Abstract
Remdesivir (GS-5734), a viral RNA-dependent RNA polymerase (RdRP) inhibitor that can be used to treat a variety of RNA virus infections, is expected to be an effective treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. On May 1, 2020, The U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for remdesivir to treat COVID-19 patients. In light of the COVID-19 pandemic, this review presents comprehensive information on remdesivir, including information regarding the milestones, intellectual properties, anti-coronavirus mechanisms, preclinical research and clinical trials, and in particular, the chemical synthesis, pharmacology, toxicology, pharmacodynamics and pharmacokinetics of remdesivir. Furthermore, perspectives regarding the use of remdesivir for the treatment of COVID-19 are also discussed.
Keywords: Remdesivir (GS-5734), RdRP inhibitor, Coronavirus, COVID-19, Antiviral
Graphical abstract
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- AGMs
African green monkeys
- AHF
Argentine haemorrhagic fever
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ATP
Adenosine triphosphate
- Bn
Benzyl
- Ces1c
Carboxylesterase 1c
- COVID-19
Coronavirus disease 2019
- DBT
Delayed brain tumour
- EBOV
Ebola virus
- Et3N
Triethylamine
- EUA
Emergency use authorization
- EVD
Ebola virus disease
- FAS
Fas cell surface death receptor
- U.S. FDA
U.S. Food and Drug Administration
- FISH
Fluorescence in situ hybridization
- HAE
Human airway epithelial
- HCoV-229E
Human coronavirus 229E
- HCoV-OC43
Human coronavirus OC43
- HLE
Human lung epithelial
- HMVE
Human umbilical vein endothelial
- ICTV
International Committee on Taxonomy of Viruses
- IFNβ
Interferon beta
- IND
Investigational new drug
- INSERM
Institut National de la Santé Et de la Recherche Médicale
- (i-Pr)2NEt
N,N-diisopropylethylamine
- i-Pr2O
Isopropyl ether
- i-PrOAc
Isopropyl acetate
- i-PrMgCl·LiCl
Isopropylmagnesium chloride lithium chloride
- IV
Intravenous injection
- JUNV
Junin virus
- LASV
Lassa fever virus
- LF
Lassa fever
- LPV
Lopinavir
- MeCN
Acetonitrile
- MERS-CoV
Middle East respiratory syndrome-related coronavirus
- MHV
Murine hepatitis virus
- NDV
Newcastle disease virus
- NHBE
Normal human bronchiolar epithelial
- NHPs
Nonhuman primates
- NIAID
National Institute of Allergy and Infectious Diseases
- NIV
Nipah virus
- NMI
N-methylimidazole
- NMP
Nucleoside monophosphate
- NTP
Nucleoside triphosphate
- Nuc
Nucleoside
- PALM
Pamoja Tulinde Maisha
- PDCoV
Porcine deltacoronavirus
- PNP
4-nitrophenyl
- R&D
Research and development
- RdRP
RNA-dependent RNA polymerase
- Ph
Benzene
- RSV
Respiratory syncytial virus
- rt
Room temperature
- RTV
Ritonavir
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SC
Subcutaneous injection
- SFC
Supercritical fluid chromatography
- SoC
Standard of care
- TBAF
Tetrabutylammonium fluoride
- TBDMS
tert-Butyldimethylsilyl chloride
- tBuMgCl
tert-Butylmagnesium chloride
- TEAEs
Treatment-emergent adverse effects
- TFA
Trifluoroacetic acid
- TfOH
Trifluoromethanesulfonic acid
- THF
Tetrahydrofuran
- TMSCl
Trimethylsilyl chloride
- TMSCN
Trimethylsilyl cyanide
- TMSOTf
Trimethylsilyl trifluoromethanesulfonate
- TNF
Tumour necrosis factor
- TPCK
L-1-tosylamido-2-phenylethylchloromethyl ketone
- WHO
World Health Organization
1. Introduction
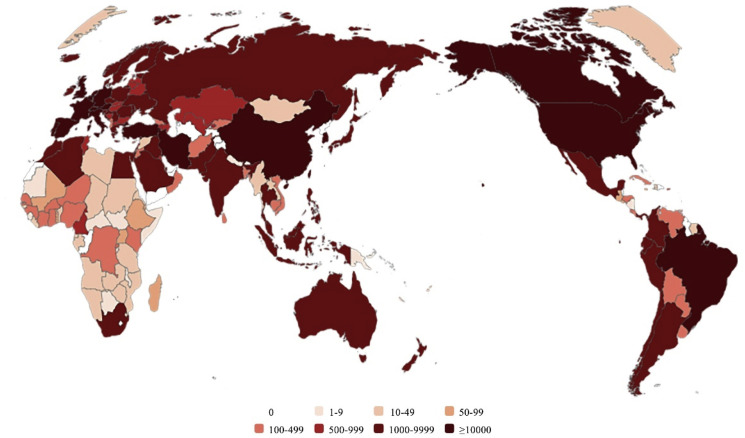
The coronavirus disease 2019 (COVID-19) coronavirus pneumonia epidemic, which began in December 2019, has developed into a global pandemic, and all populations (regardless of gender, age, or race) are generally susceptible to infection with this highly contagious virus [[1], [2], [3], [4], [5]]. The number of infected people is still increasing rapidly (Fig. 1 ). In fact, since March 2020, the number of COVID-19 patients has increased dozens of times, and these numbers in the countries affected by the epidemic have been rapidly growing [6,7]. Thus, the development of effective drugs or vaccines for the treatment or prevention of COVID-19 has become the most urgent task in the field of medical and drug research and development (R&D) throughout the world. Genetic and proteomic analyses of the new coronavirus have revealed that the nucleic acid similarity between the new coronavirus and severe acute respiratory syndrome coronavirus (SARS-CoV) is 79.5%. In addition, this new coronavirus and SARS-CoV share 94.6% similarity in the amino acid sequences of seven conserved nonstructural proteins [8,9]. Therefore, the International Committee on Taxonomy of Viruses (ICTV) determined that the new coronavirus belongs to the “severe acute respiratory syndrome-related coronavirus” species and named the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Fig. 1.
Map of the number of patients infected with SARS-CoV-2 worldwide (including death and recovery).
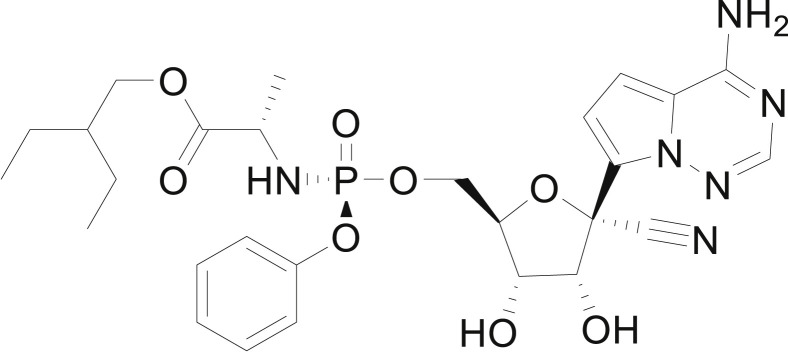
The common respiratory symptoms of COVID-19 include fever, cough, shortness of breath, and dyspnoea [[10], [11], [12]]. In the most severe cases, SARS-CoV-2 infections can lead to pneumonia, acute respiratory syndrome, and even death due to kidney and multiple organ failure [[13], [14], [15], [16]]. Currently, no drugs, monoclonal antibodies or vaccines have been approved for the treatment or prevention of SARS-CoV-2 infections [17,18], and physicians are considering “repurposing” several approved drugs to treat COVID-19 [[19], [20], [21], [22], [23], [24], [25], [26]]. In addition, remdesivir (GS-5374), which is a monophosphoramidate prodrug of an adenosine analogue (Fig. 2 ) and a competitive inhibitor of viral RNA-dependent RNA polymerase (RdRP), has been administered as a “compassionate medication” under the compassionate drug use principle even though it has not met the conditions for approval [[27], [28], [29]].
Fig. 2.
Remdesivir (GS-5734).
This review summarizes the current status, patents, mechanisms, preclinical research and clinical trial progress of remdesivir. Although the available data are not sufficient to elucidate the activity of remdesivir against SARS-CoV-2, the results showing the antiviral activity of remdesivir against other coronaviruses have provided researchers sufficient confidence and have resulted in high expectations. Laboratory and clinical studies on the use of remdesivir for the treatment of COVID-19 are still ongoing [17].
2. Current R&D status
Remdesivir is a broad-spectrum antiviral drug candidate developed by Gilead Sciences that remains at the R&D stage [30,31]. The primary clinical indication of remdesivir is for the treatment of Ebola virus (EBOV) infection, but the drug has not been approved for sale in any country in the world due to its poor efficacy in a phase III clinical trial for Ebola virus disease (EVD) [32].
Remdesivir has been demonstrated to exert activity against the coronavirus pathogens Middle East respiratory syndrome-related coronavirus (MERS-CoV) and SARS-CoV, which are structurally similar to SARS-CoV-2 [[33], [34], [35], [36]], and this finding was observed both in vitro and in vivo using animal models. Recent in vitro studies indicated that remdesivir is effective for the control of SARS-CoV-2 infection [20,37]. Specifically, remdesivir exhibited good efficacy after its administration to the first reported COVID-19 patient in the United States as a compassionate drug for experimental treatment [38]. At present, several governments, nongovernment organizations and regulatory authorities are administering remdesivir to patients with COVID-19 as an emergency treatment in the absence of any approved treatment options [39]. However, the results from individual cases of compassionate use are insufficient to evaluate the safety and efficacy of remdesivir in the treatment of COVID-19, and these findings could be validated by further prospective clinical trials.
