Abstract
Background
South Korea's aggressive responses to the coronavirus disease 2019 (COVID-19) have greatly slowed the epidemic without regional lockdowns.
Methods
The Korean Centers for Disease Control and Prevention's daily briefings were thoroughly reviewed. Information about hospital countermeasures and government coordination was collected via telephone interviews with 4 infection control team leaders, 1 emergency department nurse, and 1 infectious disease physician in Korea.
Results
After the 2015 Middle East Respiratory Syndrome outbreak, the government and hospitals prepared for the inevitable outbreak of emerging infectious diseases by reforming the epidemic preparedness system. As a result, COVID-19 diagnostic test kits were quickly developed, enabling extensive early detection of potential cases. Other key steps were tracking cases, finding exposed individuals, coordinating case assignments with health care facilities, and selective clinic screenings for visitors’ entering hospitals with mandatory mask wearing. Consequently, after overcoming the initial peak of the outbreak, which was related to a religious group, Korea has been able to maintain daily new cases at around 100 and to less than 50 daily cases in the second week of April.
Conclusions
To counter the COVID-19 pandemic, which may persist, long-term, sustained response strategies must be prepared along with coordination between government and health systems.
Key Words: Outbreak, Preparedness, Countermeasures, Screening, Tracking
Background
While the clinical features of the coronavirus disease 2019 (COVID-19) and its virus are not fully known, an estimated 20%-60% of the global adult population could eventually be infected.1 While many countries are trying to flatten the rapidly expanding epidemic curve by adopting stay-at-home orders or regional lockdowns, South Korea mitigated the initial outbreak successfully without locking down any regions. To aid the successful fight against the COVID-19 pandemic, this paper describes Korea's epidemic preparedness, summarizes the COVID-19 outbreak and national responses from the Korean Centers for Disease Control and Prevention (KCDC) daily briefings, and provides examples of several hospitals’ countermeasure strategies collected via telephone interviews with 4 infection control team leaders, 1 emergency department nurse, and 1 infectious disease physician in Korea.
Background to Korea's epidemic preparedness
Lessons from the 2015 MERS outbreak
The outbreak of Middle East Respiratory Syndrome (MERS) in Korea, which lasted from May 20 to December 23, started from a single imported case and spread via human-to-human transmission across 17 health care settings, resulting in 186 confirmed cases and 36 deaths, the largest outbreak outside the Middle East.2 With the lessons learned from the MERS outbreak, the Korean government announced countermeasures for the prevention system's reform in September 2015 and prepared 48 tasks to prevent the influx of infectious disease, detect it early, prevent its spread, and improve the medical environment and response system.2 As part of this preparation, in March 2018 the Government-wide R&D Fund project for Infectious Diseases (GFID) for 2018-2022 launched with $32.5 million,3 and the Korean government designated 165 general hospitals and 20 tertiary hospitals as specialized regional infectious disease hospitals with isolation rooms and system setups (eg, negative pressure).4 As a partial amendment to the Act on Prevention and Management of Infectious Diseases, the communicable disease reporting system was updated on January 1 from categories based on disease characteristics to those based on infectious disease severity, spreading power, isolation requirements, and reporting timeline.5 The difficult times during the 2015 MERS were a blessing in disguise for the preparation of Korea's response to COVID-19.
Environmental and cultural background
Koreans have adapted to wearing masks in daily life, because air pollution due to micro-dust has worsened for several years. Most Koreans try to avoid unhealthy behaviors, and their compliance with COVID-19 codes of conduct is relatively high: a recent survey of 1,000 Koreans found compliance rates of 67.8% for hand hygiene, 63.2% for wearing a mask, 50.0% for postponing/cancelling social events, and 41.5% for avoiding crowded spaces (“Always” responses only; excluding “Often” and “Sometimes”).6
Rapid industrialization has shaped modern Korean culture so that speediness and working overtime are routinely expected. With high-speed Internet, widespread smartphones, and text messaging apps, Koreans gather new information quickly and share it with family and peers. In the spirit of cooperation, many health care personnel (HCP) voluntarily went to Daegu, the epicenter of the outbreak, to battle the crisis. The general public also helped fight COVID-19 by volunteering in many ways (eg, working at mask factories to increase production to counter public mask shortages).
