Abstract
Accumulating evidence supports that the viral-induced hyper-inflammatory immune response plays a central role in COVID-19 pathogenesis. It might be involved in the progression to acute respiratory distress syndrome (ARDS), multi-organ failure leading to death. In this study, we aimed to evaluate the prognostic value of the immune-inflammatory biomarkers in COVID-19, then determine optimal thresholds for assessing severe and fatal forms of this disease.153 patients with confirmed COVID-19 were included in this study, and classified into non-severe and severe groups. Plasmatic levels of interleukin 6 (IL6), C-reactive protein (CRP), soluble-IL2 receptor (IL2Rα), procalcitonin (PCT) and ferritin were measured using chemiluminescence assay. Complete blood count was performed by Convergys 3X® hematology analyzer. Our results demonstrated that the peripheral blood levels of IL6, PCT, CRP, ferritin, IL2Rα, white blood cell count (WBC), neutrophil count (NEU), neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (d-NLR) were significantly higher in severe forms of COVID-19. The ROC curve analysis showed that IL6 was the most accurate inflammatory biomarker. The calculated cutoff of IL6 (42 pg/ml) could correctly classify > 90% of patients regarding their risk of severity (area under ROC curve (AUROC) = 0.972) and the threshold value of 83 pg/ml was highly predictive of the progression to death (AUROC = 0.94, OR = 184) after a median of 3 days. Besides, IL-6 was positively correlated with other inflammatory markers and the kinetic analysis highlighted its value for monitoring COVID-19 patients. PCT and NLR had also a high prognostic relevance to assess severe forms of COVID-19 with corresponding AUROC of 0.856, 0.831 respectively. Furthermore the cut-off values of PCT (0.16 ng/ml) and NLR (7.4) allowed to predict mortality with high accuracy (se = 96.3%, sp = 70.5%,OR = 61.2)’ (se = 75%, sp = 84%, OR = 14.6).The levels of these parameters were not influenced by corticosteroid treatment, which make them potential prognostic markers when patients are already undergoing steroid therapy.
Keywords: COVID-19, Inflammatory biomarker, IL6, Procalcitonin, Cytokine storm, NLR
1. Introduction
Since December 2019, the world has experienced an outbreak of pneumonia caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This infection, also called coronavirus disease 2019 (COVID-19) has a wide clinical spectrum, encompassing asymptomatic forms, mild symptoms, and severe forms that can quickly progress to death [1].
The identification of potential risk factors for disease progression of COVID-19 is very crucial in order to guide preventive and therapeutic interventions. Evidence suggests that older age and underlying diseases are more likely to be associated with COVID-19 hospitalization [2]. Similarly several observational studies have shown that aberrant immune-inflammatory response and cytokine storm might be responsible of multi-organ failure and fatal progression of COVID-19 [3], [4], [5], [6].
Reports describing the immunological features of severe and critical forms of COVID-19 have revealed a markedly high levels of circulating inflammatory cytokines, mainly interleukin-6 (IL-6) with elevated blood concentration of several inflammatory markers including the complete blood count parameters [7], [8], [9], [10], [11], [12]. Moreover, according to autopsy analyses, the elevated levels of these inflammatory markers seem to be strongly associated with extensive tissue necrosis and interstitial macrophage infiltrations in the pulmonary, cardiac and gastrointestinal tissues of COVID-19 patients [4], [13].
The aim of our study is to explore changes of the immune-inflammatory markers in peripheral blood of patients with different forms of COVID-19, and to evaluate their prognostic value, then determine optimal thresholds for assessing severity and mortality of this disease.
2. Methods
2.1. Study design
We conducted a prospective study on 153 patients recruited from Beni-Messous teaching hospital (Algiers, Algeria). All patients were diagnosed with COVID-19 by reverse transcription-polymerase chain reaction method (RT- PCR) from a nasopharyngeal swab, between March 22 and June 16, 2020. Our patients were classified based on the severity of symptoms according to the World Health Organization (WHO) interim guidance for COVID‐19 [14]‘ into mild (n = 34), moderate (n = 39), severe (n = 33) and critical (n = 47) types.
For our work, we have resembled our patients on two principal groups: non-severe group (n = 73, mild and moderate) and severe group (n = 80, severe and critical). In the severe group, note that: 21 patients received corticosteroid therapy during their hospitalization, and 38 patients have succumbed to the disease. The steroid treatment consisted of administering dexamethasone in a dosage of 8 mg intravenously per day for 10 days.
The patients were divided into three groups [Non-severe group (n = 73)’severe group without steroid therapy (n = 59) and severe group with steroid therapy (n = 21)] when taking into account the administration of corticosteroid treatment.
Demographic and clinical information were collected from medical records, and informed consent was obtained from all the participants.
In order to study the dynamic change of interleukin-6 in COVID-19 infection, the level of this cytokine was measured twice in serum samples of 27 patients (16 non-severe and 11 severe), with a delay of 5–10 days.
2.2. Laboratory examination of blood samples
Peripheral blood was obtained by venipuncture from all the patients on admission. We have analyzed: Complete Blood Count (CBC) and immune-inflammatory parameters: interleukin-6, procalcitonin (PCT), C-reactive protein (CRP), soluble-IL2 receptor (IL2Rα) and ferritin.
CBC was performed by the hematology analyzer Convergys 3X®. IL6, CRP and IL2Rα were measured by chemiluminescence immunoassay (CLIA) via IMMULITE® 2000 XPI analyzer (Siemens). The fully automated electrochemiluminescence method (ECLIA) was used to measure levels of PCT and ferritin on COBAS® E411 platform (Roche Diagnostics).
