should be encouraged to express their breast milk to establish and maintain milk supply. After each pumping session, all parts that come into contact with breast milk should be thoroughly washed, and the entire pump should be appropriately disinfected per the manufacturer's instructions. If the newborn is rooming with his/her ill mother in the same hospital room in accordance with the mother's wishes, or if this is unavoidable due to facility limitations, facilities should consider implementing measures to reduce exposure of the newborn to HCoV. If no other healthy adult is present in the room to care for the newborn, a mother who has confirmed HCoV infection or is a PUI should wear a face mask and practice hand hygiene before each feeding or other close contact with her newborn. If another healthy family or staff member is present to provide care and feeding for the newborn, they should use appropriate PPE. The face mask should remain in place during contact with the newborn.106,107
Neonatal management
A sample from the neonatal NP suction before the first breath may be collected and sent for testing for HCoV infection. Early cord clamping is recommended. The decision to temporarily separate the mother from her baby should be made on a case-by-case basis in consultation with clinicians, infection prevention and control specialists, and public health officials.
The decision should take into account disease severity, illness signs and symptoms, and results of laboratory testing for HCoV. Mothers with confirmed or suspected HCoV infection should refrain from breastfeeding until they have fully recovered or have been confirmed not to have HCoV infection.
Neonates born to mothers with confirmed HCoV infection should be considered PUIs. As such, infants should be isolated at least 14 days or until the mother's viral shedding clears. The neonate should be cared for in an isolation ward and carefully monitored for any signs of infection.
Direct breastfeeding is not recommended. If a mother and newborn do room together and the mother wishes to breastfeed, she should put on a face mask and practice hand hygiene before each feeding.42,106,107,116
Conclusions
HCoV may infect pregnant women as it infects non-pregnant women. There is no evidence that pregnant women are more susceptible to HCoV infection or that those with HCoV infection are more prone to developing severe pneumonia. Similar to non-pregnant women, pregnant women with MERS had the highest mortality, followed by those with SARS and COVID-19. Fetal infection may occur in the uterus. There is also no solid evidence of the time when vertical transmission occurs in mothers with CoVs infection. Antiviral treatment is the main management for pregnant women with three highly fatal and contagious CoVs infections, similar to non-pregnant women. Timing and the mode of delivery should be individualized. The neonate should be cared for in isolation ward and carefully monitored for any signs of infection. All staff should use PPE when caring for a patient with HCoV infection.
Acknowledgments
Figure 2 was drawn by Shengmenart.
Funding
This research was supported by the Shenzhen Science and Technology Innovation Commission (JCYJ20180228162311024).
Author Contributions
Concept and design: Shangrong Fan, Literature search: Shaomei Yan, Data acquisition: Shaomei Yan and Suhua Wang, Data analysis: Shaomei Yan and Xiaoping Liu, Statistical analysis: Shaomei Yan, Manuscript preparation, manuscript editing, and manuscript review: Shangrong Fan and Lei Huang. The manuscript has been read and approved by all the authors.
Conflicts of Interest
None.