COVID-19 outbreaks and national responses
Statistical overview
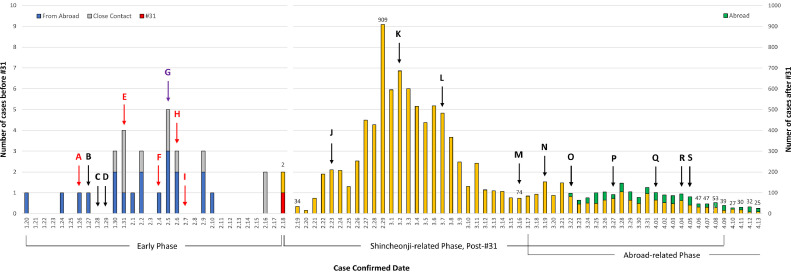
Since the first confirmed case of a Chinese tourist on January 20, as of April 13 a total of 10,537 confirmed cases (including 929 from abroad) were reported in South Korea with 217 deaths.7 Overall, the fatality rate is 2.06%: 0.09% for ages 30-39; 0.21% for 40-49; 0.72% for 50-59; 2.40% for 60-69; 9.17% for 70-79; and 21.64% for 80 years or older.7 In the outbreak's early phase until case number 30, most cases were from abroad (N = 19, 63.3%; N = 12, 40% from Wuhan) or close contacts (eg, family members).8 The epidemic curve rose rapidly with case number 31, who twice refused testing and caused dozens of cases by attending Sunday services of Shincheonji, a Korean religious movement from Daegu that is considered by many a cult and has missionary activities in Wuhan, the pandemic's origin (Fig 1 ). After around 3 weeks of the Shincheonji-related epidemic, linked sporadic cluster outbreaks were reported continuously in crowds, such as at churches and long-term-care facilities. As of March 24, confirmed cases among HCP in Daegu were 121 (ie, 14 physicians, 56 nurses, and 51 nurse aids) including 34 HCP (28%) who were Shincheonji members.9 As of April 13, 81.5% of all cases were from cluster outbreaks and 5,210 cases (49.4%) were linked with Shincheonji (ages 20-29 most affected [27.32%]); the regional distribution of confirmed cases were in Daegu (6,819; 64.71%), Gyeongsangbuk-do (the province near Daegu, 1,337; 12.69%), Gyeonggi-do (near Seoul, 631; 5.99%), and Seoul (610; 5.79%).7 As Europe and the USA entered the pandemic, cases from abroad increased in Korea since mid-March. Of 876 confirmed cases from March 30 to April 13, 56.7% were related to people coming from abroad, and 27.5% were linked with hospitals or long-term care hospitals.7
Fig 1.
Coronavirus disease 19 outbreak curve and timeline of implementation of government interventions in South Korea. Note: This histogram is based on daily briefing reports from Korea Centers for Disease Control and Prevention. The report format of case numbers and related information has been changed several times depending on the situation. For example, specific information from travelers abroad became available from March 22 and is partially reflected in the histogram. (A) Expansion of suspected case definition from pneumonia or pneumonia-suspected symptoms after Wuhan visit within 14 days, to fever or respiratory symptoms after Hubei Province visit within 14 days. Expansion of patient under investigation definition from fever or respiratory symptoms after Wuhan visit within 14 days, to pneumonia after China visit within 14 days; (B) Changed the national infectious disease crisis level from “Alert” to “Warning,” started operating the COVID 19 Emergency Headquarters; (C) Started operating 288 selective care centers and screening all number of Wuhan tourists; (D) Expanded Korean Centers for Disease Control and Prevention call center (Tel: 1339) operation; (E) Started use of real time RT-PCR test kit at the 18 locations of Research Institutes of Public Health and Environment nationwide; (F) Changed contact definition from close contact and general contact to contact within 2 meters and 1:1 assignment of a public officer to the home self-contained people; (G) Isolated the COVID-19 Korean virus (BetaCoV/Korea/CDCD03/2020) and the Korean Ministry of Food and Drug Safety recommended use of KF94 and KF99 masks for healthcare personnel; (H) Expansion of case definition to fever of respiratory symptom within 14 days or physician's opinions; (I) Authorized emergency use of COVID-19 real-time RT-PCR test kits and expanded test centers to 46 private health care facilities; (J) Raised the national infectious disease crisis level from “Warning” to “Serious,” and declared the infectious disease special management zone with Daegu and Gyeongsangbuk-do; (K) Switched from the early detection and containment strategy to one of minimizing damage strategy, and provided updated response guidelines (7th edition); (L) Started mandatory reporting of symptoms at each facility with a designated staff; (M) Started an exit quarantine for travelers to the US and expanded a special entry procedure to travelers from Europe including mobile application report of self-monitoring of COVID-19 related symptoms; (N) Expanded the Special Entry Procedure to travelers from all countries; O) Started COVID-19 tests for all travelers from Europe and 15 days of “enhanced social distancing” until April 5; (P) Started COVID-19 tests for symptomatic travelers from USA; (Q) All travelers from abroad must self-isolate at home for 14 days and report daily symptoms using a self-isolation diagnostic application for smartphones; (R) Expanded the epidemiologic investigation scope of contact individuals from 1 day prior starting symptoms to 2 days prior; (S) Extended “enhanced social distancing” for 2 weeks more until April 19, and started legal enforcement in which violations of self-containment may result in imprisonment of not more than 1 year or a find not exceeding 10 million Korean won (ie, $8,250).