2.3. Statistical analysis
Normally distributed continuous data were presented as mean ± standard deviation, a comparative analysis of this data was performed between non-severe and severe groups using unpaired 2-sided Student’s t-test. Categorical variables were described as percentages, and the significance was tested by the chi-square test. Abnormally distributed variables were expressed as medians and inter-quartile ranges (IQR), and were compared between the three above-mentioned groups using the non-parametric Kruskal Wallis test followed by pair-wise comparison by the Dunn-Bonferroni post hoc method. A p value of <0.05 was considered statistically significant.
Univariate and multivariate logistic regression analysis was used to evaluate the prognostic value of different immune-inflammatory biomarkers, by taking the severity or the mortality of COVID-19 as dependent variable. The receiver operator characteristic (ROC) curves were built to assess predictive values and the optimal discriminating cut-off values were obtained by calculating the Youden index. The Odds Ratio (OR) with their confidence intervals 95% (95% CI) were calculated according to the cut-off values by Fisher's exact test.
The correlations between interleukin-6 and other variables were analyzed by the Spearman correlation analysis. Kaplan-Meier curves were constructed for analyzing survival data. All these statistical calculations were performed using R 3.6.3 and Medcalc 19.3 software.
3. Results
3.1. Demographic and clinical characteristics
A total of 153 patients with COVID-19 were included in this study. Among them 50 (33%) were woman and 103 (67%) were man, the mean age was 61 ± 13.95 years. Hypertension (39%) and diabetes (32%) were the most common comorbidities.
Compared with non-severe group, the patients of severe group were older (65 ± 13.55 vs 57 ± 13.41 years, p = 0.0003) and had more underlying comorbidities: hypertension (50% vs 22%, p = 0.0072) and diabetes (45% vs 11%, p = 0.0006). The 38 deceased patients represent 47.5% of severe group patients (Table 1 ).
Table 1.
Demographic and clinical characteristics of patients with COVID-19.
| Variable | All patients (n = 153) | Non severe group (n = 73) | Severe group (n = 80) | P value |
|---|---|---|---|---|
| Age (years) | 61 ± 13.95 (18–88) | 57 ± 13.41 (18–80) | 65 ± 13.55 (31–88) | 0.0003 |
| Gender n (%) | ||||
| Female | 50 (33%) | 27 (37%) | 23(29%) | 0.2941 |
| Male | 103 (67%) | 46 (63%) | 57(71%) | |
| Smoking history n/N (%) | ||||
| Non-smok | 51/56(91%) | 2/29 (7%) | 3/27 (11%) | 0.6032 |
| Smok | 5/56 (9%) | 27/29 (93%) | 24/27 (89%) | |
| Any comorbidity n/N (%) | 64/94 (68%) | 19/36 (53%) | 45/58(78%) | 0.0137 |
| Hypertension | 37/94 (39%) | 8/36 (22%) | 29/58(50%) | 0.0072 |
| Diabetes | 30/94(32%) | 4/36 (11%) | 26/58(45%) | 0.0006 |
| Respiratory disease | 10/94 (11%) | 1/36 (2.7%) | 9/58(15.5%) | 0.0512 |
| Cardiopathy | 7/94 (7%) | 0/36 (0%) | 7/58(12%) | – |
| Cancer | 9/94 (9%) | 5/36 (14%) | 4/58(7%) | 0.2671 |
| Thyroiditis | 7/94 (7%) | 4/36 (11%) | 6/58(10%) | 0.8779 |
| Others | 18/94 (19%) | 4/36 (11%) | 13/58(22%) | 0.1775 |
| Disease severity status | ||||
| Mild | 34(22%) | 34(46.6%) | 0(0%) | |
| Moderate | 39(25.5%) | 39(53.4%) | 0(0%) | – |
| Severe | 33(21.5%) | 0(0%) | 36(45%) | |
| Critical | 47(31%) | 0(0%) | 44(55%) | |
| Clinical outcomes | ||||
| Recovery | 115(75%) | 73(100%) | 38(47.5%) | – |
| Death | 38(25%) | 0(0%) | 42 (52.5%) |
Data are mean ± standard deviation (range), n (%) or n/N (%) where N is the total number of patients with available data. P values indicate differences between severe and non-severe groups and were calculated using the Student's t-test or χ2 test. P < 0.05 was considered statistically significant.
3.2. Prognostic value of immune-inflammatory markers to assess the severity
We first analyzed the CBC parameters: lymphocyte count was significantly lower in the severe group when compared with the non-severe group (P < 0.0001). White blood cell count (WBC), neutrophil count (NEU), neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (d-NLR) were significantly higher in patients with severe forms (P < 0.0001 and p = 0.00071 for WBC). There were no significant differences in the hemoglobin levels (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelets count, platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) between the two groups of patients (p > 0.05).
We next compared the levels of CRP, IL6, IL2Rα, PCT and ferritin in plasma samples between severe and non-severe groups. The values of the above-mentioned parameters were significantly higher in patients with severe form of COVID-19 than in non-severe form (P < 0.0001) (Table 2 ).
Table 2.
Laboratory finding of patients with COVID-19.