References
- [1].Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1967–1976. doi:10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- [2].Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17(3):181–192. doi:10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Song Z, Xu Y, Bao L, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019;11(1):59 doi:10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am 2019;33(4):869–889. doi:10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020;323(14):1406–1407. doi:10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. World Health Organization. Update 95-SARS: Chronology of a Serial Killer. 2020. Available at: https://www.who.int/csr/don/2003_07_04/en/. Accessed April 12, 2020. [Google Scholar]
- [7]. World Health Organization. Novel Coronavirus (2019-nCoV) Situation Reports. 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4. Accessed April 12, 2020. [Google Scholar]
- [8].Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367(19):1814–1820. doi:10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- [9].Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Azhar E, Hui DSC, Memish ZA, et al. The Middle East respiratory syndrome (MERS). Infect Dis Clin North Am 2019;33(4):891–905. doi:10.1016/j.idc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lim YX, Ng YL, Tam JP, et al. Human coronaviruses: a review of virus-host interactions. Diseases 2016;4(3):E26 doi:10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Memish ZA, Perlman S, Van Kerkhove MD, et al. Middle East respiratory syndrome. Lancet 2020;395(10229):1063–1077. doi:10.1016/S0140-6736 (19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peng Y, Xu B, Sun B, et al. Importance of timely management of patients in reducing fatality rate of coronavirus disease 2019. J Infect Public Health 2020;13(6):890–892. doi:10.1016/j.jiph.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–1062. doi:10.1016/S0140-6736 (20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Knight M, Bunch K, Vousden N, et al. UK obstetric surveillance system SARS-CoV-2 infection in pregnancy collaborative group characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020;369:m2107 Published 2020 Jun 8. doi:10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mullins E, Evans D, Viner RM, et al. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol 2020;55(5):586–592. doi:10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- [17].Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020;395(10226):760–762. doi:10.1016/S0140-6736 (20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liang H, Acharya G. Novel corona virus disease (COVID-19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand 2020;99(4):439–442. doi:10.1111/aogs.13836. [DOI] [PubMed] [Google Scholar]
- [19].Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003;361(9366):1319–1325. doi:10.1016/s0140-6736 (03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727–733. doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fung TS, Liu DX. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol 2019;73:529–557. doi:10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- [22].To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol 2004;203(3):740–743. doi:10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li WH, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426(6965):450–454. doi:10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46(4):586–590. doi:10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495(7440):251–254. doi:10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zumla A, Hui DS, Azhar EI, et al. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet 2020;395(10224):e35–e36. doi:10.1016/S0140-6736 (20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection--a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020;9(1):727–732. doi:10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill 2020;25(11):2000266 doi:10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020;71(15):778–785. doi:10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sarzi-Puttini P, Giorgi V, Sirotti S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 2020;38(2):337–342. [PubMed] [Google Scholar]
- [31].Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003;200(3):282–289. doi:10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ng DL, Al Hosani F, Keating MK, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 2016;186(3):652–658. doi:10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem corebiopsies. Mod Pathol 2020;33(6):1007–1014. doi:10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jiang X, Gao X, Zheng H, et al. Specific immunoglobulin g antibody detected in umbilical blood and amniotic fluid from a pregnant woman infected by the coronavirus associated with severe acute respiratory syndrome. Clin Diagn Lab Immunol 2004;11(6):1182–1184. doi:10.1128/CDLI.11.6.1182-1184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med 2020;144(7):799–805. doi:10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- [36].Fan S, Liu P, Yan S, et al. New concept and management for sepsis in pregnancy and the puerperium. Maternal Fetal Med 2020;2(4):231–239. doi:10.1097/FM9.0000000000000058. [Google Scholar]
