Supplemental Digital Content is available in the text.
Keywords: cost-effectiveness, Europe, guideline, ischemic stroke, quality-adjusted life years, thrombectomy
Background and Purpose:
Mechanical thrombectomy (MT) has been recommended for the treatment of nonminor ischemic stroke by national and international guidelines, but cost-effectiveness evidence has been generated for only a few countries using heterogeneous evaluation methods. We estimate the cost-effectiveness of MT across 32 European countries.
Methods:
A Markov model was developed to estimate the cost-effectiveness of MT compared with standard care over a 5-year time horizon. Patients with ischemic stroke eligible for MT were identified from 2017 country-specific incidence data. A societal perspective was adopted, including health, social, and informal care costs, and productivity losses. Model outcomes were expressed as quality-adjusted life years. Sensitivity analyses were conducted to test the robustness of findings.
Results:
We identified 267 514 ischemic stroke cases that were eligible for MT treatment across 32 European countries. MT was found to be more effective and cheaper than standard care in two-thirds of the countries (21/32) and cost-effective in all but one country (Bulgaria). Across Europe, the intervention was estimated to produce over 101 327 additional quality-adjusted life years (95% uncertainty interval, 65 180–149 085) and cost savings of $981 million (€868 million, 95% uncertainty interval, −1544 to 2564) and of $1.7 billion (€1.5 billion, 95% uncertainty interval, −1.2 to 3.6) in health and social care and societal costs, respectively.
Conclusions:
MT is highly likely to be cost-effective compared with standard care across Europe as a whole and in the vast majority of European countries.
See related article, p 674
Ischemic stroke represents a major concern to European societies, accounting for > 4 in 5 of all new stroke cases.1 It is estimated that over 1 million people suffer an ischemic stroke, and around 438 000 die of the condition, every year in Europe.2 In addition, ischemic stroke survivors have a high risk of becoming disabled and having a reduced quality of life.3,4 Therefore, survivors are likely to rely heavily on the health and social care system as well as on informal carers, resulting in high societal costs.5 This is projected to be particularly the case as the European population is aging, with health and social care costs continuing to rise as a result.6 It will, therefore, become imperative for policymakers to invest in medical and social care interventions that may mitigate these rising costs while improving population health outcomes.
Mechanical thrombectomy (MT) has been recommended by European guidelines7 and by the National Institute for Health and Care Excellence, and as the preferred treatment of acute ischemic stroke, particularly for large-vessel occlusions.8 MT treatment aims to restore blood flow by removal of the clot blocking the artery in the brain, using a revascularization device. This procedure is typically administered in combination with other medical treatments such as clot-busting drugs and nonthrombolytic care, depending on the patient’s risk of vascular complications (ie, bleeding).9
Although MT has shown to be highly effective10–13 and cost-effective14–20 in some health care systems, these cost-effectiveness studies used different parameters and modeling methods, making it difficult to evaluate the generalizability of the findings to different country settings. Furthermore, there is currently limited evidence on the cost-effectiveness of MT at the population level, especially for some European regions (ie, Southern and Eastern Europe). Therefore, the aim of this study was to consistently assess the population-level impact, in terms of costs and quality-adjusted life expectancy, of routinely providing MT versus current practice for patients with nonminor ischemic stroke across Europe.
Methods
The authors declare that all supporting data are available within the article (and its Data Supplement). We assessed the cost-effectiveness of routinely providing MT in combination with intravenous thrombolysis (IVT), compared with standard care which was defined as IVT alone. For patients over the age of 80 years, to whom IVT is typically not administered,21 we compared MT on its own with nonthrombolytic treatment. A cost-utility analysis was performed for 32 European countries, namely, the current 27 State members of the European Union, and Norway, Iceland, Israel, Switzerland, and the United Kingdom. We adopted a societal cost perspective, including health and social care costs, informal care costs, and productivity losses. Outcomes consisted of quality-adjusted life years (QALYs). The time horizon was 5 years with future costs and QALYs discounted using an annual rate of 3.5%. Cost was expressed at 2017 prices and reported in USD ($). The 2017 average exchange rates were used (exchange rate: €1=$1.1297).22 No ethical approval was required.
Country-specific, age- and sex-stratified adult ischemic stroke cases were obtained from the Global Burden of Disease study.23 Based on the criteria used in previous randomized controlled trials studies10–13 and current recommendation by the National Institute for Health and Care Excellence,24 patients eligible for MT were defined as follows:
having nonminor ischemic stroke, defined using a National Institutes of Health Stroke Scale score ≥5. Data from a published model25 were used to determine the proportion of patients with ischemic stroke with nonminor ischemic stroke;
being admitted to the hospital within 6 hours from symptoms onset.24 Following a review of the literature, 60% of patients were assumed to reach hospital within this timeframe26;
not being severely disabled before the event, as defined by a modified Rankin Scale (mRS) score ≤3. The proportion of premorbidly nondisabled patients was derived from the OXVASC (Oxford Vascular Study).
Markov Model, Treatment Effectiveness, and Transition Probabilities
A cohort-level state-transition Markov model with an embedded decision tree (Figure 1) was built to simulate the natural history of stroke and the impact of the interventions under analysis. The model structure was adapted from a previously published model.18 First, all incident ischemic stroke patients were divided into those that were eligible for MT and those who were not following the criteria and data sources described above. Eligible patients with ischemic stroke were all assumed to receive MT treatment and assigned to one of the 7 mRS score at 3 months (dead, mRS score of 5 [severe disability] to mRS score of 0 [no symptoms at all]). Hence, 3 months following the incident stroke, an individual could be either dead, recover fully (mRS score of 0), or have some form of stroke-related disability (mRS score of 1–5).27 If alive at 3 months, the model then simulated the risk of death up to 5 years conditional on their 3-month mRS score, age, and sex. The effectiveness of MT was modeled via a change in all-cause mortality and mRS score at 3 months, compared with standard care.