| Variable | Normal rang | All patients (n = 153) | Non severe group (n = 73) | Severe group (n = 80) |
P valuea | P valueb | |
|---|---|---|---|---|---|---|---|
| without steroid therapy (n = 59) | with steroid therapy (n = 21) | ||||||
| WBC × 103/µl | 5–10 | 9.2 (6.7–11.4) | 7.7 (6.5–9.4) | 10.5 (7.2–12.2) | 12.59 (9.9–15.5) | 0.00071 | 0.224 |
| NEU × 103/µl | 2.5–7.5 | 6.8 (4.6–9.6) | 5(4–6.9) | 9.09 (5.9–10.8) | 10.88 (8.27–13.9) | <0.0001 | 0.75 |
| LYM × 103/µl | 1.3–4 | 1.2 (0.8–1.8) | 1.5 (1.1–2) | 0.9 (0.71–1.34) | 0.85 (0.57–1.57) | <0.0001 | 0.99 |
| MON × 103/µl | 0.16–0.7 | 0.3 (0.2–0.6) | 0.33 (0.2–0.73) | 0.24 (0.13–0.5) | 0.34 (0.13–0.58) | 0.048 | 0.617 |
| HGB g/dl | 12–17.4 | 13.1 (11.7–14.1) | 13.4 (12.4–14.2) | 13(11.8–14) | 11.5 (11–13) | 0.61 | 0.08 |
| MCV (fl) | 76–96 | 84 (81–86) | 83.7 (81–86) | 84(82–86) | 83.5 (80–86.5) | 0.99 | 0.79 |
| MCHC (g/dl) | 30–35 | 32.9 (32.1–33.5) | 32.7 (32.1–33.4) | 33(32.2–33.8) | 32.5 (31.9–33) | 1.0 | 0.86 |
| PLT × 103/µl | 150–400 | 235(167–309) | 242(174–310.5) | 196.5 (140.5–273.5) | 333(260–398.5) | 0.067 | 0.00017 |
| NLR | 1–3 | 5.6 (3–9.8) | 3.5 (2.2–5.7) | 8.2 (5.4–13.6) | 12.2 (5.3–19.6) | <0.0001 | 0.97 |
| d-NLR | – | 2.8 (1.4–5.3) | 2.2 (1.2–3.8) | 5.4 (2.3–8.4) | 1.6 (1.19–3) | <0.0001 | 0.00083 |
| PLR | – | 186.4 (125.4–295) | 160.7 (109.7–233.7) | 184.5 (129.6–315.8) | 375.3 (239.2–597.2) | 0.263 | 0.02217 |
| LMR | – | 3.8 (2.1–7) | 4.4 (2.53–7.3) | 3.5 (2.2–7) | 3.6 (1.5–5.2) | 0.90 | 0.88 |
| CRP (mg/l) | <3 | 103 (33–167) | 61(8–113) | 160 (102–215.5) | 99.1 (15–171) | <0.0001 | 0.028 |
| IL6 (pg/ml) | <5.9 | 35 (13–85) | 17(4–19.8) | 114 (64–230.5) | 16.4 (6.5–38) | <0.0001 | <0.0001 |
| IL2Rα (U/ml) | 158–623 | 918(642–1459) | 740(501–1055) | 1308 (773.5–1809.5) | 1010 (772–1442) | <0.0001 | 1.0 |
| PCT (ng/ml) | <0.05 | 0.13 (0.05–0.37) | 0.059(0.022–0.13) | 0.3 (0.151–0.71) | 0.25 (0.132–0.71) | <0.0001 | 1.0 |
| Ferritin (ng/ml) | 13–400 | 714 (362–1292) | 438(234–867) | 1045 (657–1615) | 928 (362–1768) | <0.0001 | 1.0 |
Data are presented as median (interquartile ranges).P values were calculated using the Kruskal-Wallis test followed by Dunn’s posthoc test.
Abbreviations. CRP: C-reactive protein; d-NLR = derived neutrophil-to-lymphocyte ratio (neutrophil count divided by the result of white cell count minus neutrophil count); HGB: hemoglobin; IL2Rα: soluble-interleukin-2 receptor; IL6: interleukin-6; LMR: lymphocyte-to-monocyte ratio; LYM: lymphocyte count; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; MON: monocyte count; NEU: neutrophil count; NLR: neutrophil-to-lymphocyte ratio; PCT: procalcitonin; PLR: platelet-to-lymphocyte ratio; PLT: Platelet count; WBC = white blood cell count.
P values indicate the comparison between non severe group and severe without corticosteroid therapy group.
P values indicate the comparison between severe with and without corticosteroid therapy groups.
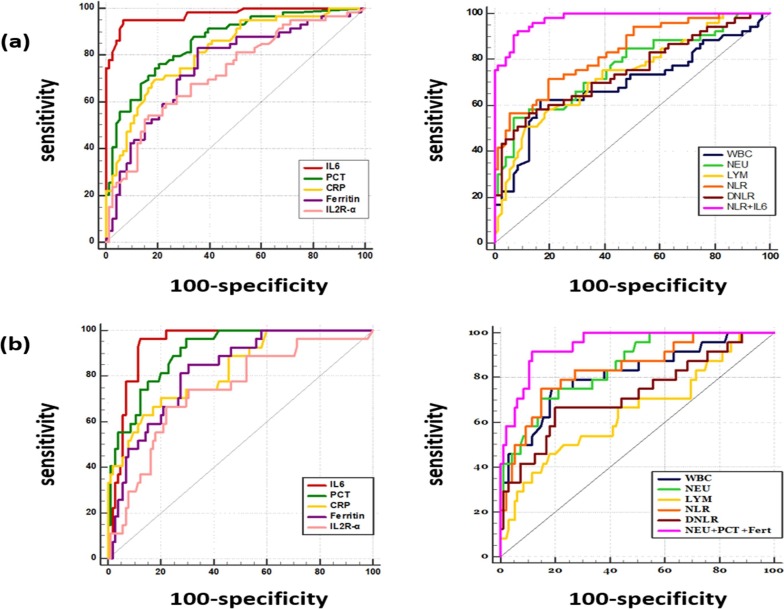
The ROC curve analysis showed that IL6 has the highest area under ROC curve (AUROC) = 0.97, followed by PCT, NLR and CRP with corresponding AUOC of 0.856, 0.831 and 0.816 respectively, reflecting a high prognostic performance of these inflammatory parameters to assess severe forms of COVID-19. The optimal cut-off values were 44 pg/ml for IL6 with an excellent accuracy (sensitivity (se) = 95%, specificity (sp) = 93%), 0.138 ng/ml (se = 76.3%’ sp = 79.5%) for PCT’ 123 mg/l (se = 70%, sp = 81%) for CRP and 5.9 (se = 72%’ sp = 80.3%) for NLR.