- [37].Lam CM, Wong SF, Leung TN, et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG 2004;111(8):771–774. doi:10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004;191(1):292–297. doi:10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Assiri A, Abedi GR, Al Masri M, et al. Middle East respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis 2016;63(7):951–953. doi:10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fassett MJ, Lurvey LD, Yasumura L, et al. Universal SARS-Cov-2 screening in women admitted for delivery in a large managed care organization. Am J Perinatol 2020;37(11):1110–1114. doi:10.1055/s-0040-1714060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med 2020;382(25):e100 doi:10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rebmann T. Severe acute respiratory syndrome: implications for perinatal and neonatal nurses. J Perinat Neonatal Nurs 2005;19(4):332–345. doi:10.1097/00005237-200510000-00008. [DOI] [PubMed] [Google Scholar]
- [43].Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol 2020;55(4):435–437. doi:10.1002/uog.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li N, Han L, Peng M, et al. Maternal and Neonatal Outcomes of Pregnant Women With Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin Infect Dis 2020;71(16):2035–2041. doi:10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020;222(6):521–531. doi:10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69(25):769–775. doi:10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ahmed I, Azhar A, Eltaweel N, et al. First COVID-19 maternal mortality in the UK associated with thrombotic complications. Br J Haematol 2020;190(1):e37–e38. doi:10.1111/bjh.16849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].AlZaghal LA, AlZaghal N, Alomari SO, et al. Multidisciplinary team management and cesarean delivery for a Jordanian woman infected with SARS-COV-2: A case report. Case Rep Womens Health 2020;27:e00212 Published 2020 May 1. doi:10.1016/j.crwh.2020.e00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020;223(2):275–278. doi:10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Alzamora MC, Paredes T, Caceres D, et al. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol 2020;37(8):861–865. doi:10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 2020;2(2):100118 doi:10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Browne PC, Linfert JB, Perez-Jorge E. Successful treatment of preterm labor in association with acute COVID-19 infection. Am J Perinatol 2020;37(8):866–868. doi:10.1055/s-0040-1709993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Blauvelt CA, Chiu C, Donovan AL, et al. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2020;136(1):46–51. doi:10.1097/AOG.0000000000003949. [DOI] [PubMed] [Google Scholar]
- [54].Cooke WR, Billett A, Gleeson S, et al. SARS-CoV-2 infection in very preterm pregnancy: experiences from two cases. Eur J Obstet Gynecol Reprod Biol 2020;250:259–260. doi:10.1016/j.ejogrb.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Carosso A, Cosma S, Borella F, et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol 2020;249:98–99. doi:10.1016/j.ejogrb.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cheng SO, Khan S, Alsafi Z. Maternal death in pregnancy due to COVID-19. Ultrasound Obstet Gynecol 2020;56(1):122 doi:10.1002/uog.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dória M, Peixinho C, Laranjo M, et al. Covid-19 during pregnancy: a case series from an universally tested population from the north of Portugal. Eur J Obstet Gynecol Reprod Biol 2020;250:261–262. doi:10.1016/j.ejogrb.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Futterman I, Toaff M, Navi L, et al. COVID-19 and HELLP: overlapping clinical pictures in two gravid patients. AJP Rep 2020;10(2):e179–e182. doi:10.1055/s-0040-1712978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ferraiolo A, Barra F, Kratochwila C, et al. Report of positive placental swabs for SARS-CoV-2 in an asymptomatic pregnant woman with COVID-19. Medicina (Kaunas) 2020;56(6):E306 doi:10.3390/medicina56060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Govind A, Essien S, Karthikeyan A, et al. Re: novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol 2020;251:272–274. doi:10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gidlöf S, Savchenko J, Brune T, et al. COVID-19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand 2020;99(7):948–949. doi:10.1111/aogs.13862. [DOI] [PubMed] [Google Scholar]
- [62].Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID-19 disease. Am J Obstet Gynecol 2020;223(1):109.e1–109.e16. doi:10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hong L, Smith N, Keerthy M, et al. Severe COVID-19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case Rep Womens Health 2020;27:e00217 Published 2020 May 7. doi:10.1016/j.crwh.2020.e00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Iqbal SN, Overcash R, Mokhtari N, et al. An uncomplicated delivery in a patient with COVID-19 in the United States. N Engl J Med 2020;382(16):e34 doi:10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Juusela A, Nazir M, Gimovsky M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM 2020;2(2):100113 doi:10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kuhrt K, McMicking J, Nanda S, et al. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by COVID-19, without vertical transmission to the babies. Am J Obstet Gynecol MFM 2020;2(3):100135 doi:10.1016/j.ajogmf.2020.