Updated case definitions
To detect potential cases earlier, case definitions have been updated 7 times with response manuals. According to the latest 7-4 version released on April 2: Cases are confirmed when testing positive, regardless of clinical symptoms; suspected cases have a fever (37.5°C or above) or respiratory symptoms (eg, cough, breathing difficulty) within 14 days of contact with a confirmed case; symptomatic patients under investigation are (1) suspected cases with unknown origin pneumonia according to physician's diagnosis, (2) cases with symptoms within 14 days of traveling abroad, and (3) cases with symptoms within 14 days epidemiologically linked with COVID-19’s collective outbreak.10
Expanded diagnostic tests
The early detection of potential cases was crucial in controlling COVID-19 outbreaks. In the very early phase, the 2-step pan-coronavirus test (screening all coronaviruses, then the novel coronavirus [SARS-CoV-2]), based on the pilot modeling of testing kits in December 2019 as a part of an R&D project started in 2017, was conducted at the KCDC headquarters. Results were available within 24-48 hours. With the expedited approval system for the urgent test kit use, a new, single-step, real-time RT-PCR test kit taking 6 hours was approved and available on January 31 at 18 Research Institute of Public Health and Environment locations.11 One week later, with expanded case definitions, this diagnostic testing became available at 46 health care facilities.
Around February 23, drive-through testing centers opened; potential cases can drive their automobiles for testing processes (eg, registration, questionnaire examination, payment, and specimen collection) in large spaces like parking lots. The drive-through testing idea originated from the initial discussion of distributing antidotes in 2018 bioterrorism response research and has several advantages: less cross-infection opportunity, reducing testing time to 10 minutes, less personal protective equipment (PPE) changes per patient, and less patient waiting time. For pedestrians, in mid-March one hospital invented a walking-through one-person safety diagnostic booth that maintains negative pressure, with gloves attached to glass walls allowing HCP to collect specimens in only 3 minutes without direct exposure to patients.12
As of April 10, 580 health care facilities and public health centers, and 73 drive-through test centers could perform diagnostic tests.13 The daily test capacity was 100,000 as of March 23, and the total accumulated test numbers on April 13 were 518,743.7 The government covers the cost of tests meeting case criteria. People who want tests despite not meeting the criteria can receive tests by paying $130; the government reimburses them for tests with positive results. Because of recent incoming cases from abroad, 40 walking-through testing clinic booths were installed at Incheon International Airport from March 25.14
Tracking exposed cases and isolation of confirmed cases
Based on the Enforcement Decree of the Infectious Disease Control and Prevention Act requirements,15 the KCDC conducts a daily briefing that includes reports on COVID-19 cases’ modes of transportation used, locations visited, and number of people contracted. Under the same Act, tracking case movement routes is possible through credit cards, cellphone GPS, and security camera records. Moreover, emergency alert messages are distributed for new cases, so neighbors who cross paths with confirmed cases can report and visit testing sites. Although some privacy concerns were raised because some individuals could attempt to triangulate case identities despite anonymized information, a social consensus was established among most people on transparently sharing information for public cooperation following the MERS experience.2
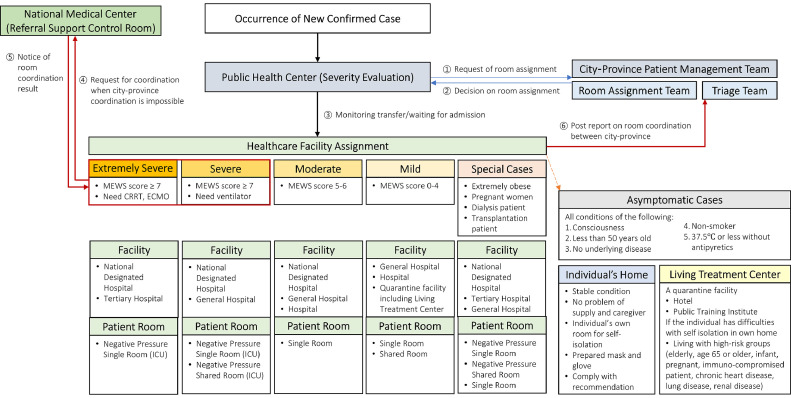
For cases with positive test results, the case isolation strategy has 5 categories (asymptomatic, mild, moderate, severe, and extremely severe) based on the modified early warning score, and assignment to a facility is managed by public health centers and city-province patient management centers (Fig 2 ).16 When over 2,000 cases were found simultaneously in Daegu, mild cases were accommodated at training facilities and transferred to hospitals with worsening symptoms. Standards for discontinuing hospital isolation are (1) Clinical: no fever without antipyretic drug AND recovered from clinical symptoms; and (2) Diagnostic: 2 instances of PCR negative results with a 24-hour interval.10 National health insurance fully covers patient medical bills for COVID-19 treatment.17 For cases isolated at home or live-in treatment facilities, symptoms are monitored twice daily by HCP or individually assigned public health officers.10
Fig 2.