Multivariate logistic regression analysis revealed that IL6 and NLR are independent predictors of severe COVID-19. The combined detection of these two markers allowed to reach an AUROC value of 0.98 (Table 3 , Fig. 1 ).
Table 3.
Area under the ROC curve and optimal cut-off values of the immune inflammatory markers according to the severity.
| Variable | AUROC | Optimal cut-off value | Sensitivity | Specificity | OR (95%CI) |
|---|---|---|---|---|---|
| NLR | 0.831 | 5.9 | 71.7% | 80.3% | 8.7 (3.85–19.7) |
| d-NLR | 0.748 | 4.6 | 57% | 87% | 7.95 (3.36–18.83) |
| WBC | 0.693 | 9.67 × 103/µl | 63% | 83.1% | 8.11 (3.53–18.66) |
| NEU | 0.765 | 8.84 × 103/µl | 55% | 93% | 15.95 (5.54–45.94) |
| LYM | 0.737 | 0.9 × 103/µl | 51% | 88.7% | 8.30 (3.34–20.66) |
| IL6 | 0.972 | 44 pg/ml | 94.9% | 93.2% | 253.86 (58.11–1109) |
| PCT | 0.856 | 0.138 ng/ml | 76.3% | 79.5% | 12.42 (5.44–28.39) |
| CRP | 0.816 | 123 mg/l | 70% | 80.8% | 9.59 (4.29–21.45) |
| Ferritin | 0.752 | 601 ng/ml | 83.1% | 64.4% | 8.86 (3.85–20.35) |
| IL2Rα | 0.724 | 1204 U/ml | 54.2% | 83.6% | 6.02 (2.69–13.45) |
| IL6 + NLR | 0.98 | – | – | – | – |
The optimal discriminating cut-off values were obtained by calculating the Youden index. The Odds Ratio with their confidence intervals 95% were calculated according to the cut-off values by Fisher's exact test.
AUOC: Area under the receiver operating characteristic curve; CI: confidence interval; OR: odds ratio.
Fig. 1.
Receiver operator characteristic curves of the immune inflammatory markers according to (a) the severity (b) the mortality in COVID-19 patients. Univariate and multivariate logistic regression analysis were conducted to evaluate the prognostic value of different immune-inflammatory biomarkers, by taking the severity or the mortality of COVID-19 as dependent variable.
3.3. Predictive value of immune-inflammatory markers to assess the mortality
The area under ROC curve of immune-inflammatory parameters were ranging between 0.65 and 0.94. IL6 and PCT were the most accurate predictive markers of mortality with AUROC > 0.9, followed by NEU, NLR, CRP, ferritin and WBC with AUROC > 0.8, indicating a high prognostic value for mortality.
Multivariate logistic regression analysis showed that PCT, neutrophil count and ferritin are independent risk factors associated with fatal outcomes. The AUROC of the risk model that combined these three markers was as high as that of the IL6 calculated in the univariate analysis (=0.94).
The thresholds for CBC markers that indicate a high risk of mortality are summarized in Table 4 . The strongest predictor biomarkers of mortality were NLR (se = 75%, sp = 84%, OR = 14.6), neutrophil count (se = 68%, sp = 85%, OR = 12.6) and white blood cell count (se = 80%, sp = 74%, OR = 11.4).
Table 4.
Area under the ROC curve and optimal cut-off values of the immune inflammatory markers according to the mortality.
| Variable | AUROC | Optimal cut-off value | Sensitivity | Specificity | OR (95%CI) |
|---|---|---|---|---|---|
| NLR | 0.839 | 7.4 | 75% | 84% | 14.64 (5.07–42.32) |
| d-NLR | 0.730 | 4.9 | 66.7% | 79% | 7.52 (2.84–19.96) |
| WBC | 0.814 | 9.67 × 103/µl | 80% | 74% | 11.38 (3.88–33.42) |
| NEU | 0.851 | 9 × 103/µl | 68% | 85% | 12.6 (4.57–34.7) |
| LYM | 0.653 | 0.82 × 103/µl | 46% | 82% | 3.62 (1.41–9.27) |
| IL6 | 0.94 | 83 pg/ml | 96.3% | 87.6% | 184 (22.98–1472.9) |
| PCT | 0.905 | 0.16 ng/ml | 96.3% | 70.5% | 61.22 (7.95–471.36) |
| CRP | 0.825 | 151mg/l | 70.4% | 80% | 9.5 (3.65–24.67) |
| Ferritin | 0.821 | 834 ng/ml | 85.2% | 69.5% | 13.11 (4.19–41.02) |
| IL2Rα | 0.735 | 1276 U/ml | 67% | 78.1% | 7.13 (2.83–17.96) |
| PCT+NEU+Ferritin | 0.94 | – | – | – | – |
The optimal discriminating cut-off values were obtained by calculating the Youden index. The Odds Ratio with their confidence intervals 95% were calculated according to the cut-off values by Fisher's exact test.
AUOC: Area under the receiver operating characteristic curve; CI: confidence interval; OR: odds ratio.