Several clinical trials on the use of remdesivir as a treatment for COVID-19 have been registered at ClinicalTrials.gov [40]. The purpose of these clinical trials is to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19 [41]. In addition, two expanded access studies of remdesivir have been initiated with the aim of establishing a treatment protocol for COVID-19. These ongoing clinical trials are expected to blind unsealed and debrief participants within two months. According to the partially released clinical results, remdesivir exhibits efficacy in the treatment of COVID-19 [[42], [43], [44]].
Because remdesivir is a promising candidate that has the potential to effectively curb the COVID-19 epidemic, many companies have begun R&D of generic remdesivir drugs. However, since 2011, Gilead Sciences has applied for 166 remdesivir-related patents in 48 countries around the world, and these patents can be classified into seven patent families. In addition, the number of patents for which Gilead Sciences has applied reached its first peak (57 patents) during the MERS epidemic in 2015, and the second peak occurred during the EVD outbreak in 2018. It is believed that after the COVID-19 pandemic, there will be more therapeutic utilization patents related to the use of remdesivir for the treatment of RNA viruses.
At present, 11 patents across three patent families have been granted, and these patents are related to the core skeleton, analogues, derivatives, polymorphs, synthetic methods, dosage form and usage of remdesivir (Table 1 ). It is worth noting that Gilead Sciences registered a patent family for the treatment of Arenaviridae and Coronaviridae infections with remdesivir in 2016, and this patent family has been granted in the United States. The claims of this patent family described the treatment of all confirmed zoonotic coronaviruses with remdesivir.
Table 1.
Granted patents of remdesivir.
| No. | Title | Applicant | Patent | Publication Date | Subject Matter | Conditions |
|---|---|---|---|---|---|---|
| 1 | Methods for treating Filoviridae virus infections | Gilead Sciences, Inc. | US 2019275063 | 2019-09-12 | Methods of use | Viral infection |
| 2 | Methods for treating Filoviridae virus infections | Gilead Sciences, Inc. | US 9949994 | 2018-04-24 | Methods of use | Ebola virus disease |
| US 2016361330 | 2016-12-15 | Marburg virus haemorrhagic fever | ||||
| 3 | Methods for treating Filoviridae virus infections | Gilead Sciences, Inc. | US 9724360 | 2017-08-08 | Methods of use | Ebola virus disease |
| US 2016122374 | 2016-05-05 | Marburg virus haemorrhagic fever | ||||
| WO 2016069826 | 2016-05-06 | |||||
| JP 2017534614 | 2017-11-24 | |||||
| 4 | Methods for treating Filoviridae virus infections | Gilead Sciences, Inc. | WO 2016069827 | 2016-05-06 | Methods of use | Ebola virus disease |
| Marburg virus haemorrhagic fever | ||||||
| 5 | Methods for treating Filoviridae virus infections | Gilead Sciences, Inc. | US 10251898 | 2019-04-09 | Methods of use | Viral infection |
| US 2018311263 | 2018-11-01 | |||||
| 6 | Methods for treating Flaviviridae virus infections | Gilead Sciences, Inc. | WO 2017184668 | 2017-10-26 | Methods of use | Viral infection |
| 7 | Methods for treating Arenaviridae and Coronaviridae virus infections | Gilead Sciences, Inc. | US 2019255085 | 2019-08-22 | Methods of use | Coronavirus acute respiratory syndrome |
| 8 | Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate | Gilead Sciences, Inc. | KR 2019141747 | 2019-12-24 | Polymorphs | Viral infection |
| CN 110636884 | 2019-12-31 | Drug substances | ||||
| US 2018346504 | 2018-12-06 | |||||
| WO 2018204198 | 2018-11-08 | |||||
| 9 | Compositions comprising an RNA polymerase inhibitor and cyclodextrin for treating viral infections | Gilead Sciences, Inc. | WO 2019014247 | 2019-01-17 | Dosage forms and compositions | Viral infection |
| US 2019083525 | 2019-03-21 | |||||
| 10 | Methods and compounds for treating Paramyxoviridae virus infections | Gilead Sciences, Inc. | CN 103052631 | 2013-04-17 | Methods of use | Viral infection |
| US 2015111839 | 2015-04-23 | |||||
| KR 2013091743 | 2013-08-19 | |||||
| US 2012027752 | 2012-02-02 | |||||
| US 2019055251 | 2019-02-21 | |||||
| CN 105343098 | 2016-02-24 | |||||
| KR 2018012336 | 2018-02-05 | |||||
| WO 2012012776 | 2012-01-26 | |||||
| US 2015152116 | 2015-06-04 | |||||
| EP 2595980 | 2013-05-29 | |||||
| IN 201948034308 | 2019-10-18 | |||||
| JP 2013535453 | 2013-09-12 | |||||
| US 10065958 | 2018-09-04 | |||||
| 11 | 1′-Substituted carba-nucleoside analogues for antiviral treatment | Gilead Sciences, Inc. | CN 102015714 | 2011-04-13 | Drug substances | Hepatitis C |
| US 2009317361 | 2009-12-24 | |||||
| KR 2016138591 | 2016-12-05 | |||||
| KR 2011004883 | 2011-01-14 | |||||
| EP 2268642 | 2011-01-05 | |||||
| JP 2011521903 | 2011-07-28 | |||||
| EP 2937350 | 2015-10-28 | |||||
| WO 2009132135 | 2009-10-29 |
3. Mechanism
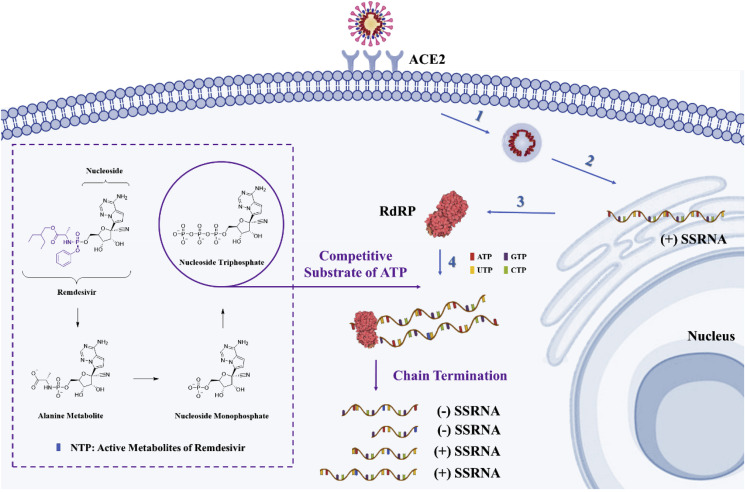
Remdesivir is an RdRP inhibitor that can achieve antiviral effects by inhibiting viral nucleic acid synthesis [26,[45], [46], [47]]. SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA virus [48], and the genomic replication process of RNA viruses is dominated by RdRP, which is encoded by the virus itself [[49], [50], [51], [52]]. After the virus invades the host cell, the viral genomic RNA is directly used as a template, and the host cell protein synthesis system is used for the translation of RdRP [[53], [54], [55], [56]]. RdRP is subsequently used to complete the transcriptional synthesis of the negative-strand subgenomic RNA, the synthesis of various structural protein-related mRNA, and the replication of viral genomic RNA [[57], [58], [59], [60]]. RdRP can accurately and efficiently synthesize tens of thousands of nucleotides and thus facilitates all other biological activities after the virus invades the host cell [[61], [62], [63]]. RdRP is an effective target of broad-spectrum antiviral drugs, and at present, most of the anti-coronavirus drugs targeting RdRP are nucleoside (Nuc) analogues or RNA interferons [64].
Remdesivir, which is a monophosphoramidate prodrug of an adenosine analogue, enters the host cell in the form of a prodrug, is converted to nucleoside monophosphate (NMP), and is then further dephosphorylated to active nucleoside triphosphate (NTP) [65]. NTP and adenosine triphosphate (ATP) have a similar structure and competitively bind to the viral RdRP with similar efficiencies. NTP is inserted into the RNA synthesis chain at position i through the recognition of RdRP, and this process leads to RNA chain termination at a position a few bases downstream of position i. This process is called “chain termination” and predominantly occurs at position i+5 [66]. Through this process, the replication of the virus is suppressed (Fig. 3 ).
Fig. 3.
Mechanism of remdesivir-mediated inhibition of coronavirus replication in host cells. The blue arrows and numbers represent the following essential viral infection steps: (1) attachment of the coronavirus particle to its specific receptor ACE2 and uncoating of the virus after adsorption into host cells; (2) establishment of replication organelles at the endoplasmic reticulum, where both genome replication and gene expression occur; (3) early translation of the incoming viral genome, which results in the generation of the RdRP; and (4) viral RNA replication. The purple wireframe and arrows represent the intracellular triphosphorylation of remdesivir and the competitive binding of NTP and ATP to RdRP to terminate viral RNA replication. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
As a broad-spectrum antiviral drug, remdesivir exerts a good antiviral effect on coronaviruses, including MERS-CoV and SARS-CoV. Previous studies have shown that almost 80% of the genome of SARS-CoV-2 is homologous to that of SARS-CoV, and almost all SARS-CoV-2 proteins are homologous to SARS-CoV proteins [67].