Facility and patient room assignment flow for confirmed cases of COVID-19. Note: MEWS, modified early warning system. This score is calculated based on pulse, systolic blood pressure, respiratory rate, body temperature, and level of consciousness; CRRT, continuous renal replacement therapies; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit. This flow is modified to reflect a Korean confirmed case's isolation assignment with severity categories based on an example of the bed assignment process from the COVID-19 Response Manual for Municipal (version 7-4, published on April 2, 2020) Appendix.
Treatment
Treatment focuses on determining which high-risk patients, specifically those over 60 years of age or with diabetes and reduced immune functions, developed into severe cases and prioritizing them for hospital placement, whereas low-risk groups are advised to self-quarantine at home. Some drugs (ie, ritonavir/lopinavir, hydroxychloroquine, ribavirin, interferon) that are potentially effective are recommended for severe cases. Clinical studies for remdesivir are ongoing in Korea, along with a clinical study of hydroxychloroquine's prevention effect on those exposed.
Public messaging
Since the KCDC's first COVID-19 recommendations on January 16, the public has been encouraged to wear masks for respiratory symptoms (particularly when visiting health care facilities), wash their hands, and report their travel history abroad.18 To increase public awareness, KCDC has provided educational materials; the latest version on March 31 contains 15 brochures, including one on correctly wearing facial masks.7
With cases multiplying, demand for masks at one point exceeded supply. To prevent panic buying and to treat every citizen fairly, since March 9 the government has implemented a 5-day rotation system for distributing masks: on designated days of the week based on birth year, citizens can purchase 2 masks weekly from pharmacies for $1.20.19 As of March 30, 11.1 million masks/day are provided20; 80% are distributed to the public and 20% to health care facilities. A good commonly available mask is the Korean Ministry of Food and Drug Safety-approved Korean filter 94 maskapproved for sanitation purposes that can filter 94% of 0.4µm-sized particles, with a less than 11% of facial leakage rate and less than 70 Pa of inhalation resistance.21
Hospital countermeasure strategies
COVID-19 designated and safe hospitals
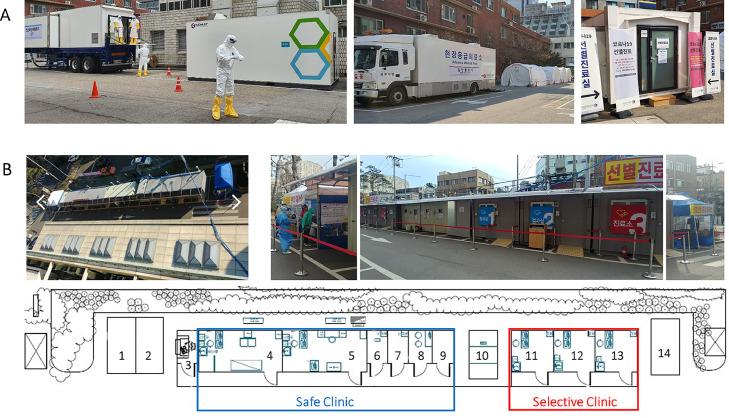
To secure isolation beds for the hospitalization of COVID-19 patients with “Moderate” severity,16 the Korean government assigned 43 COVID-19 designated hospitals on February 21 and expanded to 67 on March 10 with $31.6 million in support.22 As of March 12, 11,658 beds were vacated to prepare 7,207 equivalent isolation beds (after renovations with bulkheads and equipment) and 5,725 beds were prepared (3,595 occupied; 2,130 available).22 Some government-owned or provincial hospitals vacated 100% of beds, and other designated hospitals operated COVID-19 beds flexibly on demand. In contrast, to establish a hospital traffic control system distinguishing between respiratory-symptomatic and nonrespiratory-symptomatic patients from outpatient clinic visits to hospitalization, the government designated safe hospitals with reimbursement support if applicant hospitals met the designation criteria (Fig 3 ).23
Fig 3.