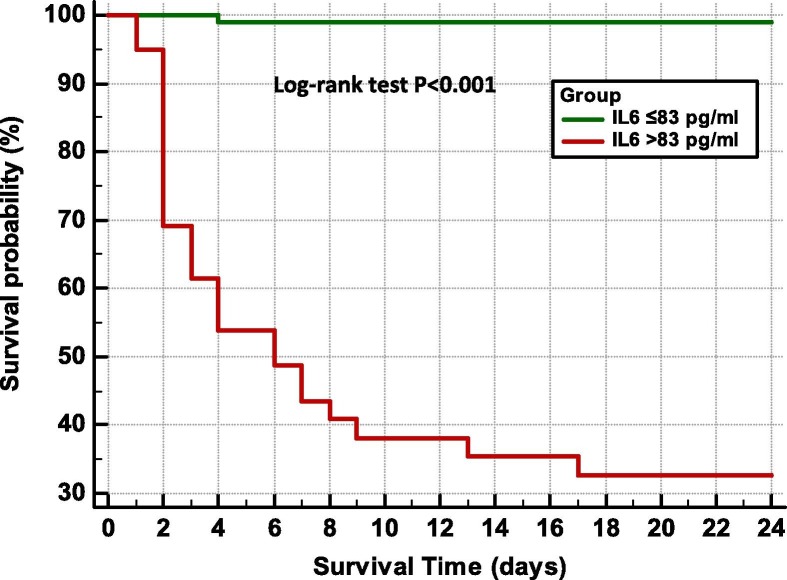
The statistically optimal cut-off value for IL6 was 83 pg/ml, this value could predict mortality with high accuracy (se = 96%, sp = 87%). The risk of mortality is 184 times higher among patients with IL6 levels above this cut-off, (OR = 184’ log-rank, P < 0.0001).Moreover the median time to death after reaching an IL6 value of 83 pg/ml, was 3 days (range 1–17 days) (Fig. 2 ).
Fig. 2.
Kaplan–Meier survival curves according to the cut-off value of interleukin-6 levels. The log-rank test indicates a significant difference between the survival curves.
The ROC curve analysis of the other plasmatic markers revealed that, the cut-off values of PCT (0.16 ng/ml) and CRP (151 mg/l) allowed to predict the progression towards the death with high sensitivity and specificity (se = 96.3%, sp = 70.5%, OR = 61.2), (se = 70.4%, sp = 80%, OR = 9.5) respectively (Table 4, Fig. 1).
3.4. Correlation between IL6’other inflammatory parameters and comorbidities
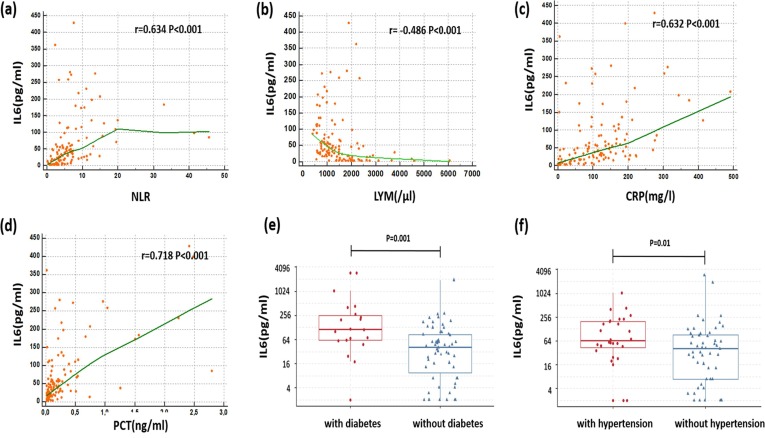
Significant positive correlations were found between the plasmatic level of IL6 and other immune-inflammatory parameters: PCT(r = 0.718, P < 0.0001), CRP (r = 0.632, P < 0.0001), NLR (r = 0.634, P < 0.0001)’ ferritin (r = 0.499, P < 0.0001)’ neutrophil count (r = 0.483, P < 0.0001).It was negatively correlated with lymphocyte count (r = −0.486, P < 0.0001) and platelet count (r = −0.212, p = 0.017). Moreover the level of IL6 was significantly higher in patients with diabetes and/or hypertension than in patients without these underlying diseases. (114 (61.5–251.5) vs 40 (10–86.5), p = 0.001) and (65 (43.7–199.5) vs 40 (7–92.5) p = 0.01) respectively (Fig. 3 ).
Fig. 3.
Correlation between interleukin-6 and (a) neutrophil-to-lymphocyte ratio, (b) lymphocyte count, (c) C-reactive protein and (d) procalcitonin in patients with COVID-19. The levels of interleukin-6 in COVID-19 patients with and without underlying disease: (e) diabetes and (f) hypertension. The Spearman rank correlation between IL6 and other inflammatory parameters is presented graphically in the form of scatter-plots, with a Local Regression Smoothing (LOESS) trend-lines for each association, r: Spearman’s rank correlation coefficient.
3.5. Dynamic change of IL6
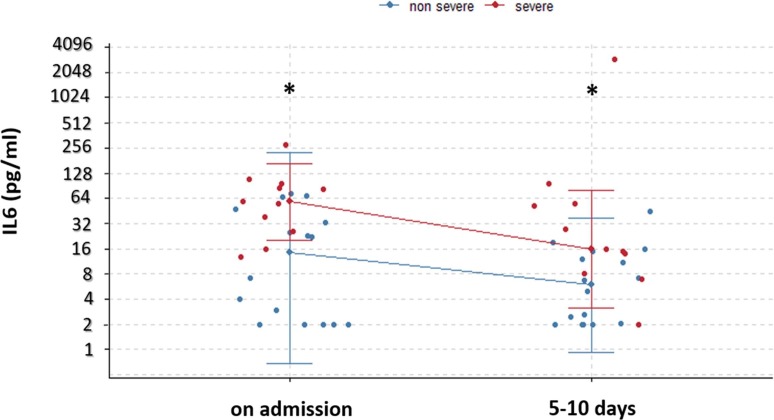
Between the two measurements, the level of IL6 had decreased from a median (IQR) of 14.5 (2–36.5) to 5.9 (2–12.7) in the non-severe group, and from 58 (32–91) to 16 (11–53.8) in the severe group. Note that the difference of the IL6 levels between the two groups remains significant in the two checkpoints with a P value of 0.005 and 0.01 respectively (Fig. 4 ).