4. Synthesis of remdesivir
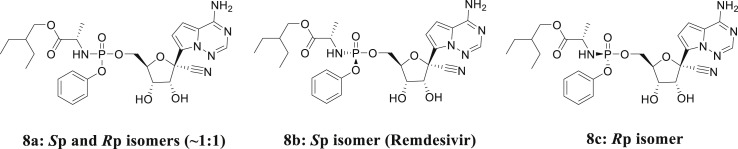
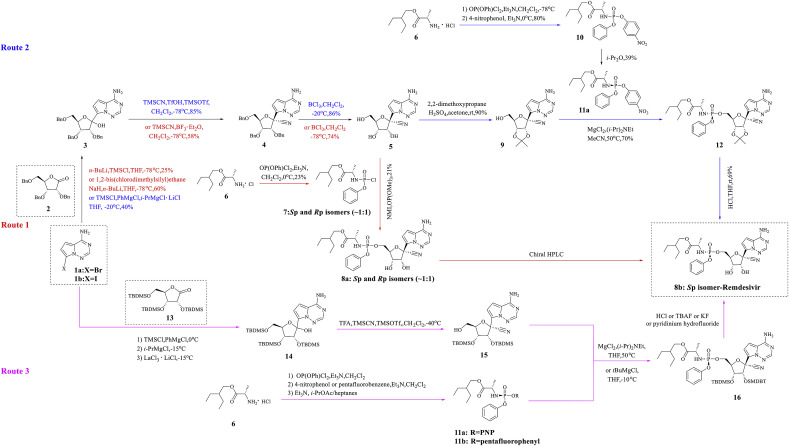
Gilead Sciences identified compound 8a (Fig. 4 , Sp and Rp isomers ∼1:1) through an anti-EBOV screening of selected compound libraries, and studies have shown that 8a exhibits potent activity against EBOV in HeLa and human umbilical vein endothelial (HMVE) cells [68]. According to a stereochemical perspective, 8a has two configurations, Sp-isomer (8b) and Rp-isomer (8c), and the difference between the two lies in the spatial configuration of 2-ethylbutyl-2-aminopropanoate and anisole. Among these, remdesivir is the Sp isomer (8b), and a previous study found that 8b exhibits higher selectivity and an appropriate therapeutic window that is suitable for antiviral therapy [68]. A comprehensive literature study identified three synthesis routes and various key synthetic intermediates, which are outlined in a scheme map (Fig. 5 ).
Fig. 4.
Diastereomers of remdesivir.
Fig. 5.
Diagram of remdesivir synthesis.
4.1. Route 1
Synthetic route 1 was reported by Richard L. Mackman [68]. First, 2,3,5-tri-O-benzyl-D-ribono-1,4-lactone (2) is combined with 7-bromopyrrolo[2,1-f][1,2,4]triazine-4-amine (1a), and trimethylsilyl chloride (TMSCl) and n-BuLi or NaH and 1,2-bis(chlorodimethylsilyl)ethane are then added for the N-protection of 1a. Subsequently, metal-halogen exchange is performed in tetrahydrofuran (THF) at −78 °C to obtain compound 3. Compound 3 is combined with trimethylsilyl cyanide (TMSCN) in CH2Cl2 and BF3 • Et2O at −78 °C, and the main product (R)-isomer (4) is then separated by chromatography. Three benzyl protecting groups are removed in CH2Cl2 and BCl3 to obtain compound 5. The diastereomer mixture of the phosphoramidyl chloride hydrochloride prodrug portion (7) is then coupled to obtain compound 8 at a yield of 21% in the form of an approximately 1:1 mixture of diastereomers. The two diastereomers were resolved by chiral HPLC to obtain the Sp isomer 8b, remdesivir.
4.2. Route 2
The second route is based on two studies by Richard L. Mackman [68] and Warren, T. K [65]. In this scheme, 7-iodopyrrolo[2,1-f][1,2,4]triazin-4-amine (1b) is sequentially treated with TMSCl, PhMgCl and isopropylmagnesium chloride lithium chloride (i-PrMgCl·LiCl) in THF at −20 °C and then condensed with lactone (2) to produce adduct 3. The cyanation of lactol (3) with TMSCN with trimethylsilyl trifluoromethanesulfonate (TMSOTf) and trifluoromethanesulfonic acid (TfOH) in CH2Cl2 at −78 °C generates the corresponding epimeric cyanide, and the desired (R)-isomer (4) is separated from this mixture through chromatography. The debenzylation of intermediate (4) in the presence of BCl3 in CH2Cl2 at −20 °C generates triol (5), whose diol moiety is protected with 2,2-dimethoxypropane using H2SO4 in acetone to yield the isopropylidene derivative (9). The condensation of primary alcohol (9) with amidophosphate (11a) in the presence of MgCl2 and N,N-diisopropylethylamine ((i-Pr)2NEt) in acetonitrile (MeCN) yields protected remdesivir (12), which is deprotected with aqueous HCl in THF to furnish the target remdesivir.
4.3. Route 3
In their disclosed patent family, Gilead Sciences described a third synthesis route for remdesivir [[69], [70], [71], [72], [73]]. The scheme is initiated by the N-protection of 7-iodopyrrolo[2,1-f][1,2,4]triazin-4-amine (1b) with TMSCl and PhMgCl, and this step is followed by metal-halogen exchange with i-PrMgCl·LiCl in THF at −15 °C. The subsequent addition of persilylated lactone (13) with LaCl3·2LiCl in THF at −15 °C yields the lactol intermediate (14), and the cyanation and selective desilylation of lactol (14) with TMSCN using TMSOTf and trifluoroacetic acid (TFA) in CH2Cl2 at −40 °C generates the corresponding epimeric cyanide. The desired (R)-isomer (15) is then separated from this mixture by preparative HPLC. The condensation of primary alcohol (15) with amidophosphate (11a) in the presence of MgCl2 and (i-Pr)2NEt in THF or with pentafluorophenyl protected derivative 11b in the presence of tert-butylmagnesium chloride (tBuMgCl) in THF produces silylated 8b, and this compound subsequently undergoes deprotection by means of HCl, tetrabutylammonium fluoride (TBAF), KF or pyridinium hydrofluoride to furnish the target remdesivir (8b).
The synthesis of intermediates 11a or 11b is a key step in all three routes. The condensation of 2-ethylbutyl l-alaninate hydrochloride (6) with phenyl dichlorophosphate using triethylamine (Et3N) in CH2Cl2 gives 2-ethylbutyl N-[chloro(phenoxy)phosphoryl]-l-alaninate (7), which upon coupling with 4-nitrophenol or pentafluorobenzene in the presence of Et3N in CH2Cl2 provides the corresponding aminophosphate derivatives. The resolution of the epimers through treatment with Et3N in isopropyl acetate (i-PrOAc)/heptanes or by recrystallization in isopropyl ether (i-Pr2O) at 0 °C yields (S)-aminophosphates 11a or 11b.
In summary, synthetic route 1 utilizes supercritical fluid chromatography (SFC) separation, which is difficult for large-scale industrial production, whereas synthetic routes 2 and 3 apply chiral synthesis to avoid SFC separation. However, these three synthesis routes have the common defect that the yield of the key intermediate 4 or 15 is relatively low, which affects the overall yield.
5. Pharmacology, toxicology and pharmacodynamics
Remdesivir exhibits potent antiviral activities against a variety of RNA viruses, including Coronaviridae, Filoviridae, Paramyxoviridae and Arenaviridae, in cultured cell lines and mouse and nonhuman primate models. However, the antiviral activity of remdesivir is selective, and this drug exerts no significant inhibitory effect on DNA viruses and certain RNA viruses, such as alphaviruses and retroviruses [65].
5.1. Coronaviridae
Coronaviruses are associated with a variety of diseases in humans and vertebrates and can cause respiratory, digestive, circulatory, and nervous system diseases [74,75]. Current research has demonstrated that coronaviruses, which exhibit family-wide genetic diversity in RdRP, are susceptible to inhibition by remdesivir. As a broad-spectrum antiviral drug, remdesivir functions against SARS-CoV-2, MERS-CoV, SARS-CoV, human coronavirus OC43 (HCoV-OC43), human coronavirus 229E (HCoV-229E), murine hepatitis virus (MHV) and porcine deltacoronavirus (PDCoV).
5.1.1. SARS-CoV-2
M. Wang et al. [20] tested the antiviral activities of remdesivir against SARS-CoV-2 in vitro. In Vero E6 cells, remdesivir effectively blocked SARS-CoV-2 infection at low concentrations (EC50 = 0.77 μM) and exhibited low cytotoxicity (CC50 > 100 μM) and a high selection index (SI > 129.87). In addition, the EC90 value of remdesivir against SARS-CoV-2 in Vero E6 cells was 1.76 μM. A. Pizzorno et al. [37] evaluated the antiviral potential of remdesivir against SARS-CoV-2 in both Vero E6 and human airway epithelial (HAE) models. The results showed that postinfection treatment with remdesivir exerts a very strong antiviral effect.