Examples of selective clinics in South Korea. Note: Picture A. A selective clinic outside the National Medical Center. From left to right, waiting rooms for the selective clinic; entrance of selective clinic; chest X-ray truck; and a computed tomography (CT) truck on the left and a generator for the CT truck. Picture B. A safe clinic and a selective clinic located outside Soonchunhyang University Seoul Hospital. Floor plan B. (1) Waiting area for a safe clinic, (2) Waiting area for a pediatric safe clinic, (3) Questionnaire checking and registration desk, (4) Negative-pressure safe clinic room, (5) Negative-pressure safe clinic room for pediatric patients, (6) Negative-pressure doffing personal protective equipment (PPE) room, (7) Negative-pressure sputum-collecting room, (8) Waiting room for health care personnel, (9) Donning PPE room, (10) Restrooms, (11) Negative-pressure test laboratory 1, (12) Negative-pressure test laboratory 2, (13) Negative-pressure selective clinic, and (14) Waiting area for selective clinic. All pictures were provided by coauthors at the above mentioned hospitals.
Incoming hospital visitor screening
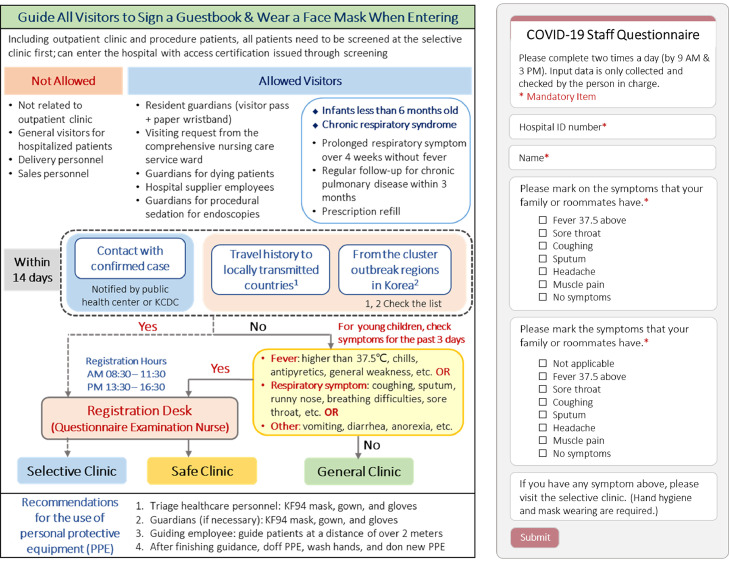
Many hospitals experienced with MERS prepared selective clinics outside emergency department (ED) entrances. Separate clinics for patients with respiratory symptoms outside EDs prevented suspected COVID-19 patients from entering EDs and increasing risks to other patients. Specifically, the National Medical Center prepared a selective clinic equipped with trucks for X-rays and computed tomography to diagnose cases outside hospitals (Fig 2A). One tertiary hospital built container-based (maintained negative pressure) clinics: a selective clinic for COVID-19 potential cases and a safe clinic for patients with respiratory symptoms unrelated to COVID-19 (Fig 2B). The hospital also developed a screening process for all hospital visitors: permitted visitors must complete the checklist of their potential exposure history and symptoms, and visit assigned clinics based on assessment results without mixing with visitors to other clinics (Fig 4 ). To enter the hospital, all visitors must also wear masks.
Fig 4.
Examples of the process of screening all hospital visitors and the smartphone application screen for screening staff's daily symptoms. Note. The figure on the left is a modified English version of the process guideline for healthcare personnel from Soonchunhyang University Seoul Hospital. These guidelines are posted at the hospital entrance, and all hospital visitors must follow the screening process to the designated clinics as shown in Figure 2B. The questionnaire on the right is a modified English version of the smartphone application screen for hospital staff's daily symptom screening which is required to check twice a day from the Dankook University Hospital.