Fig. 4.
. Temporal changes of interleukin-6 in patients with COVID-19. Median with range were presented. *P < 0.05 between non-severe and severe groups.
In contrast, we have observed an important elevation of IL6 level for two deceased patients (from 109 pg/ml to 2950 pg/ml and from 38 pg/ml to 97 pg/ml respectively). It should be mentioned that the second measurement was performed the day before their death.
3.6. The effect of corticosteroid treatment on immune-inflammatory markers
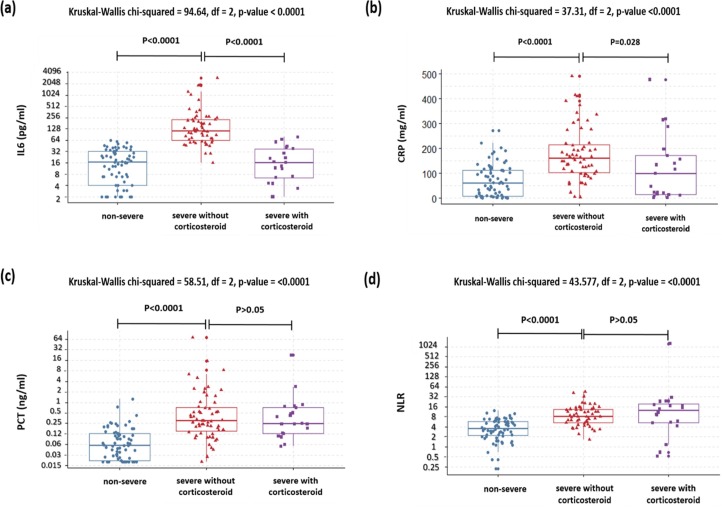
Among the severe group, the patients who received corticosteroid treatment had significantly lower levels of IL6 and CRP, and higher platelets count compared to patients who didn’t receive steroid treatment: (16.4 pg/ml vs 114 pg/ml, P < 0.0001), (99.1 mg/l vs 160 mg/l, P = 0.02) and (333 × 103/µl vs 96.5 × 103/µl, P < 0.0001) respectively.
In the other hand, there were no significant differences in the levels of: PCT (0.25 ng/ml vs 0.3 ng/ml), NLR (12.2 vs 8.2), IL2Rα (1010 UI/ml vs 1308 UI/ml) and ferritin (928 ng/ml vs 1045 ng/ml), between the patients with and without steroid therapy, with P values > 0.05 (Table 2, Fig. 5 ).
Fig. 5.
The levels of (a) interleukin-6, (b) C-reactive protein, (c) procalcitonin and (d) neutrophil-to-lymphocyte ratio in patients with COVID-19: comparison between non severe group, severe group with and without corticosteroid therapy. Differences were tested using the Kruskal-Wallis test followed by Dunn’s posthoc test.
4. Discussion
The inflammatory response plays a central role in COVID-19 pathogenesis. Accumulating evidence supports that the unbalanced pro-inflammatory immune response might be involved in the progression towards severe and critical forms of the disease [15], [16], [17].
Several studies have reported that the peripheral blood levels of immune-inflammatory and CBC markers such as IL6, PCT, CRP, neutrophil count and NLR increase considerably in severe forms of COVID-19. The levels of these markers reflect the intensity of cytokine-mediated hyper-inflammatory response, and seem to be strongly associated with poor outcome [7], [8], [9], [10], [11], [12].
In our study, we first analyzed CBC test considering that it is affordable and broadly available exam in routine laboratories. We have observed that white blood cell count, neutrophil count, NLR and d-NLR were significantly higher in patients with severe forms of COVID-19, while lymphocyte count was decreased in severe group.
NLR combines two CBC parameters: neutrophil count and lymphocyte count. The increase of neutrophil count reflects the intensity of systemic inflammation whereas the lymphopenia reflects lymphocytes sequestration in inflammation site and their apoptosis. The combination of these two biomarkers may improve the prediction of severe COVID-19 [18], [19].
The ROC curve analysis in our study showed that NLR has the highest accuracy among CBC markers to assess severity and mortality in COVID-19, with cut-off values of 5.9 and 7.4 respectively. The proposed cut-off values reported in previous studies were ranging between 3 and 6 (3.13 [12]‘ 3.3 [20], 3.63 [21]‘ 4 [8]‘ 5 [22]‘ 5.5 [23] 5.87 [24]).
Concerning the plasmatic inflammatory markers, the levels of CRP, IL6, IL2Rα, PCT and ferritin were significantly higher in patients with severe form of COVID-19. These results are consistent with the cytokine release syndrome (CRS) hypothesis [15].
The plasmatic levels of PCT are usually undetectable in physiological condition. It increases considerably in the bacterial and fungal infections compared to the viral infections, which makes it a differential diagnosis marker between them [25]. In our study, the proposed cut off values were 0.138 ng/ml for severity and 0.16 ng/ml for mortality. In line with prior studies, our results suggest that the secondary bacterial and fungal infections, expressed by high level of PCT, are associated with severe and potentially fatal forms of COVID-19, justifying the necessity of rational use of antibiotics in COVID-19. However, the thresholds suggested are variable according to the studies going from: 0.07 ng/ml to 0.5 ng/ml [26], [27], [28], [29].
CRP is an acute-phase protein of inflammation. Its role as predictive marker of severe forms of the COVID-19 was clearly proven by several studies. In our series we have defined threshold values of: 123 mg/l to assess severe forms and 151 mg/l to predict mortality with high accuracy. These cut-off values are relatively higher than those proposed by similar studies: 20.42 mg/l [30]‘ 41.4 mg/l [31]‘ 60 mg/l [5] and 97 mg/l [32].
