Abstract
Sars-Cov-2 or Novel coronavirus infection (COVID-19) has become a global challenge, affecting elderly population at large, causing a burden on hospitals. It has been affecting the world from a health and economic perspective after its emergence since October 2019 at Wuhan province of China. Later on it became a pandemic, with aged people most affected. Surprisingly, the infants and children were not severely infected and mortality among them was reported infrequently. If they died it was due to some comorbidity or congenital heart problems. Why the rate of infection varies in different age groups around the world and what is the protective mechanism in children remains a mystery.
Based on our neuropathological experience at the “Lino Rossi Research Center for the study and prevention of the unexpected perinatal death and Sudden Infant Death Syndrome (SIDS)” of the University of Milan, Italy, we hypothesize that the decreased severity of the disease in infants compared to the elderly may be due to alteration at neurotransmitter levels especially of the Substance P (SP) and of the spinal trigeminal nucleus in the brainstem that is responsible for its secretion.
This neurotransmitter may be directly related to the respiratory illness as is in COVID-19 infection. It is responsible for the increased inflammation and the characteristic symptoms associated with this disease. It is the main switch that must be urgently turned off using the NK-1R antagonist which is the receptor of SP and responsible for its functionality, especially in the elderly.
Keywords: COVID-19, Substance P, Neurokinin-1 receptor antagonist, Respiratory illness, Infectious disease, Coronavirus treatment
Highlights
-
•
COVID-19 is the biggest public health crisis of recent times.
-
•
Children are not significantly affected in the same way as adults by COVID-19.
-
•
The infant's immune system might protect from the ‘cytokine storm’ of adults.
-
•
Increased expression of substance P in brains is here proposed as a possible cause.
-
•
Aprepitant could be a promising therapeutic approach to COVID-19 infection in adults.
Coronavirus Disease-2019 (COVID-19) is an acute respiratory infection and emerging disease. As per the World Health Organization's (WHO) update dated 12th December 2020, there have been 69,521,294 confirmed COVID-19 infected cases and 1,582,674 deaths worldwide [1]. Many authors to date report that the number of children affected by the infection is much lower than that of adults and, if infected, the disease is not severe [[2], [3], [4], [5]]. In particular, in a study performed in China on 728 (34.1%) laboratory-confirmed COVID-19 infants and 1407 (65.9%) suspected cases, with a median age of 7 years, even if children of all ages appeared susceptible to COVID-19, the clinical manifestations were generally less severe than those of the adult patients [2]. Likewise, in another study from USA on a total of 32,437 patients, 168 (0.5%) belonging to age group 04 years and 425 (1.3%) to 5–17 years of age, mortality rate was observed to 0.1% in children (3 pediatric deaths, all had associated to underlying conditions) while the overall rate was 2.27% [3]. All these findings suggest that disease severity is not the same in different age groups and that children are less affected and have milder illness than adults. The pathophysiology in this regard is not known so far, even if several hypotheses about this different manifestation of COVID-19 between children and adults have been proposed.
First of all, while in adults COVID-19 leads to an overproduction of immune cells and their activating compounds, the cytokines, that are the endogenous mediators of the immune system directly involved in the activation of cells at the inflammatory site, children generally produce low levels of inflammatory mediators. Therefore, they are protected against the ‘cytokine storm’ (a cascade process whereby the immune system overreacts to an infection) that is responsible for uncontrolled inflammatory responses in adults [6]. Once the protection from microorganisms that the mother exerts with her antibodies in the early stages of the newborn's life is depleted, the children are defenseless against possible new infections. It follows that the frequent diseases that occur in the first few years of life serve to build the memory T and B cell pool to prevent reinfection or the development of diseases. Thus, the pediatric immune system is prepared and ready to react to new pathogens, a function that could be reduced in adults and elderly [5]. In addition, considering that the lower airway is the primary target of the COVID-19 infection [7], children could be less infected since they have healthier respiratory tracts not having been exposed to as much cigarette smoke and air pollution as adults. A further explanation of the different behavior of COVID-19 in pediatric cases may lie in the different immune process mediated by the thymus. The thymus and T lymphocytes, the cells matured in the thymus with precise roles in immunomodulation, are highly active only during the early periods of life and lose their function with age. This implies a difficulty in adults to control the immune response to COVID-19 with production of inflammatory storms [8].