Figure 1.
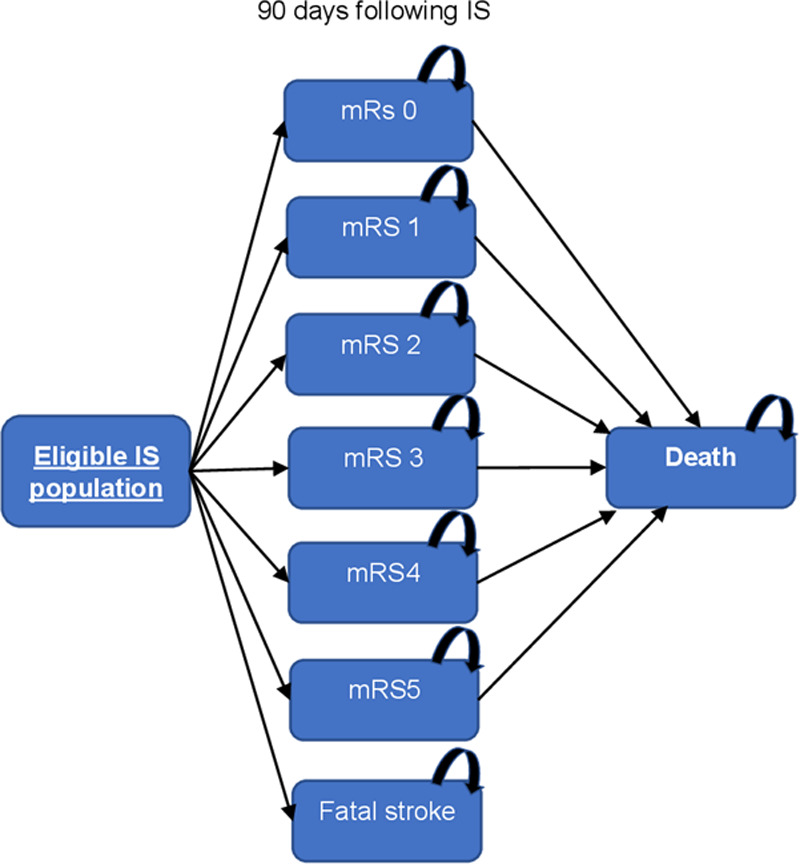
Markov model structure. IS indicates ischemic stroke; and mRS, modified Rankin Scale.
Appendix I in the Data Supplement reports the parameters and data sources used to populate the model. Briefly, country, age, and sex-specific numbers of incident ischemic stroke cases were derived from the Global Burden of Disease study.23 A meta-analysis of 5 randomized controlled trials20 informed the effectiveness of MT on the mRS score distribution and case fatality at 3 months poststroke compared with standard care. Data from OXVASC27 were used to simulate all-cause 5-year mortality risks following a nonfatal event at 3 months. Appendix II in the Data Supplement provides details on the countries informing key model parameters and how country-level variation was captured in our analysis. Model cycle length was 1 year following the first 12 months of simulation. This was sufficiently short to capture all relevant outcomes and costs in each cycle.
Survival and Quality of Life
Based on OXVASC, we estimated parametric survival equations and quality of life values. Quality of life was measured using the Euroqol-5 dimensions-3 levels instrument,28 where Euroqol-5 dimensions responses were collected from patients with ischemic stroke at 1 to 3, 6, 12, and 60 months and converted into utilities using UK population tariffs.29 We also used published regression analyses estimating the changes in utility values over time conditional on 3-month mRS score, age, and sex.28 We assumed UK utility and survival values to apply to the remaining 31 countries, conditional on the patient’s mRS score at 3 months, age, and sex. Estimated QALYs varied across countries due to the different population age/sex distributions. Hence, identical patients (in terms of age, sex, and mRS score) would experience the same mortality risk and quality of life, irrespective of the country of origin, but the population mortality risk and utility values varied due to the different age/sex distributions across countries.
Treatment Costs
Costs of MT and standard care (with and without IVT) were derived from published studies. We assumed that the unit costs of devices and consumables were the same across countries (ie, MT cost: $4981, IVT cost: $685, and nonthrombolytic treatment: $140). We also assumed that the same treatment protocol was followed across all countries. The protocol consisted of what was reported in 2 previous studies in the United Kingdom18 and Italy20 and (Appendix III in the Data Supplement). Staff time was valued using country-specific unit costs.2 All interventions under analysis included the nonthrombolytic treatment. Hence, across the 32 countries, MT in combination with IVT amounted to $7543 ($4981+$685+$140+$1737), MT alone (patients over the age of 80 years) amounted to $6661 ($4981+$140+$1540), standard care in combination with IVT amounted to $1078 ($685+$140+$253), and standard care alone (patients over the age of 80 years) amounted to $196 ($140+$56). Appendix IV in the Data Supplement reports the costs of the interventions by country.
Health and Social Care Costs
Health and social care resource use following ischemic stroke were derived from OXVASC, conditional on age, sex, and 3-month mRS score.2 As part of 5-year follow-up after stroke, resource use included were: hospital stay and day cases (inpatient costs), outpatient visits, accident and emergency visits, and nursing/residential care.