B. Williamson et al. [76] used a rhesus macaque model of SARS-CoV-2 infection to evaluate the effect of remdesivir treatment on COVID-19 outcome. In contrast to vehicle-treated animals, animals treated with remdesivir did not exhibit signs of respiratory disease and showed reduced pulmonary infiltrates on radiographs. Significant reductions in the virus titres in bronchoalveolar lavages were observed as early as 12 h after the first treatment was administered. At necropsy on day 7 after inoculation, the lung viral loads of the remdesivir-treated animals were significantly lower, and a clear reduction in lung tissue damage was observed. In summary, therapeutic treatment with remdesivir initiated early during infection exerts a clear clinical benefit in SARS-CoV-2-infected rhesus macaques. These data support the early initiation of remdesivir treatment for COVID-19 patients to prevent progression to severe pneumonia.
5.1.2. MERS-CoV
MERS-CoV can cause severe lower respiratory tract infections in people, and the fatality rate can reach as high as 36% [[77], [78], [79]], and remdesivir can inhibit MERS-CoV replication in multiple in vitro models. T.P. Sheahan et al. [80] evaluated the antiviral activity of remdesivir in continuous human lung epithelial (HLE) cells and found a IC50 value of 0.025 μM. Furthermore, primary HAE cell cultures constitute the most biologically relevant in vitro lung model. The antiviral activity of remdesivir against MERS-CoV in HAE culture yielded an IC50 value of 0.074 μM. A similar test was performed by M.L. Agostini et al. [81], who treated MERS-CoV-infected HAE cultures with remdesivir and obtained an EC50 value of 0.074 μM.
T.P. Sheahan et al. [80] reported a similar dose-dependent decrease in both the intracellular genomic and subgenomic viral RNA in MERS-CoV-infected HAE cells in response to increasing concentrations of remdesivir. Moreover, the in vitro toxicity was evaluated by measuring the expression of tumour necrosis factor (TNF) and the Fas cell surface death receptor (FAS) in remdesivir-treated HAE cells and normal human bronchiolar epithelial (NHBE) cells. However, a dose-dependent effect of remdesivir on increased transcription of these apoptotic factors was not observed. The effective concentration of remdesivir in HAE cells was found to be at least 100-fold lower than the observed cytotoxic concentration.
T.P. Sheahan et al. [82] compared the therapeutic efficacy of remdesivir against MERS-CoV with that of a combination of lopinavir, ritonavir and interferon beta (LPV/RTV-IFNβ). This study showed that remdesivir and IFNβ exert superior in vitro antiviral activities compared with those of LPV and RTV. In a mouse model, both prophylactic and therapeutic doses of remdesivir improved pulmonary function, reduced the lung viral loads and relieved severe lung pathology, and these therapeutic effects were better than those of the LPV/RTV-IFNβ combination.
E. de Wit et al. [83] tested the efficacy of prophylactic and therapeutic remdesivir treatment using a rhesus macaque primate model of MERS-CoV infection. The initiation of prophylactic remdesivir treatment 24 h prior to inoculation completely prevented the MERS-CoV-induced symptoms, strongly inhibited MERS-CoV replication in lung tissues, and prevented respiratory lesions. The initiation of therapeutic remdesivir treatment 12 h postinoculation also provided significant clinical benefits, including reduced clinical symptoms, decreased virus replication in the lungs, and decreased severity of lung lesions.
5.1.3. SARS-CoV
SARS-CoV is a zoonotic pathogen that can cause severe respiratory disease in humans [84,85]. M.L. Agostini et al. [81] assessed the activity of remdesivir against SARS-CoV in primary HAE cells and found an EC50 value of approximately 0.069 μM. In addition, the administration of remdesivir 24 h after SARS-CoV infection also resulted in decreased viral titres of SARS-CoV at 48 and 72 h postinoculation, and no measurable remdesivir-induced cellular toxicity was observed in the HAE cultures. In a mouse model of SARS-CoV pathogenesis, the prophylactic and early therapeutic administration of remdesivir significantly reduced the lung viral load, improved respiratory function, including bronchiolitis, perivascular inflammatory infiltration, and intra-alveolar oedema associated with diffuse alveolar damage, and improved prognosis [80].
5.1.4. HCoV-OC43
HCoV-OC43 is one of the seven coronaviruses to which humans are susceptible, and these viruses typically cause upper respiratory infection in children and severe lower respiratory infection in adult and elderly patients with underlying respiratory conditions [86,87]. Remdesivir is a potent antiviral drug against HCoV-OC43 (EC50 = 0.15 μM, CC50 > 10 μM, SI > 66). According to a HCoV-OC43 focus forming assay, a dose-dependent reduction in HCoV-OC43 antigen foci could be observed in remdesivir-treated Huh7 cells. As demonstrated by RNA fluorescence in situ hybridization (FISH) microscopy, the HCoV-OC43 FISH signal disappeared after treatment with the highest dose of remdesivir (0.25 μM), but decreases in the remdesivir dose were associated with gradual increases in virus replication to levels similar to those found in untreated cells. Therefore, remdesivir can reduce the HCoV-OC43 genomic RNA level in a dose-dependent manner [45].
5.1.5. HCoV-229e
HCoV-229E is a common pathogen that causes upper respiratory tract infection in humans [88]. Patients infected with HCoV-229E can also present with gastrointestinal diseases and nervous system-related symptoms [89]. Treatment with remdesivir leads to a dose-dependent reduction in HCoV-229E replication without drug-induced cytotoxicity (EC50 = 0.024 μM, CC50 > 10 μM, SI > 400) [45].
5.1.6. MHV
MHV can induce a highly infectious disease in mice, which exhibit various manifestations including hepatitis, encephalitis and enteritis [90]. M.L. Agostini et al. [81] studied the inhibitory activity of remdesivir against MHV in delayed brain tumour (DBT) cells and found that remdesivir exhibits concentration-dependent inhibition: after treatment with a remdesivir concentration higher than 0.5 μM, no virus could be detected in the DBT cells, and the EC50 and CC50 were 0.03 and 39 μM, respectively. An assessment of the effect of resistance to remdesivir on viral fitness indicated that mutations that confer resistance to remdesivir impair the competitive fitness of MHV. In addition, drug resistance could be overcome by increased concentrations of remdesivir. This study supports the development of remdesivir as a broad-spectrum therapeutic.
5.1.7. PDCoV
PDCoV is an emerging swine enteropathogenic coronavirus that causes acute diarrhoea and mortality in piglets [91]. A.J. Brown et al. [45] reported that remdesivir is highly effective against PDCoV. In Huh7 cells cultured in L-1-tosylamido-2-phenylethylchloromethyl ketone (TPCK) trypsin-containing and serum-free media, PDCoV replication was reduced by remdesivir in a dose-dependent manner, and the EC50 value was 0.02 μM.
5.2. Filoviridae
Filoviridae is a type of single-stranded, negative-stranded RNA virus named for its filamentous appearance [92]. Currently, only two genera of the Filoviridae family have been identified: EBOV and Marburg virus. T.K. Warren et al. [65] studied the efficacy of remdesivir against filoviruses in cellular and animal models. As demonstrated using cell-based assays, remdesivir exhibits activity against a broad range of filoviruses, including Marburg virus and several variants of EBOV, such as Ebola Makona virus, Ebola Kikwit virus, Bundibugyo Ebola virus and Sudan viruses. Currently, the in vivo and in vitro activities of remdesivir against EBOV are supported by relatively sound research.
5.2.1. EBOV
EBOV is the pathogen that causes EVD in humans and nonhuman primates [93]. The initial EVD epidemic outbreak in West Africa included a total of 28,616 patients and exhibited a mortality rate of nearly 50% between December 2013 and January 2016 [94,95]. T.K. Warren et al. [65] showed that remdesivir could inhibit EBOV replication in multiple human cell lines, including endothelial cells, liver Huh-7 cells, primary macrophages, foreskin fibroblasts and HeLa cells, with EC50 values ranging from 0.06 to 0.14 μM.
Subsequent studies using the rhesus monkey model of EBOV infection revealed that the once-daily intravenous administration of 10 mg/kg remdesivir for 12 days resulted in profound suppression of EBOV replication and protected 100% of the EBOV-infected animals by preventing lethal syndrome and ameliorating clinical symptoms and pathophysiological markers. Even when the remdesivir treatments were initiated three days after viral exposure, systemic viral RNA was detected in only two of the six treated rhesus monkeys. These experiments showed that remdesivir treatment provided substantial protection in nonhuman primates (NHPs) after EBOV exposure.
5.3. Paramyxoviridae
Paramyxoviridae harbour single-stranded, negative-strand RNA, and Paramyxoviridae viruses are characterized by a unique affinity for mucin, a high mutation rate and a short incubation time [96,97]. Among these viruses, Nipah virus (NIV), respiratory syncytial virus (RSV) and Newcastle disease virus (NDV) can be contagious to humans and animals, and studies conducted to date have revealed that remdesivir exerts inhibitory activities against NIV and RSV [65,98].
5.3.1. NIV
NIV is an emerging pathogen in the Paramyxoviridae family [99]. In infected individuals, NIV causes a range of illnesses ranging from asymptomatic (subclinical) infection to acute respiratory illness and fatal encephalitis [100]. M.K. Lo et al. [98] reported the antiviral activities of remdesivir against NIV. According to a virus titre reduction assay, an antigen reduction assay, a reporter assay and a cytopathic effect assay, the EC50 values of remdesivir against NIV ranged from 0.029 to 0.047 μM. Further research indicated that submicromolar concentrations of remdesivir inhibit NIV minigenome replication in a dose-dependent manner.