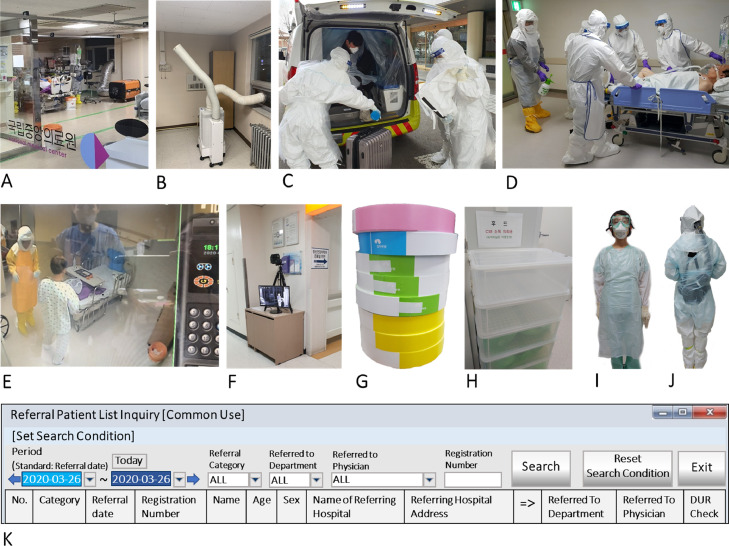
One tertiary Daegu hospital installed exothermic detectors at entrances to screen visitors for fever (Fig 5 E). For effective screening linked with the Drug Utilization Review database (to check other hospital visits), one hospital developed a referral checking program in their electronic medical record system (Fig 5K). Hospitals prepared more isolation rooms by installing portable negative-pressure machines through window vents (Fig 5A and B). With no screening capacity, one 300-bed long-term care hospital changed its admission policy to receive only referred elderly patients with negative COVID-19 test results and to protect resident patients by disallowing visitors.
Fig 5.
Examples of Korean hospitals’ COVID-19 response. Note: (A) A portable negative pressure machine with high efficiency particulate air (HEPA) filter was set up through the window to convert the intensive care unit to negative pressure. Pictures A through E were taken at the National Central Hospital; (B) A portable negative pressure machine with HEPA filter was installed at the isolated patient room. One vent is up toward the patient to draw in contaminated air and the other vent is linked to the outside through the window to emit the filtered air; (C) Health care personnel preparing to receive the arrival from the airport of a traveler who tested positive. A disinfection solution being sprayed is diluted sodium hypochlorite (500 ppm); (D) A case patient is transferred to an operating room for a surgery. In this picture, due to intubation, the patient could not wear a face mask. However, all patients must wear the mask inside a hospital building; (E) Monitoring an isolated patient room outside through CCTV; (F) All hospital visitors are screened for fever with an exothermic detector at one of the entrances at Daegu Catholic University Hospital; (G) For the daily screening of patients’ visitors (residents or frequent visitors), paper wrist bands with different colors are used at Soonchunhyang University Seoul Hospital. The color of the wrist band is changed every day to screen all visitors; H) Used hoods are collected for disinfection at the central supply room, by putting them inside double layered green bags at each plastic cabinet drawer. Pictures H to K were taken at Dankook University Hospital; (I) Four personal protective equipment (PPE) items (disposable gown, gloves, KF94 mask, and goggle) for taking care of probable cases; (J) Protecting the hose and battery for the powered air purifying respirator (PAPR) by wearing a disposable plastic gown back (also, front); (K) An English version of an electronic medical record (EMR) program screen for checking any missing travel information (including other hospitals using drug utilization review, DUR), for sharing information, and in case the patient doesn't provide the right information. All pictures were provided by coauthors at the above mentioned hospitals.
EDs also reorganized areas to separate patients from COVID-19 cases. One 2,705-bed tertiary hospital ED assigned infectious disease fellows (2-3 for day, 1 for night) to symptom assessment and COVID-19 triage, following their selective process protocol. They prepared a screening ED unit (where patients for admission can await test results), an acute care unit (including an 8-bed, 2 negative-pressure room emergency intensive care unit for confirmed cases), and an intensive isolation room (with an anteroom and a shower room for an extremely severe case).
PPE
Korean hospitals have received Level D coveralls from the KCDC based on the daily number of hospitalized cases and HCP. Some hospitals have reserved additional PPE storage. A 613-bed NMC, the leading public disaster-preparedness hospital in Seoul, maintained the largest amount of PPE stockpiles (around 16,000 Level D coveralls, 5,200 Level C coveralls, and 2,000 powered air purifying respirator [PAPR] hoods). After consuming 80% of the Level D coveralls in two months, they found alternative supplies of them with distributors to other industries (eg, wastewater treatment plants). When PPE supplies were insufficient, hospitals changed Level D coveralls to disposable plastic gowns, and N95 respirators to KF94 masks. Although KF94 masks cannot ensure airtight seals due to the ear-loop structure, their filtration capacity is similar to the N95, and the KCDC recommended the KF94 or KF99 for HCP.24 Currently, Level D coveralls and N95 respirators (KF94 masks or PAPR) are recommended for confirmed cases and high-risk aerosol-generating procedures. As Level D supplies are depleted, a four-item PPE set (disposable plastic gown, gloves, mask, and goggles or face shield; Fig 5I) is used more often for patient care.