We accounted for country-level heterogeneity by using country-specific resource use informed by UK/EU adjustment factors.2 For inpatient days, weights were calculated as the ratio of mean number of days in hospital following stroke in the United Kingdom over the respective mean number of days in hospital for the country under analysis. For example, if the average number of days spent in hospital after stroke was 18 days in the United Kingdom and 19 days in Austria, in the Austrian version of the model we corrected the hospital stay cost estimates using a factor of 1.06 (ie, 19/18). For outpatient and accident and emergency visits, weights were calculated as the ratio between estimates of per-capita visits due to stroke in the United Kingdom over per-capita stroke-related visits in the country under study. For nursing/residential care, per-capita rates of institutionalization in those aged 65 years or more in the United Kingdom were divided by the same per-capita rate for the country under analysis. Appendix V in the Data Supplement reports the weights used to adjust resource use for each country.
Informal Care
We assumed that half of those with an mRS score of 3 and all of patients with an mRS score of 4 and 5 would require informal care. We estimated informal care costs using country and age-/sex-specific numbers of days of care received, derived from the SHARE study (Survey of Health, Ageing and Retirement in Europe).30 For countries not included in the SHARE study, we used proxy costs. More specifically, we classified SHARE countries into 4 macroregions of Europe, that is, Scandinavia, Central Europe; Eastern Europe and Southern Europe. We then estimated regional level averages of age-/sex-specific numbers of days of care received in each macroregion. We then attributed these region-level averages to those countries not included in SHARE according to the macroregion they belonged to.
Productivity Losses
Loss of productivity was calculated in terms of mortality and morbidity in ischemic stroke patients under the age of 65 years, in line with a previous published study.2 In terms of mortality, we estimated the potential working years lost due to premature death, adjusting for country-specific employment rates.31,32 In terms of morbidity, for stroke survivors with a 3-month mRS score ≤2, we assumed that their absence from work would be temporary. Country-specific average days off work due to stroke were then used. For those patients with an mRS score >2, we assumed that their absence from work would be permanent. These estimates were then friction-adjusted, whereby only the first 90 days of work absence were considered. Working days lost due to mortality and morbidity were then valued using country-specific, sex-stratified earnings.2,31
Analysis
Country-specific cohorts of eligible patients with ischemic stroke were used to simulate the population receiving MT or standard care. To capture the heterogeneity of impact due to age and sex, a simulation was run for each of the defined 28 age group/sex combinations (fourteen 5-year age groups, ie, 20–24, 25–29 up to ≥85 years old, and defined for 2 sex groups, female/male). Results were subsequently combined based on group proportions for the defined country/group of countries.
In the base case analysis, we adopted a societal perspective, and MT was judged to be cost-effective if the incremental cost-effectiveness ratio was below the country-specific gross domestic product (GDP) per capita.33 As sensitivity analyses, we adopted a health care perspective and a cost-effectiveness threshold of $22 594 (£20 000) per QALY gained.34 The incremental cost-effectiveness ratio was estimated by dividing the difference in mean costs by the difference in mean QALYs for MT compared with standard care. Incremental cost-effectiveness ratios were thus calculated under a health and social care and a societal perspective (ie, including informal care and productivity losses). Estimates for the whole European Union (ie, the 27 countries) and for the whole of Europe (ie, all the 32 countries) were calculated as weighted averages (ie, taking into account population size and age/sex distribution).
Internal validity of the model was checked using sensitivity analysis (extreme and zero values) and in terms of its mathematical logic by checking whether a change in parameter values would have predictable effects on results. Probabilistic sensitivity analysis was conducted to characterize the uncertainty surrounding model outputs and the decision.35 A thousand iterations were simulated to represent the full distribution of uncertain parameters and to assess the likelihood of the intervention to be the most cost-effective option.
Finally, we conducted a series of 1-way sensitivity analyses to examine the robustness of the results to different assumptions concerning key model parameters: (1) the effectiveness of MT was varied to match the lower and upper uncertainty interval (UI) of the meta-analysis, as well as using the effectiveness from the individual trials; (2) we assumed that all those with an mRS score of 3 received informal care (100%) or did not receive it at all (0%); and (3) for survival poststroke, health utilities, and costs (treatment, health care, social, and informal care) we varied the base case model parameters by ± 20%.