M.K. Lo et al. [101] tested the efficacy of remdesivir against NIV using African green monkeys (AGMs). AGMs were inoculated with a lethal dose of NIV, and treatment based on once-daily intravenous administrations of remdesivir was initiated 24 h after inoculation and continued for 12 days. Mild respiratory symptoms were observed in two of the four treated animals, whereas all the control animals developed symptoms of severe respiratory disease. Finally, all the remdesivir-treated animals survived. This in vivo experiment indicated that remdesivir is a promising antiviral therapeutic that can protect AGMs from lethal NIV infection.
5.3.2. RSV
RSV is the most common pathogen that causes acute lower respiratory tract infections in infants worldwide [102]. T.K. Warren et al. [65] utilized replication-competent RSV-infected cells to determine the antiviral activity of remdesivir, and the EC50 and EC90 values were found to equal 0.019 and 0.051 μM, respectively. Among the currently reported data regarding the effective concentrations against different RNA viruses, the lowest EC50 value of remdesivir was found against RSV, which likely indicates that remdesivir exerts its most potent in vitro antiviral activity against RSV.
5.4. Arenaviridae
Currently, four of the thirteen reported arenaviruses are known to cause human viral haemorrhagic fever [103]. Among the four pathogenic arenaviruses, Junin virus (JUNV) can cause severe argentine haemorrhagic fever (AHF) with 20%–30% mortality rates, whereas Lassa fever virus (LASV) can cause fatal Lassa fever (LF) in humans. T.K. Warren et al. [65] reported that the EC50 values of remdesivir against JUNV and LASV were 0.47 and 1.48 μM, respectively. In addition, the EC90 values for the remdesivir-mediated inhibition of JUNV and LASV replication were 1.33 and 2.80 μM, respectively.
In summary, these studies on the antiviral activity of remdesivir further enhance the cognitive depth, confidence and ability to using this drug to combat the emerging SARS-CoV-2.
6. Pharmacokinetics
The high serum esterase activity in many rodent models degrades the remdesivir promoiety and adversely impacts its pharmacokinetic profile. Thus, NHPs are considered the most suitable animal model for evaluating the pharmacokinetic properties of remdesivir [104]. Pharmacokinetics experiments using monkeys orally administered remdesivir indicated a low bioavailability due to the high first-pass hepatic extraction of phosphoramidates. Moreover, the oral administration of this drug to coronavirus-infected patients might not be ideal because severe gastrointestinal symptoms could limit the effective absorbed dose. Therefore, intravenous injection (IV) or subcutaneous injection (SC) is the appropriate administration route of remdesivir [68].
T.K. Warren et al. [65] studied the pharmacokinetics, metabolism and distribution of remdesivir in both healthy rhesus monkeys and healthy crab-eating monkeys. Rhesus monkeys were intravenously injected with remdesivir (10 mg/kg), and the half-life of remdesivir in plasma was short, namely, T1/2 = 0.39 h. After its administration, remdesivir was rapidly distributed into peripheral blood mononuclear cells and converted to the main metabolite NTP within 2 h, and the half-life of NTP in plasma was 14 h. The plasma concentration of NTP was continuously higher than the EC50 and exerted antiviral effects within 24 h. After the intravenous injection of remdesivir (10 mg/kg) into crab-eating monkeys, remdesivir metabolites distribute to the testis, epididymis, eyes and central nervous systems at different concentrations. A low concentration is detected in the central nervous system at the early stages of treatment, and 7 days after injection, the metabolite concentration was higher in the cerebrospinal fluid and even higher in plasma.
T.P. Sheahan et al. [80] studied the pharmacokinetics of remdesivir in SARS-CoV-infected secreted carboxylesterase 1c (Ces1c) gene-knockout mice. These mice cannot secrete Ces1c to improve the plasma stability of remdesivir. When subcutaneously administered at 50 mg/kg once a day or at 25 mg/kg twice a day, the obtained pharmacokinetic profiles demonstrated characteristics similar to those previously observed in rhesus monkeys. Both administration strategies improved the weight loss of the mice due to SARS-CoV and significantly decreased the viral titres in the lungs, although the twice-daily administration of 25 mg/kg exerted better results. The half-life of NTP in mouse lung tissue is approximately 3 h, whereas that in primate lung tissue is 14 h. A 25 mg/kg twice-daily administration regimen can maintain the effective concentration in the knockout mice during the administration interval.
Additional studies have shown that the peak concentration of NTP in the plasma of rhesus monkeys after the administration of 10 mg/kg remdesivir is 30–40 μmol/L [65], whereas that in the lung tissue of mice is 2–10 μmol/L [80]. In summary, the pharmacokinetic data indicate that the experimental dose of remdesivir could provide sustained and effective intracellular NTP levels and thus exerts intracellular antiviral effects in sites of viral accumulation.
7. Clinical trials
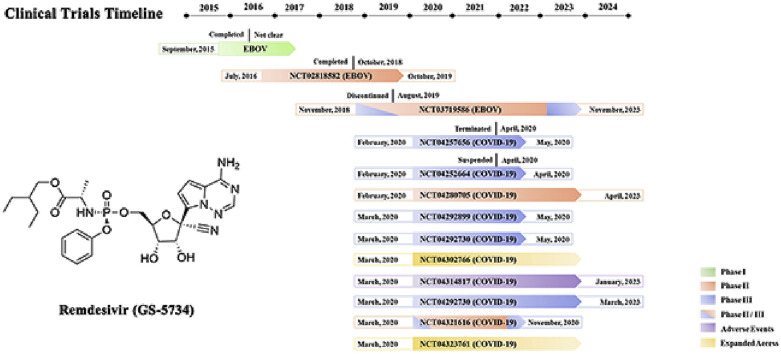
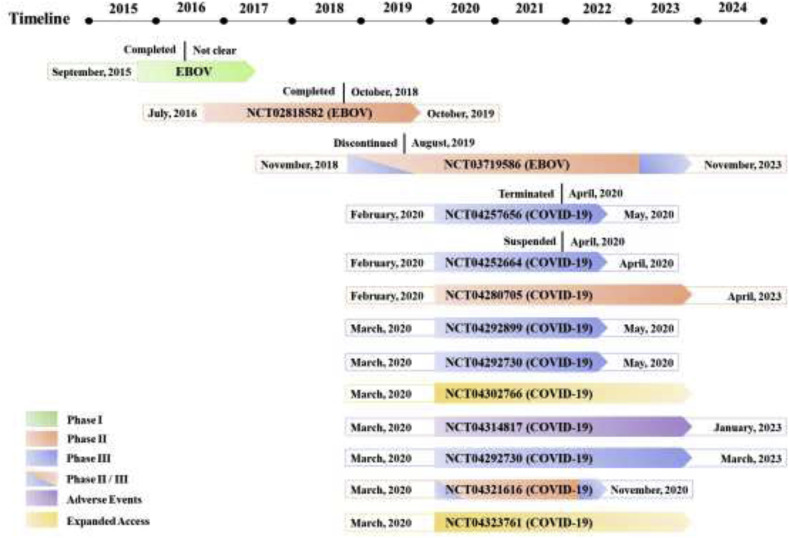
Remdesivir has been developed for the treatment of EVD by Gilead Sciences for nearly 10 years and remains in clinical trials. The clinical dates of the phase I and II trials on the treatment of EVD with remdesivir have not been fully disclosed. During the EVD phase III trial, the mortality rate was not significantly decreased by remdesivir treatment, and the curative effect did not reach a statistically significant difference. Therefore, the clinical development of remdesivir was suspended until the outbreak of COVID-19. Phase II and III clinical trials on the treatment of COVID-19 are currently being performed based on the compassionate drug use principle. A phase I trial of remdesivir was not conducted partially because a phase I trial was performed during the EVD epidemic (Fig. 6 ).
Fig. 6.
Information on the clinical trials of remdesivir detailed in ClinicalTrials.gov.
7.1. EVD
7.1.1. Phase I trial
The purpose of the blinded, randomized, and placebo-controlled phase I trial of remdesivir was to evaluate its safety, tolerability, and pharmacokinetics after its IV administration in healthy adult volunteers. The single-dose IV administration of 3–225 mg of remdesivir was well tolerated and did not lead to dose-limiting toxicity. The multiple-dose IV administration of 150 mg of remdesivir once daily for 7 or 14 days was generally well tolerated. No subjects experienced grade 3 or 4 treatment-emergent adverse effects (TEAEs) or exhibited laboratory test abnormalities during the clinical trial. Several participants showed reversible grade 1 or 2 elevations in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) but no abnormalities in total bilirubin, alkaline phosphatase (ALP), or albumin. Remdesivir did not cause any adverse effects on renal or liver function in either the single-dose study or the multiple-dose study.
7.1.2. Phase II trial
The phase II clinical trial [NCT02818582] evaluated the antiviral activity, longer-term EVD clearance and safety of remdesivir in male EVD survivors who exhibit evidence of EVD persistence in their semen [105]. This study was a double-blind, randomized, two-phase (treatment and longer-term follow-up), two-arm trial of remdesivir versus placebo in 38 male EVD survivors. The participants were randomized 1:1 to receive either 100 mg of remdesivir or placebo once a day via IV administration for 5 days. The antiviral activity, safety and tolerability of remdesivir were assessed during the treatment phase. The longer-term clearance of EVD was assessed during the 5-month follow-up phase. In 2019, a phase II trial of remdesivir for the treatment of EVD was performed, but the clinical results have not been released.