Here, we propose an innovative neuropathological explanation of the protective mechanism in children consisting in the involvement of neurotransmitters and specific brainstem nuclei. We suggest that the neurotransmitter Substance P (SP) and the spinal trigeminal nucleus in the brainstem may be explored further in this context. The SP, encoded by the tachykinin 1 (TAC1) gene, is a member of the tachykinin family, that is a group of peptides acting as neurotransmitters or modulators in the human central and peripheral nervous system. The biological functions of the SP are performed through binding to the receptor neurochinin-1 (NK1R), resulting in a SP/NK1R complex [9,10].
Basically, the trigeminal ganglion controls the cardiorespiratory activity by releasing its neurotransmitters such as SP, CGRP, serotonin etc. [9,10,22]. SP in particular regulates the respiratory rhythm at central and peripheral level, also by modulating the functionality of the airways [11,12]. SP is in fact one of the most common neuropeptides in the airways. It's prevalently located in bronchopulmonary C fibers with the main purpose to protect the lungs against any noxious stimuli, as inhaled irritants or viral infections.
An increased release of SP from airway epithelium can lead to respiratory illness, as asthma, persistent cough, lung damage, inflammation, pneumonia and acute respiratory syndrome [13,14]. Then it is evident that the symptoms of COVID-19 infection are similar to those conditions that are caused by SP imbalance.
The SP/NK-1R complex is even widely distributed in the cardiovascular system, exerting direct effects on cardiomyocytes, cardiac fibroblasts, and cardiac inflammatory cells [15,16]. In humans SP is expressed in intrinsic cardiac ganglia, blood vessels and musculature [17]. Experimental studies have shown that viral infection of the heart results in dramatic increases in myocardial SP levels leading to hypertrophy, necrosis, cytokine mediated apoptosis and inflammation of cardiac cells [18]. COVID-19 infected patients also undergo cardiac system malfunctioning or heart failure in severe cases. It is important to point out that however, these manifestations are prevented in SP-deficient mice [18,19].
In addition, SP released from trigeminal ganglion provides innervation through trigeminal nerve to ophthalmic, maxillary and mandibular regions. An altered SP secretion is responsible for orofacial symptoms such as headache, sore throat, loss of sense of smell and taste, ocular pain, flu and neurological and psychiatric alterations, all symptoms that are common with those related to COVID-19 infection [20].
Also non-neuronal cells, such as immune cells (in particular granulocytes neutrophils, lymphocytes, dendritic cells), besides to produce inflammatory mediators and cytokines such as TNF-alpha, IL-6, IL-1, BDNF, IL-8, PGE2, histamines, serotonin, can release SP as a result of inflammation [21].
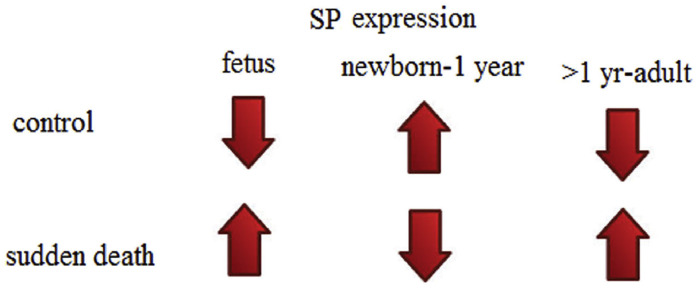
In our previous studies related to sudden death, we have reported that SP expression, if raised in trigeminal ganglion before birth may lead to fetal death, if decreased after birth may cause sudden infant death [22,23], while in adults, higher SP expression may cause death [24] (Fig. 1).
Fig. 1.
Substance P (SP) expression in human brainstems at different ages and its relation to sudden deaths.
Keeping this in view, if SP is enhanced as in case of viral infection, while newborns and infants, in which the level of neurotransmitter is naturally high, are protected and they do not have serious concerns, this condition on the contrary may be fatal for healthy adults, which have normally low SP levels, even more risk in older age groups which may be the case in COVID-19 patients.