As previously mentioned, IL6 was the most accurate inflammatory biomarker observed in our study. First, the calculated cut-off for IL6 levels (42 pg/ml) could correctly classify >90% of patients regarding their risk of severity (AUROC = 0.972). In addition, the proposed threshold value of IL6 (83 pg/ml) was highly predictive of progression to death (AUROC = 0.94, OR = 184) after a median of 3 days. The thresholds of severity reported in different studies are very variable ranging between 24 and 32 pg/ml [26], [33], [34]. A metanalysis based on a total of nine studies included 1426 patients, suggested a cut-off value of 55 pg/ml to identify severe forms [35].Concerning the mortality’ only few studies have analyzed the predictive value of IL6 to assess critical and fatal forms. The study of Herold et al has defined a value of 80 pg/ml of the need for mechanical ventilation with a median time of 1.5 days [32], [36], In addition Chen et al have reported that the median level of IL6 was 72 pg/ml with IQR (35.6–146.8), in 113 deceased patients with COVID-19 [37].
Indeed, the IL6 values in severe COVID-19 are 10- to 200-fold lower compared to those observed in patients with the hyper-inflammatory phenotype of acute respiratory distress syndrome (ARDS) and septic shock reported in previous series [38], [39], [40], [41]. It has been hypothesized that the hyper-inflammatory response is primarily induced in lung tissues with a high local concentrations of IL6 compared with their circulating plasma levels [42], [43].
Otherwise, despite the high predictive value of IL6, its implication in pathogenesis of severe COVID-19 remains unclear. It is well known that IL6 is a pleiotropic pro-inflammatory cytokine which can induce a variety of acute-phase proteins [44]. Besides’ it is considered as a crucial regulator of neutrophil trafficking during acute inflammatory response via STAT3 signaling pathway [45]. In our study, IL6 was positively correlated with other inflammatory markers including CRP, neutrophil count and NLR. It was negatively correlated with lymphocyte count. According to autopsy studies IL6 was directly involved in the decrease of lymphocyte count observed in COVID-19 [46]. In addition the treatment with tocilizumab (anti-IL6 receptor) was able to correct the lymphopenia [47].
Furthermore, our severe group was older, had more underlying diseases: hypertension and diabetes. Also, the IL6 levels were significantly higher in patients with these comorbidities. This relationship might be linked to the low expression of angiotensin-converting enzyme 2 (ACE-2) in elderly patients with chronic diseases. This induces the accumulation of angiotensin-II which has pro-inflammatory proprieties and may contribute to the progression towards severe and fatal forms of the COVID-19 [48].
In addition to its prognostic value, the kinetic analysis of IL6, has demonstrated that this cytokine might be a good marker for the monitoring of COVID-19 patients. These results are consistent with previous studies [49], [50], [51].
Only 14% of our patients, all included in the severe group, received the corticosteroid therapy. This practice is still widely used in COVID-19. Our study demonstrated that the plasmatic levels of IL6 and CRP might be influenced by steroid therapy in contrast with PCT and NLR, which may constitute good alternative markers to assess severe forms when patients are already undergoing steroid therapy.
5. Conclusion
Severe and fatal forms of COVID-19 are associated with high levels of immune-inflammatory markers in peripheral blood. These results, obtained from our Algerian cohort study, support the finding largely reported in previous series, since the beginning of COVID-19 pandemic.
Furthermore, it is concluded that interleukin-6, procalcitonin, NLR and CRP may constitute potential markers to assess severe forms and fatal progression of SARS-CoV-2 infection in the context of cytokine-mediated hyper-inflammatory response.
The determination of cut-offs may allow to identify with a high accuracy and at an early stage the patients requiring immunosuppression, particularly the best candidates for IL6 blockade therapy. Moreover, the levels of procalcitonin can guide the antibiotic therapy decisions.
Lastly, IL6 might be a good marker to monitor COVID-19 patients and guide treatment. However, PCT and NLR are accurate and low-cost alternative markers considering that their blood levels are not influenced by corticosteroid treatment.
CRediT authorship contribution statement
Wafa Sayah: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing - original draft. Ismahane Berkane: Conceptualization, Methodology, Investigation, Validation, Writing - original draft. Imène Guermache: Investigation, Methodology, Writing - original draft. Mohamed Sabri: Investigation, Writing - original draft. Fatma Zahra Lakhal: Investigation, Writing - original draft. Sarah Yasmine Rahali: Investigation, Writing - original draft. Asma Djidjeli: Investigation, Writing - original draft. Lydia mahammad Lamara: Conceptualization, Writing - review & editing. Fatma Merah: Conceptualization, Writing - review & editing. Brahim Belaid: Conceptualization, Writing - review & editing. Lilya Berkani: Conceptualization, Writing - review & editing. Nouzha Zhor Lazli: Conceptualization, Writing - review & editing. Lylia Kheddouci: Conceptualization, Writing - review & editing. Ahmed Kadi: Resources, Visualization. Mourad Ouali: Resources, Visualization. Rachida Khellafi: Resources, Visualization. Dalila Mekideche: Resources, Visualization. Assia Kheliouen: Resources, Visualization. Réda Malek Hamidi: Resources, Visualization. Soraya Ayoub: Resources, Visualization. Nabil Beramtane raaf: Resources, Writing - review & editing. Fawzi Derrar: Resources, Writing - review & editing. Merzak Gharnaout: Resources, Writing - review & editing. Ines Allam: Supervision, Writing - review & editing. Réda Djidjik: Conceptualization, Methodology, Validation, Project administration, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko J.Y., Danielson M.L., Town M., Derado G., Greenlund K.J., Daily Kirley P., et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. 2020;2020(02) [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soraya G.V., Ulhaq Z.S. Social Science Research Network; Rochester, NY: 2020. Crucial Laboratory Parameters in COVID-19 Diagnosis and Prognosis: An Updated Meta-Analysis (Internet) Mar (cited 2020 Aug 30). Report No.: ID 3576912. Available from: https://papers.ssrn.com/abstract=3576912. [Google Scholar]
- 8.Ciccullo A., Borghetti A., Zileri Dal Verme L., Tosoni A., Lombardi F., Garcovich M., et al. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int. J. Antimicrob. Agents. 2020;56(2):106017. doi: 10.1016/j.ijantimicag.2020.106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight (Internet) 2020;5(10) doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.B. Zhou, J. She, Y. Wang, X. Ma, Utility of Ferritin, Procalcitonin, and C-reactive Protein in Severe Patients with 2019 Novel Coronavirus Disease, 2020 Mar 19 (cited 2020 Aug 30); Available from: https://europepmc.org/article/ppr/ppr122473.