7.1.3. Phase II/III trial
On November 21, 2018, a phase II/III trial [NCT03719586] of four investigational therapies for EVD was conducted in the Democratic Republic of Congo. The researchers evaluated the safety and efficacy of the triple monoclonal antibody ZMapp (the control group), the antiviral agent remdesivir, the single monoclonal antibody MAb114, and the triple monoclonal antibody REGN-EB3 in 681 EVD carriers through the Pamoja Tulinde Maisha (PALM) trial. All four agents used in the trial were administered intravenously. The primary end point was the fatality rate after 28 days, and the minor end point was the time from enrolment to the time when the Ebola nucleic acid test results became negative.
The results showed that the case fatality rate of the patients in the remdesivir group was 53%, which was significantly higher than those in the MAb114 (35%) and REGN-EB3 groups (33%) and higher than that in the ZMapp control group (49%) [32]. The median viral nucleic acid negative conversion time in the MAb114 (16 days) and REGN-EB3 groups (15 days) was shorter than that in the ZMapp group (27 days). Because the case fatality rate of the remdesivir group was higher than 50%, the median viral nucleic acid negative conversion time might be longer than 28 days. The results suggested that the anti-EBOV efficacy of remdesivir did not meet expectations, thus, an in-depth study on the treatment of EVD with remdesivir has not been performed.
7.2. COVID-19
7.2.1. Phase II trial
The U.S. National Institute of Allergy and Infectious Diseases (NIAID) has initiated a phase II [NCT04280705] trial of remdesivir. This study is an adaptive, randomized, double-blind, placebo-controlled trial that aims to evaluate the safety and efficacy of remdesivir in hospitalized adult patients diagnosed with SARS-CoV-2 infection. The study is also a multicentre trial that will be conducted at up to 50 sites worldwide. The participants were assigned 1:1 to receive either placebo or remdesivir. The regimen involves the intravenous administration of 200 mg of remdesivir on Day 1 followed by a maintenance regimen of 100 mg per day during the 10-day course of hospitalization. This study is expected to end on April 1, 2023.
7.2.2. SOLIDARITY trial (phase II/III trial)
The World Health Organization (WHO) has developed a master protocol for COVID-19 clinical trials in an effort to standardize the design of clinical trials conducted around the world such that researchers can compile data obtained from different clinical trials and obtain clearer and stronger evidence. On March 19, the WHO announced that it and its partners will jointly launch a large-scale global clinical trial, called the SOLIDARITY trial, to evaluate the clinical efficacy and safety of different investigational therapeutics in hospitalized patients with COVID-19. The SOLIDARITY trial is a global, multi-arm, randomized, controlled, adaptive design clinical trial that included a control group and four treatment groups. The patients in the control group will receive the standard of care (SoC) in their country, and the patients belonging to the other four treatment groups will be treated with remdesivir, LPV/RTV, LPV/RTV-IFNβ or hydroxychloroquine. Each country and hospital can choose one or more drugs for the clinical trial according to their own circumstances.
Oslo University Hospital joined the SOLIDARITY trial and initiated a multicentre, adaptive, randomized, open clinical trial [NCT04321616] to evaluate the safety and efficacy of hydroxychloroquine, remdesivir and SoC in hospitalized adult patients diagnosed with COVID-19. This trial will follow the core WHO protocol but has additional efficacy, safety and explorative endpoints.
7.2.3. Phase III trial
Gilead Sciences initiated two phase III clinical studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19. These randomized, open-label, multicentre studies will enrol approximately 1000 patients at medical centres primarily across Asian countries and in other countries around the world with high numbers of diagnosed patients beginning in March 2020. The study [NCT04292899] will evaluate the safety and efficacy of both a 5-day (200 mg of remdesivir on day 1 and 100 mg of remdesivir on days 2, 3, 4, and 5) and a 10-day (200 mg of remdesivir on day 1 and 100 mg on days 2–10) remdesivir dosing regimen accompanied by SoC in patients with severe manifestations of COVID-19. Another study [NCT04292730] will utilise the same remdesivir dosing regimens with the SoC for patients with moderate COVID-19 manifestations and will compare this treatment with SoC alone. The primary end point of both trials will be the proportion of participants whose body temperature and oxygen saturation return to normal levels on the 14th day, and the secondary end point will be the proportion of emergency adverse events that lead to drug withdrawal during the study. These two clinical trials are expected to be completed in May 2020.
In addition, Chinese health authorities have initiated two clinical trials of remdesivir as a candidate drug using patients who have been infected with SARS-CoV-2. The two studies are being coordinated by the China-Japan Friendship Hospital and conducted at multiple sites in Hubei Province. The study [NCT04257656] recruited patients who are suffering from severe clinical manifestations of COVID-19, such as difficulty breathing that requires supplemental oxygen. The treatment plan involves a 200-mg loading dose of remdesivir on day 1 followed by a 100-mg once-daily maintenance dose administered IV for 9 days. Another study [NCT04252664] includes patients with confirmed SARS-CoV-2 infection who were hospitalized but exhibited mild clinical manifestations and did not require any breathing support. The dosing regimens were consistent with those used in the other study [NCT04257656].
Additionally, the Institut National de la Santé Et de la Recherche Médicale (INSERM) in France initiated a study [NCT04321616] evaluating remdesivir and other potential treatments on March 20, 2020, using the master protocol developed by the WHO. COVID-19 patients will be randomized among four treatment arms, and each treatment will be administered in addition to the usual SoC in the participating hospital. The treatment drugs will include remdesivir, LPV/RTV, LPV/RTV-IFNβ and hydroxychloroquine, and the primary endpoint will be the subject’s clinical status (on a 7-point ordinal scale) at day 15.
7.2.4. Studies of adverse events
The outbreak of COVID-19 encouraged the initiation of several clinical trials and treatment experiments worldwide. Groupe Hospitalier Pitie-Salpetriere initiated a clinical trial [NCT04314817] of adverse events related to the treatments used to treat COVID-19 since March 17, 2020. This study investigates the reports of adverse events related to the molecules used for treatment, which include but are not limited to LPV/RTV, chloroquine, azithromycin, remdesivir and IFNβ.
7.2.5. Expanded access studies
The U.S. Army Medical Research and Development Command initiated an expanded access study of remdesivir [NCT04302766] following the U.S. Food and Drug Administration’s (FDA) rapid review and acceptance of Gilead Sciences’ investigational new drug (IND) filing on March 10, 2020. Subsequently, Gilead Sciences also initiated an expanded access trial of remdesivir for the treatment of SARS-CoV-2 infection on March 27 [NCT04323761]. The primary intent of these expanded access studies is to provide treatment for COVID-19 patients rather than to collect data about remdesivir. Patients who cannot participate in a clinical trial of remdesivir but have severe COVID-19 may benefit from treatment with remdesivir.
8. Future perspectives
The clinical trials of remdesivir have attracted worldwide attention. On March 12, 2020, S.A. Kujawski and colleagues first released a small sample clinical report on medRxiv.org [106], and this report describes the epidemiological and clinical course and virological characteristics of the first twelve COVID-19 patients diagnosed in the United States. All the patients experience good prognosis and were declared cured or improving. Three of the twelve patients were treated with remdesivir combined with antibiotics on days 7–10, 11–15, or 11–20 and exhibited improved respiratory symptoms. However, the patients who received remdesivir experienced gastrointestinal side effects. F.-X. Lescure et al. [43] reported the relevant characteristics of the first confirmed cases of COVID-19 in Europe, and three of the patients were treated with remdesivir. During their treatment, these patients experienced varying degrees of viral load declines accompanied by an elevation in the alanine aminotransferase level and a rash. However, no conclusions could be drawn on the potential efficacy of remdesivir on the treatment of SARS-CoV-2 infections, and whether this adverse event is related to remdesivir remains unclear.
J. Grein et al. [42] reported that the majority of patients with severe coronavirus symptoms treated with remdesivir showed clinical improvement in a compassionate use trial. specifically, in this cohort of patients, clinical improvement was observed in 36 of 53 patients (68%), and seven patients (13%) died. The most common adverse events were increased hepatic enzymes, diarrhoea, rash, renal impairment, and hypotension. These compassionate-use data have some limitations but are of great significance to patients who have achieved symptom improvement.
Unlike general antiviral drugs, such as oseltamivir, which are more effective at the early stage of viral infection, a recent study demonstrated that remdesivir could also be effective for the treatment of COVID-19 patients with severe clinical symptoms [44].
In addition, two phase III clinical trials of remdesivir led by Chinese health authorities are targeting mainly severe COVID-19 adult cases [NCT04257656, NCT04252664], and the results are expected to be disclosed on April 27th, 2020. However, because the epidemic of COVID-19 has recently been well controlled in China, no more eligible patients could be enrolled. As a result, these two clinical studies have been suspended. The clinical results of the severe group [107] showed that the treatment with remdesivir did not shorten the duration of illness or reduce mortality from COVID-19 compared with the placebo in hospitalized patients in the trial. However, while not statistically significant, analysis of the pre-specified secondary outcomes found that the time to clinical improvement and the duration of invasive mechanical ventilation were shorter in people who were treated with remdesivir within 10 days of showing symptoms compared with the durations in people who received standard care. In summary, the true effectiveness of the antiviral drug remdesivir remains unclear, and future studies of remdesivir are needed to better understand its potential effectiveness.