Therefore, in our opinion, an increase of SP can initiate through its NK-1R the nociception in response to viral infection and trigger the immune response leading to cytokine storming and organ failure in critical cases. Understanding the pathophysiological mechanisms underlying the disease severity in various age groups may be important in identifying the high risk individuals and planning an effective therapeutic strategy for them.
Based on our considerations, we intend here to propose a possible strategy to counteract the serious outcome of COVID-19 in adulthood.
It is known that the SP/NK1R complex is involved in a variety of human pathological processes, including neuroinflammation due to various causes (as bacterial, viral, parasitic infections and neurodegenerative diseases) [25]. In particular we know that elevated levels of this complex play a major pathogenetic role in the interaction between the nervous and immune systems and in the activation of genes controlling inflammatory mechanisms [26].
For about twenty years the potential clinical utility of Neurokinin receptor antagonists have been highlighted [27]. Between the different compounds that have been developed by pharmaceutical companies to block specifically the binding of SP to its receptor, only the “Aprepitant” (Emend) has been approved for the clinical use in humans [28]. This drug is well tolerated and normally used as antiemetics to combat chemotherapy-induced nausea in cancer patients [29]. Furthermore, investigators have focused on the use of Aprepitant as anti-inflammatory drug with a specific antiviral effect. In vitro and in vivo studies have shown in particular that the Aprepitant can represent a potential therapeutic agent with effective immunomodulatory and anti-inflammatory role in HIV infected patients, which are characterized by significantly higher plasma SP levels compared with uninfected controls [30,31].
We retain that the Aprepitant could be an emerging and promising therapeutic approach for treatment also of COVID-19 infection particularly in adulthood and therefore could be used in clinical practice to prevent the fatal consequences. Further studies are however required to support the validity and efficiency of this proposed therapeutic intervention.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Authors declare no conflict of interest.
References
- 1.World Health Organization Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ available at.
- 2.Dong Y.M.X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Coronavirus Disease 2019 (COVID-19) Center for Disease Control and Prevention (CDC) 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/04102020/labs-regions.html Available at: (Accessed 14 April 2020)
- 4.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., Waddington C., Thomas J., Russell S., van der Klis F., Koirala A., Ladhani S., Panovska-Griffiths J., Davies N.G., Booy R., Eggo R.M. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2020;25 doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P.-I., Hu Y.-L., Chen P.-Y., Huang Y.-C., Hsueh P.-R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;10(11):1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subbarao K., Mahanty S. Respiratory virus infections: understanding COVID-19. Immunity. 2020;52(6):905–909. doi: 10.1016/j.immuni.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Güneş H., Dinçer S., Acıpayam C., Yurttutan S., Özkars M.Y. What chances do children have against COVID-19? Is the answer hidden within the thymus? Eur. J. Pediatr. 2020;13:1–4. doi: 10.1007/s00431-020-03841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Recio S., Gascon P. Biological and pharmacological aspects of the NK1-receptor. Biomed. Res. Int. 2015;2015:495704. doi: 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantyh P.W. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;11:6–10. [PubMed] [Google Scholar]
- 11.Ptak K., Hunt S.P., Monteau R. Substance P and central respiratory activity: a comparative in vitro study in NK1 receptor knockout and wild-type mice. Pflugers Arch. 2000;440(3):446–451. doi: 10.1007/s004240000300. [DOI] [PubMed] [Google Scholar]
- 12.Szereda-Przestaszewska M., Kaczynska K. Serotonin and substance P: synergy or competition in the control of breathing. Auton. Neurosci. 2020;225 doi: 10.1016/j.autneu.2020.102658. [DOI] [PubMed] [Google Scholar]