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Khan S, Peng L, Siddique R, et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol 2020;41(6):748–750. doi:10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kalafat E, Yaprak E, Cinar G, et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol 2020;55(6):835–837. doi:10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- [69].Lee DH, Lee J, Kim E, et al. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J Anesthesiol 2020;73(4):347–351. doi:10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lowe B, Bopp B. COVID-19 vaginal delivery - a case report. Aust N Z J Obstet Gynaecol 2020;60(3):465–466. doi:10.1111/ajo.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lucarelli E, Behn C, Lashley S, et al. Mechanical ventilation in pregnancy due to COVID-19: a cohort of three cases. Am J Perinatol 2020;37(10):1066–1069. doi:10.1055/s-0040-1713664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lyra J, Valente R, Rosário M, et al. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Med Port 2020;33(6):429–431. doi:10.20344/amp.13883. [DOI] [PubMed] [Google Scholar]
- [73].Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect 2020;[published online ahead of print, 2020 Mar 4]. doi:10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li J, Wang Y, Zeng Y, et al. Critically ill pregnant patient with COVID-19 and neonatal death within two hours of birth. Int J Gynaecol Obstet 2020;150(1):126–128. doi:10.1002/ijgo.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Martinelli I, Ferrazzi E, Ciavarella A, et al. Pulmonary embolism in a young pregnant woman with COVID-19. Thromb Res 2020;191:36–37. doi:10.1016/j.thromres.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mehta H, Ivanovic S, Cronin A, et al. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Rep Womens Health 2020;27:e00220 Published 2020 May 16. doi:10.1016/j.crwh.2020.e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ochiai D, Kasuga Y, Iida M, et al. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet 2020;150(2):268–269. doi:10.1002/ijgo.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Peng Z, Wang J, Mo Y, et al. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health 2020;13(5):818–820. doi:10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rabice SR, Altshuler PC, Bovet C, et al. COVID-19 infection presenting as pancreatitis in a pregnant woman: a case report. Case Rep Womens Health 2020;27:e00228 doi:10.1016/j.crwh.2020.e00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rosen MH, Axelrad J, Hudesman D, et al. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis 2020;26(7):971–973. doi:10.1093/ibd/izaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Silverstein JS, Limaye MA, Brubaker SG, et al. Acute respiratory decompensation requiring intubation in pregnant women with SARS-CoV-2 (COVID-19). AJP Rep 2020;10(2):e169–e175. doi:10.1055/s-0040-1712925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wen R, Sun Y, Xing QS. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China. J Microbiol Immunol Infect 2020;53(3):499–500. doi:10.1016/j.jmii.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang X, Zhou Z, Zhang J, et al. A case of 2019 Novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis 2020;71(15):844–846. doi:10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zambrano LI, Fuentes-Barahona IC, Bejarano-Torres DA, et al. A pregnant woman with COVID-19 in Central America. Travel Med Infect Dis 2020;36:101639 doi:10.1016/j.tmaid.2020.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Baud D, Greub G, Favre G, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020;323(21):2198–2200. doi:10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Malik A, El Masry KM, Ravi M, et al. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis 2016;22(3):515–517. doi:10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ng WF, Wong SF, Lam A, et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology 2006;38(3):210–218. doi:10.1080/00313020600696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Pathol 2020;154(1):23–32. doi:10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol 2020;23(3):177–180. doi:10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gagneur A, Dirson E, Audebert S, et al. Materno-fetal transmission of human coronaviruses: a prospective pilot study. Eur J Clin Microbiol Infect Dis 2008;27(9):863–866. doi:10.1007/s10096-008-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323(18):1846–1848. doi:10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020;323(18):1848–1849. doi:10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang C, Zhou Y, Yang H, et al. Intrauterine vertical transmission of SARS-CoV-2: what we know so far. Ultrasound Obstet Gynecol 2020;55(6):724–725. doi:10.1002/uog.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhang ZJ, Yu XJ, Fu T, et al. Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J 2020;55:2000697 doi:10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020;52(6):427–429. doi:10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG 2020;127(9):1116–1121. doi:10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kimberlin DW, Stagno S. Can SARS-CoV-2 infection be acquired in utero? More definitive evidence is needed. JAMA 2020;323(18):1788–1789. doi:10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- [98].Poon LL, Guan Y, Nicholls JM, et al. The aetiology, origins, and diagnosis of severe acute respiratory syndrome. Lancet Infect Dis 2004;4(11):663–671. doi:10.1016/S1473-3099 (04)01172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323(18):1843–1844. doi:10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kelly JC, Dombrowksi M, O’neil-Callahan M, et al. False-negative COVID-19 testing: considerations in obstetrical care. Am J Obstet Gynecol MFM 2020;2(3):100130 doi:10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bradley BT, Bryan A. Emerging respiratory infections: the infectious disease pathology of SARS, MERS, pandemicinfluenza, and Legionella. Semin Diagn Pathol 2019;36(3):152–159. doi:10.1053/j.semdp.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 2020;92(9):1518–1524. doi:10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7(1):4 doi:10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Moro F, Buonsenso D, Moruzzi MC, et al. How to perform lung ultrasound in pregnant women with suspected COVID-19. Ultrasound Obstet Gynecol 2020;55(5):593–598. doi:10.1002/uog.22028. [DOI] [PubMed] [Google Scholar]
- [105].Inchingolo R, Smargiassi A, Moro F, et al. The diagnosis of pneumonia in a pregnant woman with coronavirus disease 2019 using maternal lung ultrasound. Am J Obstet Gynecol 2020;223(1):9–11. doi:10.1016/j.ajog.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China [published correction appears in J Autoimmun. 2020 Jul;111:102487]. J Autoimmun 2020;109:102434 doi:10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chen D, Yang H, Cao Y, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet 2020;149(2):130–136. doi:10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Centers for Disease Control and Prevention. Considerations for Inpatient Obstetric Healthcare Settings. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html. Accessed June 2020. [Google Scholar]
- [109]. RCOG. COVID-19 Virus Infection and Pregnancy: Occupational Health Advice for Employers and Pregnant Women During the COVID-19 Pandemic. 2020. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-31-occupational-health-advice-for-employers-and-pregnant-women-during-the-covid-19-pandemic.pdf. Accessed August 5, 2020. [Google Scholar]
- [110].Chan BC, Lee CP, Tang GW. Universal SARS preventive measures in an obstetrics unit: experience of health care staff. Am J Infect Control 2004;32(7):417–420. doi:10.1016/j.ajic.2004.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen R, Zhang Y, Huang L, et al. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing cesarean delivery: a case series of 17 patients. Can J Anaesth 2020;67(6):655–663. doi:10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ti LK, Ang LS, Foong TW, et al. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance. Can J Anaesth 2020;67(6):756–758. doi:10.1007/s12630-020-01617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wong J, Goh QY, Tan Z, et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth 2020;67(6):732–745. doi:10.1007/s12630-020-01620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Poon LC, Yang H, Lee JCS, et al. ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol 2020;55(5):700–708. doi:10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chua MSQ, Lee JCS, Sulaiman S, et al. From the frontlines of COVID-19 - how prepared are we as obstetricians: a commentary. BJOG 2020;127(7):786–788. doi:10.1111/1471-0528.16192. [DOI] [PubMed] [Google Scholar]
- [116].Qi H, Luo X, Zheng Y, et al. Safe delivery for pregnancies affected by COVID-19. BJOG 2020;127(8):927–929. doi:10.1111/1471-0528.16231. [DOI] [PubMed] [Google Scholar]
- [117].Favre G, Pomar L, Qi X, et al. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect Dis 2020;20(6):652–653. doi:10.1016/S1473-3099 (20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol 2020;222(5):415–426. doi:10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Maxwell C, McGeer A, Tai KFY, et al. No. 225-management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). J Obstet Gynaecol Can 2017;39(8):e130–e137. doi:10.1016/j.jogc.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth 2020;67(5):568–576. doi:10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wang C, Chen D, Yang H. Updates on COVID-19 infection during pregnancy. Maternal-Fetal Med 2020;2(2):65–67. doi:10.1097/FM9.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med 2020;382(24):2327–2336. doi:10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ye XT, Luo YL, Xia SC, et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease. Eur Rev Med Pharmacol Sci 2020;24(6):3390–3396. doi:10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- [124].Wong SF, Chow KM, de Swiet M. Severe acute respiratory syndrome and pregnancy. BJOG 2003;110(7):641–642. doi:10.1046/j.1471-0528.2003.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Poon LC, Yang H, Kapur A, et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynaecol Obstet 2020;149(3):273–286. doi:10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Pereira A, Cruz-Melguizo S, Adrien M, et al. Clinical course of coronavirus disease-2019 (COVID-19) in pregnancy. Acta Obstet Gynecol Scand 2020;382(24):2327–2336. doi:10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]