In addition, it was reported that the phase trial [NCT04280705] of remdesivir for the treatment of COVID-19 initiated by the NIAID has obtained promising data, and the trial has met its primary endpoint. Meanwhile, Gilead Sciences announced positive results from the phase III trial [NCT04292899] evaluating 5-day and 10-day dosing durations of remdesivir in hospitalized patients with severe COVID-19. The study demonstrated that patients receiving a 10-day treatment course of remdesivir achieved similar improvements in clinical status compared with those taking a 5-day treatment course (odds ratio: 0.75 [95% CI 0.51–1.12] on day 14).
Recently, due to the public health emergency, the FDA has granted an emergency use authorization (EUA) for the investigational antiviral remdesivir for the treatment of hospitalized patients with severe COVID-19 in the United States. Subsequently, the Japanese Ministry of Health, Labour and Welfare granted regulatory approval of remdesivir as a treatment for SARS-CoV-2 infection, through an exceptional approval pathway. These approvals are based on clinical data from the NIAID’s global phase trial, Gilead Sciences’ phase III trial in patients with severe COVID-19, and the available data from the compassionate use program. In addition, multiple additional clinical trials are ongoing to generate more data on the safety and efficacy of remdesivir as a treatment for COVID-19.
The present review provides a comprehensive summary of the available information related to the development and clinical trials of remdesivir. It is undeniable that the development of effective anti-SARS-CoV-2 drugs over a short period of time faces substantial challenges and unknown risks. However, R&D and clinical trials of other drugs in addition to the promising antiviral candidate drug remdesivir are also progressing in parallel. Researchers from scientific research institutions and pharmaceutical companies as well as front-line physicians should strengthen their cooperation to jointly promote the pharmaceutical, preclinical and clinical trials of relevant anti-coronavirus drugs.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81602967 and 81803784), the China Postdoctoral Science Foundation (Grant Nos. 2016M592898XB and 2019M663921XB), the Basic Research Program of Natural Science of Shaanxi Province (Grant Nos. 2019JQ-779, 2020CGXNG-044, and 19JC006), the Basic Research Plan of the Education Department of Shaanxi Province (Grant No. 19JC006), and the College Students’ Innovative Entrepreneurial Training Program (Grant Nos. 201510708172, 201610708019, and 2019107080827). The authors are very grateful for the free language polishing service provided by American Journal Experts.
References
- 1.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed.: Atenei Parmensis. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., Fricchione M.J., Chugh R.K., Walblay K.A., Ahmed N.S., Stoecker W.C., Hasan N.F., Burdsall D.P., Reese H.E., Wallace M., Wang C., Moeller D., Korpics J., Novosad S.A., Benowitz I., Jacobs M.W., Dasari V.S., Patel M.T., Kauerauf J., Charles E.M., Ezike N.O., Chu V., Midgley C.M., Rolfes M.A., Gerber S.I., Lu X., Lindstrom S., Verani J.R., Layden J.E., Illinois C.-I. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heymann D.L., Shindo N., Bedford J., Enria D., Giesecke J., Heymann D., Ihekweazu C., Kobinger G., Lane C., Memish Z., Myoung-don O., Sall A.A., Ungchusak K., Wieler L., W.H.O.S.T.A.G. Infect COVID-19: what is next for public health? Lancet. 2020;395:542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng S.-Q., Peng H.-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020;9:575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Bruenink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:23–30. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., Liao J., Yang H., Hay W., Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., Tsoi H.-W., Lo S.K.-F., Chan K.-H., Poon V.K.-M., Chan W.-M., Ip J.D., Cai J.-P., Cheng V.C.-C., Chen H., Hui C.K.-M., Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J.a., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D., Chen B., Zhang Z., Guan W., Ling Z., Jiang R., Hu T., Ding Y., Lin L., Gan Q., Luo L., Tang X., Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imag. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295:16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug discoveries & therapeutics. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 18.Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J. Infect. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Q., Huang W., Zhao J., Yang Z. Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo. J. Ethnopharmacol. 2020;252:112584. doi: 10.1016/j.jep.2020.112584. [DOI] [PubMed] [Google Scholar]
- 24.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X. 2020. Favipiravir versus Arbidol for COVID-19: a Randomized Clinical Trial. medRxiv. Published online 15 April 2020. [DOI] [Google Scholar]
- 25.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elfiky A.A. 2020. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp): A Molecular Docking Study, Life sciences. 117592-117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science (New York, N.Y.) 2020;367:1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 28.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 29.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O., Mackman R.L., Hui H.C., Perron M., Ray A.S., Cihlar T., Nichol S.T., Spiropoulou C.F. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7:7. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMullan L.K., Flint M., Chakrabarti A., Guerrero L., Lo M.K., Porter D., Nichol S.T., Spiropoulou C.F., Albarino C. Characterisation of infectious Ebola virus from the ongoing outbreak to guide response activities in the Democratic Republic of the Congo: a phylogenetic and in vitro analysis. Lancet Infect. Dis. 2019;19:1023–1032. doi: 10.1016/S1473-3099(19)30291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulangu S., Dodd L.E., Davey R.T., Jr., Mbaya O.T., Proschan M., Mukadi D., Manzo M.L., Nzolo D., Oloma A.T., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.-J., Sivahera B., Camara M., Kojan R., Walker R., Dighero-Kemp B., Cao H., Mukumbayi P., Mbala-Kingebeni P., Ahuka S., Albert S., Bonnett T., Crozier I., Duvenhage M., Proffitt C., Teitelbaum M., Moench T., Aboulhab J., Barrett K., Cahill K., Cone K., Eckes R., Hensley L., Herpin B., Higgs E., Ledgerwood J., Pierson J., Smolskis M., Sow Y., Tierney J., Sivapalasingam S., Holman W., Gettinger N., Vallee D., Nordwall J., Grp P.W., P.C.S. Team. Randomized A. Controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoenen T., Groseth A., Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat. Rev. Microbiol. 2019;17:593–606. doi: 10.1038/s41579-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 34.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzorno A., Padey B., Julien T., Trouillet-Assant S., Traversier A., Errazuriz-Cerda E., Fouret J., Dubois J., Gaymard A., Lescure X. Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia. bioRxiv. 2020 doi: 10.1101/2020.03.31.017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K., Washington State-nCo V.C.I. First case of 2019 novel coronavirus in the United States, new England. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020;55:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NLM, Clinical Trials of Remdesivir, in: ClinicalTrials.gov, https://clinicaltrials.gov/ct2/results?recrs=&cond=&term=remdesivir&cntry=&state=&city=&dist=, 2020 (accessed 20 April 2020).