- 13.Chu H.W., Kraft M., Krause J.E., Rex M.D., Martin R.J. Substance P and its receptor neurokinin 1 expression in asthmatic airways. J. Allergy Clin. Immunol. 2000;106(4):713–722. doi: 10.1067/mai.2000.109829. [DOI] [PubMed] [Google Scholar]
- 14.Bai T.R., Zhou D., Weir T., Walker B., Hegele R., Hayashi S., McKay K. Substance P (NK1)- and neurokinin A (NK2)-receptor gene expression in inflammatory airway diseases. Am J Physiol. 1995;269:L309–L317. doi: 10.1152/ajplung.1995.269.3.L309. [DOI] [PubMed] [Google Scholar]
- 15.Mistrova E., Kruzliak P., Chottova Dvorakova M. Role of substance P in the cardiovascular system. Neuropeptides. 2016;58:41–51. doi: 10.1016/j.npep.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Dehlin H.M., Levick S.P. Substance P in heart failure: the good and the bad. Int J Cardiol. 2014;170(3):270–277. doi: 10.1016/j.ijcard.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weihe E., Reinecke M., Opherk D., Forssmann W.G. Peptidergic innervation (substance P) in the human heart. J Mol Cell Cardiol. 1981;13:331–333. doi: 10.1016/0022-2828(81)90321-7. [DOI] [PubMed] [Google Scholar]
- 18.Robinson P., Garza A., Moore J. Substance P is required for the pathogenesis of EMCV infection in mice. Int J Clin Exp Med. 2009;2:76–86. [PMC free article] [PubMed] [Google Scholar]
- 19.Melendez G.C., Li J., Law B.A., Janicki J.S., Supowit S.C., Levick S.P. Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells. Cardiovasc Res. 2011;92(3):420–429. doi: 10.1093/cvr/cvr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biadsee A., Biadsee A., Kassem F., Dagan O., Masarwa S., Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163:722–728. doi: 10.1177/0194599820934380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashaghi A., Marmalidou A., Tehrani M., Grace P.M., Pothoulakis C., Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73(22):4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavezzi A.M., Mehboob R., Matturri L. Developmental alterations of the spinal trigeminal nucleus disclosed by substance P immunohistochemistry in fetal and infant sudden unexplained deaths. Neuropathol. 2011;31(4):405–413. doi: 10.1111/j.1440-1789.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- 23.Mehboob R. Substance P/Neurokinin 1 and trigeminal system: a possible link to the pathogenesis in sudden perinatal deaths. Front Neurol. 2017;8:82. doi: 10.3389/fneur.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehboob R., Shazad S.A., Hashmi A.M., Ahmad F.J. Vertebrate specific oncogenic TAC1 has unconventional networking properties. Healthmed. 2014;8(7):843–849. [Google Scholar]
- 25.Martinez A.N., Philipp M.T. Substance P and Antagonists of the Neurokinin-1 Receptor in Neuroinflammation Associated with Infectious and Neurodegenerative Diseases of the Central Nervous System. J Neurol Neuromed. 2016;1(2):29–36. doi: 10.29245/2572.942x/2016/2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilisaar J., Kawabe K., Braitch M., Aram J., Furtun Y., Fahey A.J. Reciprocal regulation of substance P and IL-12/IL-23 and the associated cytokines, IFNγ/IL-17: a perspective on the relevance of this interaction to multiple sclerosis. J Neuroimmune Pharmacol. 2015;10:457–467. doi: 10.1007/s11481-015-9589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swain C.J. Neurokinin receptor antagonists. Prog Med Chem. 1998;35:57–81. doi: 10.1016/s0079-6468(08)70034-1. [DOI] [PubMed] [Google Scholar]
- 28.Quartara L., Altamura M. Tachykinin receptors antagonists: from research to clinic. Curr Drug Targets. 2006;7:975–992. doi: 10.2174/138945006778019381. [DOI] [PubMed] [Google Scholar]
- 29.Herrstedt J., Muss H.B., Warr D.G. Efficacy and tolerability of aprepitant for the prevention of chemotherapy induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer. 2005;104:1548–1555. doi: 10.1002/cncr.21343. [DOI] [PubMed] [Google Scholar]
- 30.Douglas S.D., Ho W.Z., Gettes D.R., Cnaan A., Zhao H., Leserman J., Petitto J.M., Golden R.N., Evans D.L. Elevated substance P levels in HIV-infected men. AIDS. 2001 Oct 19;15(15):2043–2045. doi: 10.1097/00002030-200110190-00019. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg Z.F., Fauci A.S. Immunopathogenesis of HIV infection. FASEB J. 1991;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]