Results
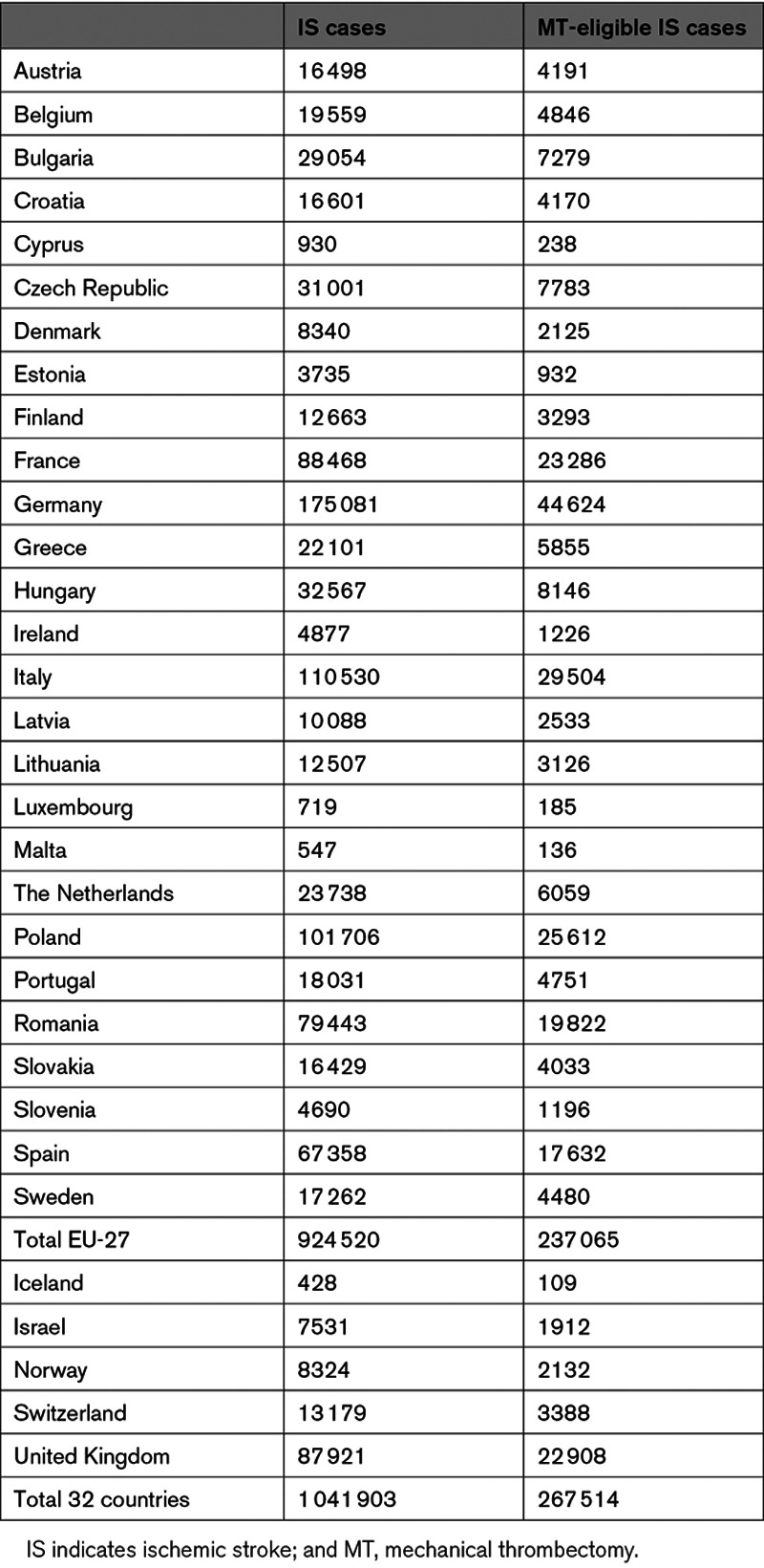
In 2017, just over 1 million people aged 20 years and over suffered an ischemic stroke across Europe, with Germany, Italy, and Poland having the highest number of cases (Table 1). Of these, 267 514 (27%) were estimated to be eligible for MT treatment in the 32 countries under study.
Table 1.
IS Cases Among Individuals Aged 20 Years and Over in 2017
Quality-Adjusted Life Years
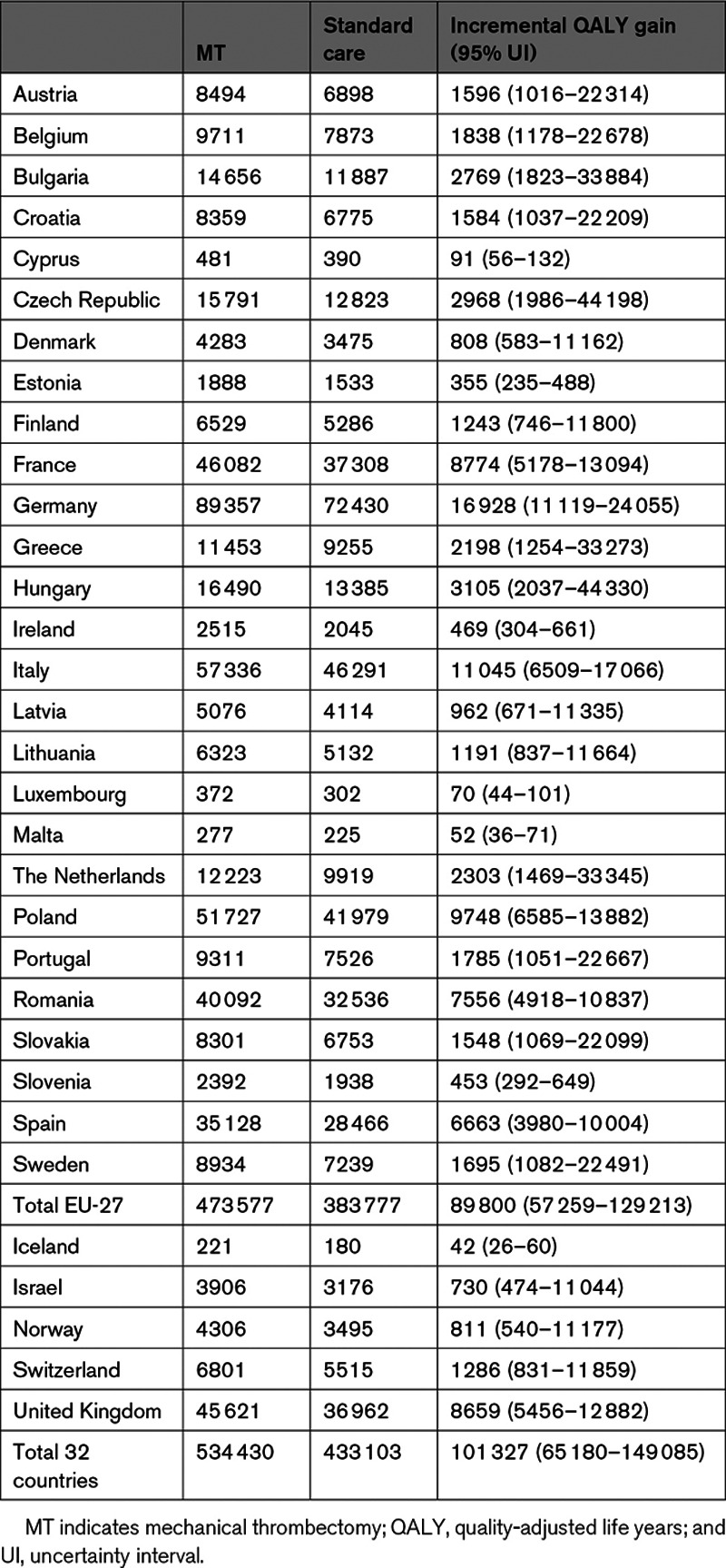
MT generated significantly higher number of QALYs, when compared with standard care, in all 32 European countries (Table 2). Overall, for the 32 European countries under study, MT generated additional QALYs of 101 327 (95% UI, 65 180–149 085).