With limited mask supplies managed by the government based on the number of HCP, HCP assigned to confirmed cases can use more than one mask daily, but other HCP are asked to use only one. Reusing N95 is limited to the same HCP after safe storage (eg, zipper bag with name marked) with more hand hygiene emphasis after touching masks. When facing a mask crisis, surgical masks may be an alternative to N95 or KF94, as the HCP infection rate was unincreased after switching to surgical masks during Singapore's 2009 novel swine-origin influenza A pandemic.25 Some out-of-stock PPE items are reused until losing functionality after disinfection at the central supply department (CSD), despite no disinfection guidelines available for PPE reuse. Used PAPR hoods are sent to the CSD for Ethylene Oxide gas Sterilization (Fig 5H). Used goggles are sent to the CSD, soaked in enzyme-based detergent, and disinfected with effervescent chlorine tablets.
HCP exposure management
HCP working hours wearing Level D sets are recommended at 2-3 hours. Nurse to patient staffing ratios are 1:2 for confirmed cases, 1:1 for severe cases, and 1:3 for mild cases. Selective clinic testing staff rotate every 3 hours. To assess occupational exposures, HCP must report related symptoms twice daily (before work and after lunch) through checklists or smartphone applications (Fig 4). If HCP have symptoms, they must visit selective clinics for testing. Until test results become available, HCP with positive symptoms are excluded from work; they can return only with negative tests. If an HCP encountered or stayed in a confined space with a confirmed case without appropriate PPE, the HCP is excluded from work for 14 days (home quarantine). If an HCP has a family member who encountered a confirmed case, they can work by checking daily symptoms.
Limitations
This study has several limitations. Although we tried to summarize Korea's responses based on the available data, given the ongoing COVID-19 situation and data limitations, this summary may lack important information of interest. For example, data was unavailable on how many Shincheonji HCP were infected by occupational exposure versus by attending their religious services (potentially community acquired) due to their overlapping routes. In addition, our hospital response examples may not represent all Korean hospitals.
Conclusions
Korea's last 85 days of aggressive response to COVID-19 were successful in lowering new cases to currently less than 50 per day after overcoming the initial peak through rapidly expanded diagnostic tests, vigorous tracking of cases’ locations and finding exposed individuals, sharing daily updated information transparently, and the public's mask wearing and cooperation. However, Korea still has challenges with continuously occurring sporadic cluster outbreaks and incoming cases from countries in the middle of the pandemic. Korea's efficiency-maximizing environment, which induces dense contact within confined spaces, can ignite risks of sporadic cluster outbreaks. COVID-19 could reappear as a second wave or in seasonal outbreaks because it has negative outbreak-control aspects, such as a highly contagious early disease phase, mutation possibility, and a clinical characteristic of PCR positive results lasting longer even after clearance in computed tomography results. Returning to prepandemic routines may be impossible until effective treatments and vaccines are available and herd immunity is established worldwide.
Under the scenario of a long-lasting pandemic, long-term sustaining response strategies must be urgently prepared. The mass production of PPE to protect front-line HCP and medical devices (eg, ventilators) for severe cases is necessary by collaborating with manufacturing companies. To prepare for greatly increasing cases, municipalities and hospitals should develop systems that can convert general patient rooms into isolation rooms, and long-term hospitals into quarantine hospitals. Moreover, while practicing social distancing, calls to action for global citizens and experts must be made in finding solutions together to return to daily life while minimizing damage from COVID-19.
Acknowledgments
We would like to thank two people at Harvard T.H. Chan School of Public Health for their help: Dr Jesse B. Bump, Executive Director, Takemi Program, Department of Global Health and Population, for his suggestion to share the Korean experience; and Donald D. Halstead, Lecturer on Epidemiology and Director of Writing Programs, for his comments on the manuscript. This work was supported by “Overseas Training Expenses for Humanities & Social Sciences” through Seoul National University and conducted with the support of the Takemi Program in International Health at Harvard T.H. Chan School of Public Health. All authors have no potential conflicts of interest to disclose.
Footnotes
Conflicts of interest: All authors have no potential conflicts of interest to disclose.
References
- 1.Shaw J. Cooperating to combat coronavirus. Harvard Magazine. Published March 3, 2020 https://harvardmagazine.com/2020/03/fighting-sars-2 Available at: Accessed March 19, 2020. [Google Scholar]
- 2.Ministry of Health and Welfare . 2016. The 2015 MERS outbreak in the Republic of Korea: Learning from MERS. [Google Scholar]
- 3.GFID. Promotional brochure for the Government-wide R & D fund project for infectious diseases. Published 2018. Available at: https://www.gfid.or.kr/kr/customer/work_view.php?idx=466. Accessed March 26, 2020.
- 4.KCDC. Information on the project for specialized regional infectious disease hospitals. Published 2017. Available at: http://www.cdc.go.kr/board.es?mid=a20504000000&bid=0014&act=view&list_no=128351. Accessed March 25, 2020.