- 12.J. Liu, Y. Liu, P. Xiang, L. Pu, H. Xiong, C. Li, et al. Neutrophil-to-Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. medRxiv, 2020 Feb 12;2020.02.10.20021584.
- 13.Xh Y., Ty L., Zc H., Yf P., Hw L., Sc Y., et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 14.W.H. Organization, Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. 2020 (cited 2020 Aug 30); Available from: https://apps.who.int/iris/handle/10665/330893.
- 15.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerboua K.E. NLR: a cost-effective nomogram to guide therapeutic interventions in COVID-19. Immunol. Invest. 2020:1–9. doi: 10.1080/08820139.2020.1773850. [DOI] [PubMed] [Google Scholar]
- 20.Yang A.-P., Liu J., Tao W., Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Yang, Wu Wenjing, Du Mingshan, et al., Neutrophil-to-Lymphocyte Ratio may Replace Chest Computed Tomography to Reflect the Degree of Lung Injury in Patients with Corona Virus Disease 2019 (COVID-19). 2020 Apr 16 [cited 2020 Aug 31]; Available from: https://www.researchsquare.com/article/rs-23201/v1.
- 22.B. Zhang, X. Zhou, Y. Qiu, F. Feng, J. Feng, Y. Jia, et al., Clinical characteristics of 82 death cases with COVID-19. medRxiv, 2020 Feb 27, 2020.02.26.20028191.
- 23.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C.-Y. Song, J. Xu, J.-Q. He, Y.-Q. Lu, COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv, 2020 Mar 8, 2020.03.05.20031906.
- 25.Creamer A.W., Kent A.E., Albur M. Procalcitonin in respiratory disease: use as a biomarker for diagnosis and guiding antibiotic therapy. Breathe. 2019;15(4):296–304. doi: 10.1183/20734735.0258-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;1(127):104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chim. Acta. Int. J. Clin. Chem. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuetz P., Bolliger R., Merker M., Christ-Crain M., Stolz D., Tamm M., et al. Procalcitonin-guided antibiotic therapy algorithms for different types of acute respiratory infections based on previous trials. Expert Rev. Anti. Infect. Ther. 2018;16(7):555–564. doi: 10.1080/14787210.2018.1496331. [DOI] [PubMed] [Google Scholar]
- 30.Tan C., Huang Y., Shi F., Tan K., Ma Q., Chen Y., et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol (Internet) 2020 doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., et al. Prognostic value of C-reactive protein in patients with COVID-19 (Internet) Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1101/2020.03.21.20040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Y. Gao, T. Li, M. Han, X. Li, D. Wu, Y. Xu, et al., Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. (Internet). (cited 2020 May 8); n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.25770. [DOI] [PMC free article] [PubMed]
- 34.Grifoni E., Valoriani A., Cei F., Lamanna R., Gelli A.M.G., Ciambotti B., et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M. Aziz, R. Fatima, R. Assaly, Elevated interleukin-6 and severe COVID-19: A meta-analysis, J. Med. Virol. (Internet). (cited 2020 Aug 27), n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.25948. [DOI] [PMC free article] [PubMed]
- 36.Herold T., Jurinovic V., Arnreich C., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv. 2020;2020(04) [Google Scholar]
- 37.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Internet) 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surbatovic M., Popovic N., Vojvodic D., Milosevic I., Acimovic G., Stojicic M., et al. Cytokine profile in severe gram-positive and gram-negative abdominal sepsis. Sci. Rep. 2015;5(1):11355. doi: 10.1038/srep11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calfee C.S., Delucchi K., Parsons P.E., Thompson B.T., Ware L.B., Matthay M.A. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Famous K.R., Delucchi K., Ware L.B., Kangelaris K.N., Liu K.D., Thompson B.T., et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am. J. Respir. Crit. Care Med. 2016;195(3):331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha P., Delucchi K.L., Thompson B.T., McAuley D.F., Matthay M.A., Calfee C.S., et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intens. Care Med. 2018;44(11):1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26(7):1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020;2020(02) [Google Scholar]
- 44.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect. Biol. (Internet) 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fielding C.A., McLoughlin R.M., McLeod L., Colmont C.S., Najdovska M., Grail D., et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008;181(3):2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 46.Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. (Internet) 2020;130(9) doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.J. Chen, Q. Jiang, X. Xia, K. Liu, Z. Yu, W. Tao, et al., Individual Variation of the SARS-CoV2 Receptor ACE2 Gene Expression and Regulation, 2020 Mar 12 [cited 2020 Aug 29]; Available from: https://www.preprints.org/manuscript/202003.0191/v1. [DOI] [PMC free article] [PubMed]
- 49.Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z., et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;1(95):332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.T. Liu, J. Zhang, Y. Yang, H. Ma, Z. Li, J. Zhang, et al., The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv, 2020 Mar 10, 2020.03.01.20029769.
- 51.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]