Table 2.
QALY Gained for Each Intervention
Costs
Over 5 years, treating all eligible patients with MT was estimated to cost society $13.8 billion (95% UI, 10.3–15.4), compared with $15.5 billion (95% UI, 10.7–17.9) under standard care. This corresponded to potential cost savings of average $1.7 (€1.5 billion, 95% UI, −1.2 to 3.6) to society. Health and social care costs accounted for most total costs, although the proportion varied across countries and interventions, ranging from 58% ($26 out of $45 million) in Israel under standard care to 96% ($264 out of $277 million) in Finland under MT.
Germany was estimated to face the highest societal costs of all the countries under study, with average $4 billion under MT, compared with around $4.7 billion under standard care, but also the highest cost savings at $665 million (95% UI, −118 to 1175). In 20 more countries, MT was associated with societal cost savings over a 5-year time horizon when compared with standard care (Figure 2). On a per-treated patient basis, MT generated the highest cost savings in Switzerland ($25 504) and Switzerland ($23 620) when compared with standard care. However, none of the cost savings were found to be statistically significant (Appendix VI in the Data Supplement).
Figure 2.
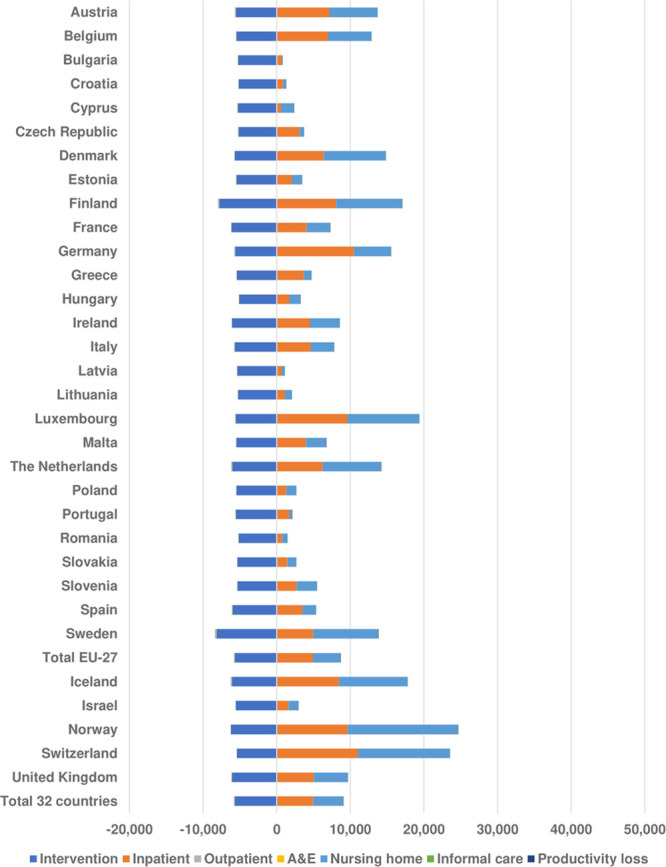
Breakdown of average $ savings per patient treated. A&E indicates accident and emergency.
Considering a health and social care perspective, for Greece, Czech Republic, Spain, and Israel, the exclusion of informal care costs and productivity losses shifted the results direction, from generating cost savings to additional costs. For 15 countries, including the 4 countries just mentioned above, MT was found to generate additional costs. However, for Greece (95% UI, −$18 million to $38 million) and Spain (95% UI, −$51 million to $115 million), the incremental estimates crossed zero.
Cost-Effectiveness of MT Compared With Standard Care
In the base case, that is adopting a societal perspective and using the country-specific GDP threshold, MT was found to be cost-effective in all but one European country (Bulgaria, Table 3). In 21 countries (62%), MT was found to be dominant over standard care (ie, it generated cost savings and was more effective). Table 3 reports the probability of MT being the cost-effective treatment across all European countries. At a $35 689 per QALY gained threshold (GDP per capita), MT had a probability of 100% of being cost-effective, compared with standard care (Figure 3). We also found MT to be cost-effective (>74% probability) in all countries, except Bulgaria (2.1% probability with at GDP per capita of $8247).
Table 3.