- 5.KCDC. In order to respond to the infectious disease crisis more quickly, the reporting system for communicable diseases will be changed from next year. Published 2019. Updated December 26, 2019. Available at: https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015. Accessed March 13, 2020.
- 6.Lee M, You M. Psychological and behavioral responses in South Korea during the early stages of Coronavirus Disease 2019 (COVID-19) Int J Environ Res Public Health. 2020;17:1–14. doi: 10.3390/ijerph17092977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KCDC. COVID-19 domestic occurrence status (April 13, Regular Briefing). Published April 13, 2020. Available at:https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015. Accessed April 13, 2020.
- 8.KCDC. COVID-19 domestic occurrence status (February 17, Regular Briefing). KCDC. Published February 17, 2020. Available at: https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015. Accessed March 17, 2020.
- 9.Kim T, Eum S, Lee Y, Seo Y. 34 COVID-19 infected Daegu healthcare workers were Shincheonji... Is this clue for tracking transmission route. News1. Published March 28, 2020 [Google Scholar]
- 10.Central Accident Investigation Headquarters, Central Discharge Countermeasures Headquarters. Coronavirus disease-19 response manual (for Municipal). In. 7-4 ed 2020.
- 11.KCDC. The amount of time for the diagnosis of novel coronavirus will be shortened by a public-private partnership. Published January 30, 2020. Available at: https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015. Accessed March 12, 2020.
- 12.Kim J. “Walking-through” this time: Test can be done within 3 minutes inside of a transparent booth. JTBC News. Published March 16, 2020 http://news.jtbc.joins.com/html/366/NB11940366.html Available at: Accessed March 18, 2020. [Google Scholar]
- 13.Ministry of Health and Welfare. Current status of selective clinics for the COVID-19 test available (As of April 10, 2020, 6 pm). Published April 13, 2020. Available at: https://www.mohw.go.kr/react/popup_200128_4.html. Accessed April 13, 2020.
- 14.Cho J. 'Walking-through' selective clinic established at the Incheon Airport... 6∼7 minutes for 1 person. TBS News. Published March 23, 2020 http://tbs.seoul.kr/news/newsView.do?typ_800=1&idx_800=2389033&seq_800=10382403 Available at: Accessed April 13, 2020. [Google Scholar]
- 15.Ministry of Health and Welfare . National Law Information Center; 2020. Enforcement Decree of the Infectious Disease Control and Prevention Act. [Google Scholar]
- 16.Central Accident Investigation Headquarters, Central Discharge Countermeasures Headquarters. Coronavirus disease-19 response manual (for Municipal) Appendix. In. 7-4 ed2020.
- 17.Ministry of Health and Welfare. Information on the cost of treatment for new infectious disease syndrome (new coronavirus infection). In: Insurance Benefits Division, ed2020.
- 18.KCDC. KCDC, strengthening community response to new coronavirus. Published January 22, 2020. Updated January 23, 2020. Available at:https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015. Accessed March 12, 2020.
- 19.Ministry of Food and Drug Safety. Q and A for the purchasing process of common good masks. Published 2020. Available at:https://www.mfds.go.kr/brd/m_659/list.do?page=1&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=. Accessed March 25, 2020.
- 20.Ministry of Food and Drug Safety. Purchasing sites and amounts of common good masks (as of March 30). Published March 30, 2020. Available at:http://ncov.mohw.go.kr/maskBoardList.do?brdId=3&brdGubun=36&dataGubun=&ncvContSeq=&contSeq=&board_id=. Accessed March 30, 2020.
- 21.Ministry of Food and Drug Safety. Safe use information for sanitation masks. Published March 5, 2019. Available at:https://www.mfds.go.kr/brd/m_580/view.do?seq=18&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1. Accessed March 31, 2020.
- 22.Ministry of Health and Welfare. Financial support of KRW 39 billion for 69 COVID-19 designated hospitals. Published March 13, 2020. Available at:http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&CONT_SEQ=353539&page=1. Accessed May 21, 2020.
- 23.Health Insurance Review and Assessment Service. Business manual on operations support for infectious disease designated hospitals. HIRA;. Published March 3, 2020. Available at:http://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=7816. Accessed May 22, 2020
- 24.KCDC. COVID-19 domestic occurrence status (February 5, Regular Briefing). Published February 5, 2020. Available at: https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015. Published Accessed March 15, 2020.
- 25.Ang B, Poh BF, Win MK, Chow A. Surgical masks for protection of health care personnel against pandemic novel swine-origin influenza A (H1N1)-2009: results from an observational study. Clin Infect Dis. 2010;50:1011–1014. doi: 10.1086/651159. [DOI] [PubMed] [Google Scholar]