Probability of MT of Being Cost-Effective Relative to Standard Care
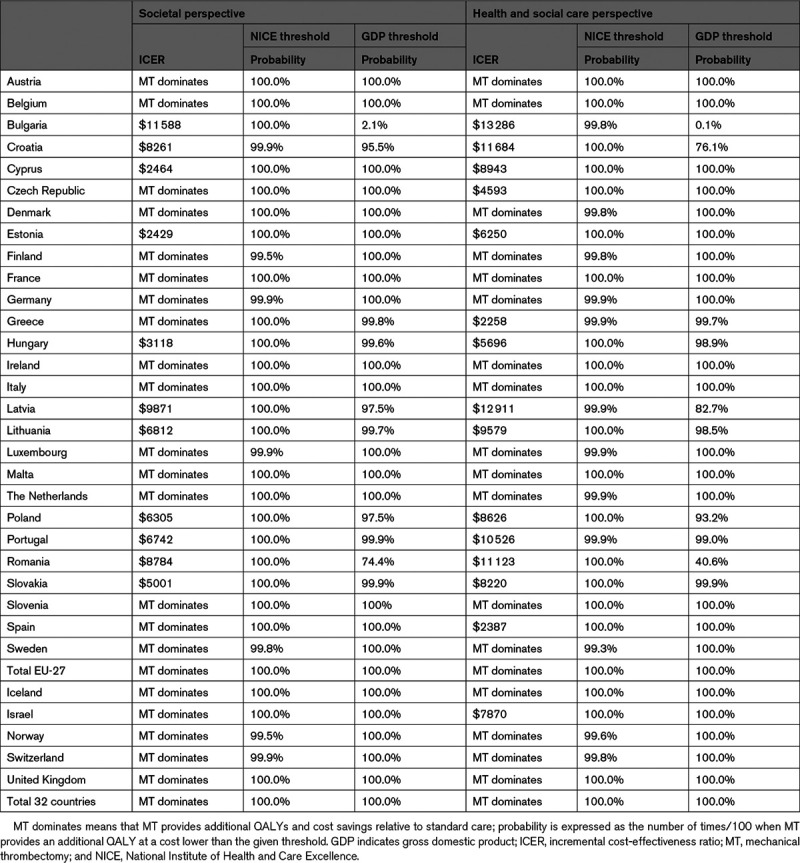
Figure 3.
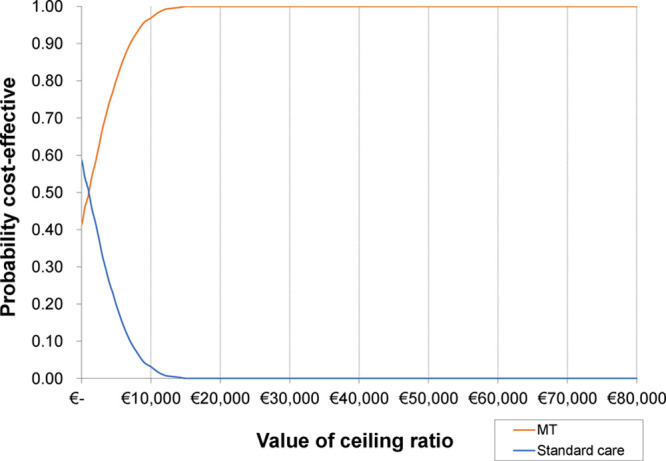
Cost-effectiveness acceptability curve for Europe. MT indicates mechanical thrombectomy.
Adopting a health and social care perspective, MT was found highly likely to be cost-effective in all but 2 European countries (Bulgaria: 0.1% and Romania: 40.6%, GDP per capita=$10 845). Instead, using the National Institute for Health and Care Excellence cost-effectiveness threshold (€22 727, that is, $25 675) per QALY gained, MT was found to be cost-effective for all European countries in both the base case analysis (societal perspective) and adopting a narrower perspective (health and social care). Further sensitivity analyses showed the cost-effectiveness results to be robust to variations to key parameters and assumptions (Appendix VII in the Data Supplement).
Discussion
MT is highly effective at preventing disability and death after nonminor ischemic stroke.21 Cost-effectiveness analyses undertaken in Western countries have shown that it can provide very good value for money.15–20 However, to the best of our knowledge, no study has evaluated the cost-effectiveness of MT across the whole of Europe. In addition, it was unknown whether MT, a relatively costly intervention, would provide good value for money in Eastern European countries characterized by lower levels of health care funding. This study has found that a routine provision of MT across Europe is highly likely to be cost-effective.
For wealthier Western European countries, the findings of our study are in line with those of published cost-effectiveness studies, which also evaluated the cost-effectiveness of MT over 5 years and found that MT generated cost savings and additional health benefits when compared with standard care in the United Kingdom and Italy.18,20 For France, our results also indicate similar conclusions to a previous cost-effectiveness analysis.17 While MT would generate additional health and social care costs compared with standard care, the total costs to society would be negative. We also show that MT is the optimal intervention not only in wealthier Western European countries but also in less affluent European countries. The only outlier appears to be Bulgaria for which, due to the relatively low per-capita GDP, MT might not be a cost-effective strategy.
Given the number of parameters and countries included in this study, our modeling approach used data from a wide array of sources. Clinical effectiveness data used in this analysis were derived from the results of a meta-analysis of randomized controlled trials.21 This strengthens our analysis and findings, as they are informed by high-quality evidence that is likely to be generalizable to the eligible ischemic stroke population. In addition, unlike previous studies which used hypothetical cohorts, our simulations were based on country-specific demographic data,2 making the results more relevant to inform population-level decisions.
We also explored the uncertainty surrounding the average results using probabilistic sensitivity analyses36 and using different willingness-to-pay for an extra QALY thresholds to assess cost-effectiveness. Two thresholds (country-specific GDP and National Institute for Health and Care Excellence) and 2 perspectives on costs were explored to evaluate whether MT was cost-effective. This represents a more robust approach than simply relying on deterministic results and only one cost-effectiveness criterion. Furthermore, unlike previous studies, we took a societal perspective on costs, broadening the evaluative space which is commonly restricted to a health and social care viewpoint.37,38
Estimates of long-term health and social care resource use and quality of life were obtained from the OXVASC. This is a large population-based cohort study, including all ischemic stroke, regardless of age or presentation to medical attention.39 We used published regression analyses evaluating the impact of 3-month mRS score on subsequent health and social care costs, mortality, and quality of life, controlling for both age and sex. Given that different European countries have differing patterns of resource use and lengths of stay, United Kingdom estimates from OXVASC were adjusted using country-specific health and social care resource use levels. In addition, resource use was valued using country-specific unit costs.
However, our results need to be interpreted in light of some limitations. Although a 5-year time horizon might capture most of the interventions’ impact on costs and quality of life, this time frame may not be long enough to capture all the relevant differences between the 2 intervention options under study. Quality of life data collected from UK patients and utility values calculated using UK tariffs might not be fully generalizable to other countries. Published evidence has suggested that populations in different countries may have differing valuations for any given health state.40
Furthermore, our analysis was based on evidence from a limited number of good quality studies that were used to extrapolate country-specific findings to many other countries. While country-level heterogeneity was captured, where possible, using country-specific data and weights, these may still not reflect fully the real differences in stroke populations across Europe. In addition, due to lack of relevant data, we made many pragmatic assumptions, such as one on the proportion of patients receiving informal care following the stroke, where half of those with an mRS score of 3 would receive it. We also assumed that capital costs required to provide the intervention (ie, device, drugs, and consumables) as well as staff time and type would not vary across countries. There is evidence that clinical staff, health care provision and system configuration may vary considerably across Europe.41 However, while these assumptions may influence the estimation of the total costs and QALYs associated with implementing the intervention, their impact when comparing MT to standard care is likely to be limited. In fact, our several sensitivity analyses showed the cost-effectiveness findings to be robust to varying assumptions, parameter values, and data sources. Future availability of data will allow research to address these limitations.
Conclusions
Routine implementation of MT for the acute treatment of nonminor ischemic stroke is highly likely to provide good value for money across Europe. In a context of increasing burden of stroke and pressure on the health and social care resources, it is recommended that European countries consider the full implementation of MT in all eligible cases.
Acknowledgments
R. Luengo-Fernandez and Dr Leal were responsible for securing funding and the overall concept of the study. Drs Candio, Luengo-Fernandez, and Leal were responsible for designing the study and development of the economic model. Dr Violato produced some empirical evidence underlying the economic model. All authors contributed to the writing, read, and approved the final version of the article.
Sources of Funding
The study was funded by an unrestricted grant from the Stroke Alliance for Europe.
Disclosures
None.
Supplemental Materials
Appendix I–VII
Supplementary Material
Nonstandard Abbreviations and Acronyms
- GDP
- gross domestic product
- IVT
- intravenous thrombolysis
- mRS
- modified Rankin Scale
- MT
- mechanical thrombectomy
- OXVASC
- Oxford Vascular study
- QALY
- quality-adjusted life year
- UI
- uncertainty interval
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.031027.
For Sources of Funding and Disclosures, see page 672.
Contributor Information
Paolo Candio, Email: paolo.candio@ndph.ox.ac.uk.
Mara Violato, Email: mara.violato@dph.ox.ac.uk.
Jose Leal, Email: jose.leal@dph.ox.ac.uk.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X [DOI] [PubMed] [Google Scholar]
- 2.Luengo-Fernandez R, Violato M, Candio P, Leal J. Economic burden of stroke across Europe: a population-based cost analysis. Eur Stroke J. 2020;5:17–25. doi: 10.1177/2396987319883160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM; Oxford Vascular Study. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81:1588–1595. doi: 10.1212/WNL.0b013e3182a9f45f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, Cuthbertson FC, Rothwell PM; Oxford Vascular Study. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44:2854–2861. doi: 10.1161/STROKEAHA.113.001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B; CDBE2010 study group; European Brain Council. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x [DOI] [PubMed] [Google Scholar]
- 6.Stevens E, Emmett E, Wang Y, McKevitt C, Wolfe C. The Burden of Stroke in Europe. 2017 https://kclpure.kcl.ac.uk/portal/files/103120905/TheBurdenOfStrokeInEuropeReport.pdf.
- 7.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by stroke alliance for europe (SAFE). Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute of Health and Care Excellence. Clinical Commissioning Policy: Mechanical Thrombectomy for Acute Ischaemic Stroke (all ages). 2018. London: National Institute of Health and Care Excellence [Google Scholar]
- 9.Hilditch CA, Nicholson P, Murad MH, Rabinstein A, Schaafsma J, Pikula A, Krings T, Pereira VM, Agid R, Brinjikji W. Endovascular management of acute stroke in the elderly: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39:887–891. doi: 10.3174/ajnr.A5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkhemer OA, Fransen PS, Beumer D, van de Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJH, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 11.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F; THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Menon BK, van Zwam BH, Dieppel DVG, Mitchell PJ, Demchuk AM, Davalos A, Maioie CBLM, van der Lugt A, de Miquel MA, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 13.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 14.Achit H, Soudant M, Hosseini K, Bannay A, Epstein J, Bracard S, Guillemin F; THRACE Investigators. Cost-effectiveness of thrombectomy in patients with acute ischemic stroke: the THRACE randomized controlled trial. Stroke. 2017;48:2843–2847. doi: 10.1161/STROKEAHA.117.017856 [DOI] [PubMed] [Google Scholar]
- 15.Aronsson M, Persson J, Blomstrand C, Wester P, Levin LÅ. Cost-effectiveness of endovascular thrombectomy in patients with acute ischemic stroke. Neurology. 2016;86:1053–1059. doi: 10.1212/WNL.0000000000002439 [DOI] [PubMed] [Google Scholar]
- 16.Arora N, Makino K, Tilden D, Lobotesis K, Mitchell P, Gillespie J. Cost-effectiveness of mechanical thrombectomy for acute ischemic stroke: an Australian payer perspective. J Med Econ. 2018;21:799–809. doi: 10.1080/13696998.2018.1474746 [DOI] [PubMed] [Google Scholar]
- 17.Kaboré N, Marnat G, Rouanet F, Barreau X, Verpillot E, Menegon P, Maachi I, Berge J, Sibon I, Bénard A. Cost-effectiveness analysis of mechanical thrombectomy plus tissue-type plasminogen activator compared with tissue-type plasminogen activator alone for acute ischemic stroke in France. Rev Neurol (Paris). 2019;175:252–260. doi: 10.1016/j.neurol.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 18.Lobotesis K, Veltkamp R, Carpenter IH, Claxton LM, Saver JL, Hodgson R. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischemic stroke in the UK. J Med Econ. 2016;19:785–794. doi: 10.1080/13696998.2016.1174868 [DOI] [PubMed] [Google Scholar]
- 19.Pan Y, Cai X, Huo X, Zhao X, Liu L, Wang Y, Miao Z, Wang Y. Cost-effectiveness of mechanical thrombectomy within 6 hours of acute ischaemic stroke in China. BMJ Open. 2018;8:e018951.doi: 10.1136/bmjopen-2017-018951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggeri M, Basile M, Zini A, Mangiafico S, Agostoni EC, Lobotesis K, Saver J, Coretti S, Drago C, Cicchetti A. Cost-effectiveness analysis of mechanical thrombectomy with stent retriever in the treatment of acute ischemic stroke in Italy. J Med Econ. 2018;21:902–911. doi: 10.1080/13696998.2018.1484748 [DOI] [PubMed] [Google Scholar]
- 21.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 22.European Central Bank. Euro foreign exchange reference rates 2018. https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-gbp.en.html
- 23.Institute of Health Metrics. Global Burden of Disease 2017. http://www.healthdata.org/gbd
- 24.National Institute of Health and Care Excellence. Stroke (update), Evidence review Thrombectomy 2018.
- 25.Xu XM, Vestesson E, Paley L, Desikan A, Wonderling D, Hoffman A, Wolfe CD, Rudd AG, Bray BD. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J. 2018;3:82–91. doi: 10.1177/2396987317746516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 27.Li L, Geraghty OC, Mehta Z, Rothwell PM; Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390:490–499. doi: 10.1016/S0140-6736(17)30770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making. 2010;30:341–354. doi: 10.1177/0272989X09349961 [DOI] [PubMed] [Google Scholar]
- 29.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 30.Börsch-Supan A, Brandt M, Hunkler C, Kneip T, Korbmacher J, Malter F, Schaan B, Stuck S, Zuber S; SHARE Central Coordination Team. Data resource profile: the survey of health, ageing and retirement in Europe (SHARE). Int J Epidemiol. 2013;42:992–1001. doi: 10.1093/ije/dyt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Central Bureau of Statistics. Employed persons by industry, age, population group and sex. http://www.cbs.gov.il/publications17/lfs15_1684/pdf/t02_03.pdf
- 32.EuroStat. Employment by sex, age and citizenship (1 000) [lfsa_egan]. https://ec.europa.eu/eurostat/web/lfs/data/database
- 33.Eurostat. GDP and main components (output, expenditure and income) (nama_10_gdp). https://ec.europa.eu/eurostat/web/national-accounts/data/database
- 34.National Institute for Health and Care Excellence. Social Value Judgements: principles for the Development of NICE Guidance. 2013. [PubMed] [Google Scholar]
- 35.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. 2006. Oxford: OUP [Google Scholar]
- 36.Baio G, Dawid AP. Probabilistic sensitivity analysis in health economics. Stat Methods Med Res. 2015;24:615–634. doi: 10.1177/0962280211419832 [DOI] [PubMed] [Google Scholar]
- 37.Neumann PJ. Costing and perspective in published cost-effectiveness analysis. Med Care. 2009;47(7 suppl 1):S28–S32. doi: 10.1097/MLR.0b013e31819bc09d [DOI] [PubMed] [Google Scholar]
- 38.Weatherly H, Drummond M, Claxton K, Cookson R, Ferguson B, Godfrey C, Rice N, Sculpher M, Sowden A. Methods for assessing the cost-effectiveness of public health interventions: key challenges and recommendations. Health Policy. 2009;93:85–92. doi: 10.1016/j.healthpol.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 39.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, et al. ; Oxford Vascular Study. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1 [DOI] [PubMed] [Google Scholar]
- 40.Heijink R, Reitmeir P, Leidl R. International comparison of experience-based health state values at the population level. Health Qual Life Outcomes. 2017;15:138.doi: 10.1186/s12955-017-0694-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papanicolas I, Smith PC. Health System Performance Comparison. An Agenda for Policy, Information and Research (2013). 2013. Open University Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