- 41.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. 2020;48:1–26. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 42.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V., Houhou-Fidouh N., Valette M., Mailles A., Lucet J.-C., Mentre F., Duval X., Descamps D., Malvy D., Timsit J.-F., Lina B., van-der-Werf S., Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillaker E., Belfer J.J., Bondici A., Murad H., Dumkow L.E. Delayed Initiation of Remdesivir in a COVID-19 Positive Patient. Pharmacotherapy. 2020;40:592–598. doi: 10.1002/phar.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., III, Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin W., Mao C., Luan X., Shen D.-D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M. Structural Basis for the Inhibition of the RNA-Dependent RNA Polymerase from SARS-CoV-2 by Remdesivir. Science. 2020 doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X., Liu Y., Weiss S., Arnold E., Sarafianos S.G., Ding J. Molecular model of SARS coronavirus polymerase: implications for biochemical functions and drug design. Nucleic Acids Res. 2003;31:7117–7130. doi: 10.1093/nar/gkg916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terret-Welter Z., Bonnet G., Moury B., Gallois J.L. Analysis of tomato spotted wilt virus RNA-dependent RNA polymerase adaptative evolution and constrained domains using homology protein structure modelling. J. Gen. Virol. 2020;101:334–346. doi: 10.1099/jgv.0.001380. [DOI] [PubMed] [Google Scholar]
- 51.Blevins T., Podicheti R., Pikaard C.S. Analysis of siRNA precursors generated by RNA polymerase IV and RNA-dependent RNA polymerase 2 in Arabidopsis. Methods Mol. Biol.(Clifton, N.J.) 2019;1933:33–48. doi: 10.1007/978-1-4939-9045-0_2. [DOI] [PubMed] [Google Scholar]
- 52.Ben Ouirane K., Boulard Y., Bressanelli S. The hepatitis C virus RNA-dependent RNA polymerase directs incoming nucleotides to its active site through magnesium-dependent dynamics within its F motif. J. Biol. Chem. 2019;294:7573–7587. doi: 10.1074/jbc.RA118.005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trobaugh D.W., Klimstra W.B. MicroRNA regulation of RNA virus replication and pathogenesis. Trends Mol. Med. 2017;23:80–93. doi: 10.1016/j.molmed.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenni S., Salgado E.N., Herrmann T., Li Z.L., Grant T., Grigorieff N., Trapani S., Estrozi L.F., Harrison S.C. In situ structure of rotavirus VP1 RNA-dependent RNA polymerase. J. Mol. Biol. 2019;431:3124–3138. doi: 10.1016/j.jmb.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richaud A., Frezal L., Tahan S., Jiang H.B., Blatter J.A., Zhao G.Y., Kaur T., Wang D., Felix M.A. Vertical transmission in Caenorhabditis nematodes of RNA molecules encoding a viral RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 2019;116:24738–24747. doi: 10.1073/pnas.1903903116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fouad A.M., Soliman H., Abdallah E.S.H., Ibrahim S., El-Matbouli M., Elkamel A.A. In-vitro inhibition of spring viremia of carp virus replication by RNA interference targeting the RNA-dependent RNA polymerase gene. J. Virol. Methods. 2019;263:14–19. doi: 10.1016/j.jviromet.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Comas-Garcia M. Packaging of genomic RNA in positive-sense single-stranded RNA viruses: a complex story. Viruses. 2019;11:253. doi: 10.3390/v11030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf Y.I., Kazlauskas D., Iranzo J., Lucía-Sanz A., Kuhn J.H., Krupovic M., Dolja V.V., Koonin E.V. Origins and evolution of the global RNA virome. mBio. 2018;9 doi: 10.1128/mBio.02329-18. e02329-02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald S.M. RNA synthetic mechanisms employed by diverse families of RNA viruses. Wiley Interdiscipl. Rev. Rna. 2013;4:351–367. doi: 10.1002/wrna.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin Y., Zhang H.J., Song W.B., Si S.Y., Han Y.X., Jiang J.D. Identification and characterization of Zika virus NS5 RNA-dependent RNA polymerase inhibitors. Int. J. Antimicrob. Agents. 2019;54:502–506. doi: 10.1016/j.ijantimicag.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Ravichandran L., Venkatesan A., Dass J.F.P. Epitope-based immunoinformatics approach on RNA-dependent RNA polymerase (RdRp) protein complex of Nipah virus (NiV) J. Cell. Biochem. 2019;120:7082–7095. doi: 10.1002/jcb.27979. [DOI] [PubMed] [Google Scholar]
- 63.Shi J.J., Perryman J.M., Yang X.R., Liu X.R., Musser D.M., Boehr A.K., Moustafa I.M., Arnold J.J., Cameron C.E., Boehr D.D. Rational control of poliovirus RNA-dependent RNA polymerase fidelity by modulating motif-D loop conformational dynamics. Biochemistry. 2019;58:3735–3743. doi: 10.1021/acs.biochem.9b00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.-S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., Perry J., Perron M., Stray K.M., Barauskas O., Feng J.Y., Xu Y., Lee G., Rheingold A.L., Ray A.S., Bannister R., Strickley R., Swaminathan S., Lee W.A., Bavari S., Cihlar T., Lo M.K., Warren T.K., Mackman R.L. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo(2,1-f triazin-4-amino adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 69.Chun B.K., Clarke M.O.N.H., Doerffler E., Hui H.C., Jordan R., Mackman R.L., Parrish J.P., Ray A.S., Siegel D. 24 April 2018. Methods for Treating Filoviridae Virus Infections. US9949994B2. [Google Scholar]
- 70.Chun B.K., Clarke M.O.N.H., Doerffler E., Hui H.C., Jordan R., Mackman R.L., Parrish J.P., Ray A.S., Siegel D. 09 April 2019. Methods for Treating Filoviridae Virus Infections. US10251898B2. [Google Scholar]
- 71.Chun B.K., Clarke M.O.N.H., Doerffler E., Hui H.C., Jordan R., Mackman R.L., Parrish J.P., Ray A.S., Siegel D. 12 September 2019. Methods for Treating Filoviridae Virus Infections. US20190275063A1. [Google Scholar]
- 72.Chun B.K., Clarke M.O.N.H., Doerffler E., Hui H.C., Jordan R., Mackman R.L., Parrish J.P., Ray A.S., Siegel D. 06 May 2016. Methods for Treating Filoviridae Virus Infections. WO2016069827A1. [Google Scholar]
- 73.Brak K., Carra E.A., Heumann L.V., Larson N. 08 November 2018. Crystalline Forms of (S) 2 Ethylbutyl 2 (((S) (((2R,3S,4R,5R) 5 (4 Aminopyrrolo[2,1-F] [1,2,4]triazin-7-Yl)-5-Cyano-3,4-Dihydroxytetrahydrofuran-2 Yl)methoxy)(phenoxy) Phosphoryl)amino)propanoate. WO2018204198A1. [Google Scholar]
- 74.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters H.L., Jochmans D., de Wilde A.H., Posthuma C.C., Snijder E.J., Neyts J., Seley-Radtke K.L. Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorg. Med. Chem. Lett. 2015;25:2923–2926. doi: 10.1016/j.bmcl.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williamson B., Feldmann F., Schwarz B., Meade-White K., Porter D., Schulz J., van Doremalen N., Leighton I., Yinda C.K., Perez-Perez L. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.04.15.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S., Zhou P., Wang P., Li Y., Jiang L., Jia W., Wang H., Fan A., Wang D., Shi X., Fang X., Hammel M., Wang S., Wang X., Zhang L. Structural definition of a unique neutralization epitope on the receptor-binding domain of MERS-CoV spike glycoprotein. Cell Rep. 2018;24:441–452. doi: 10.1016/j.celrep.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harcourt J.L., Rudoler N., Tamin A., Leshem E., Rasis M., Giladi M., Haynes L.M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health. 2018;65:749–754. doi: 10.1111/zph.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janies D., Habib F., Alexandrov B., Hill A., Pol D. Evolution of genomes, host shifts and the geographic spread of SARS-CoV and related coronaviruses. Cladistics. 2008;24:111–130. doi: 10.1111/j.1096-0031.2008.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falsey A.R., Walsh E.E., Hayden F.G. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J. Infect. Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oong X.Y., Ng K.T., Takebe Y., Ng L.J., Chan K.G., Chook J.B., Kamarulzaman A., Tee K.K. Identification and evolutionary dynamics of two novel human coronavirus OC43 genotypes associated with acute respiratory infections: phylogenetic, spatiotemporal and transmission network analyses. Emerg. Microb. Infect. 2017;6:1–13. doi: 10.1038/emi.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corman V.M., Baldwin H.J., Tateno A.F., Zerbinati R.M., Annan A., Owusu M., Nkrumah E.E., Maganga G.D., Oppong S., Adu-Sarkodie Y., Vallo P., da Silva L., Leroy E.M., Thiel V., van der Hoek L., Poon L.L.M., Tschapka M., Drosten C., Drexler J.F. Evidence for an ancestral association of human coronavirus 229E with bats. J. Virol. 2015;89:11858–11870. doi: 10.1128/JVI.01755-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia S., Xu W., Wang Q., Wang C., Hua C., Li W., Lu L., Jiang S. Peptide-based membrane fusion inhibitors targeting HCoV-229E spike protein HR1 and HR2 domains. Int. J. Mol. Sci. 2018;19:487. doi: 10.3390/ijms19020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koo M., Bendahmane M., Lettieri G.A., Paoletti A.D., Lane T.E., Fitchen J.H., Buchmeier M.J., Beachy R.N. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7774–7779. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niederwerder M.C., Hesse R.A. Swine enteric coronavirus disease: a review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transboundary Emerg. Dis. 2018;65:660–675. doi: 10.1111/tbed.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Emanuel J., Marzi A., Feldmann H. Advances in Virus Research. Elsevier; 2018. Filoviruses: ecology, molecular biology, and evolution; pp. 189–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spence E.H., Huff M., Shattuck K., Vickers A., Yun N., Paessler S. Ebola virus and Marburg virus in human milk are inactivated by holder pasteurization. J. Hum. Lactation. 2017;33:351–354. doi: 10.1177/0890334416685564. [DOI] [PubMed] [Google Scholar]
- 94.Rogers K.J., Shtanko O., Vijay R., Mallinger L.N., Joyner C.J., Galinski M.R., Butler N.S., Maury W. Acute plasmodium infection promotes interferon-gamma-dependent resistance to Ebola virus infection. Cell Rep. 2020;30:4041. doi: 10.1016/j.celrep.2020.02.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suder E., Furuyama W., Feldmann H., Marzi A., de Wit E. The vesicular stomatitis virus-based Ebola virus vaccine: from concept to clinical trials. Hum. Vaccines Immunother. 2018;14:2107–2113. doi: 10.1080/21645515.2018.1473698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson D.E., Pfeffermann K., Kim S.Y., Sawatsky B., Pearson J., Kovtun M., Corcoran D.L., Krebs Y., Sigmundsson K., Jamison S.F. Comparative loss-of-function screens reveal ABCE1 as an essential cellular host factor for efficient translation of Paramyxoviridae and Pneumoviridae. mBio. 2019;10 doi: 10.1128/mBio.00826-19. e00826-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stokes K.L., Currier M.G., Sakamoto K., Lee S., Collins P.L., Plemper R.K., Moore M.L. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J. Virol. 2013;87:10070–10082. doi: 10.1128/JVI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassan M.Z., Sazzad H.M.S., Luby S.P., Sturm-Ramirez K., Bhuiyan M.U., Rahman M.Z., Islam M.M., Stroher U., Sultana S., Kafi M.A.H., Daszak P., Rahman M., Gurley E.S. Nipah virus contamination of hospital surfaces during outbreaks, Bangladesh, 2013-2014. Emerg. Infect. Dis. 2018;24:15–21. doi: 10.3201/eid2401.161758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ang B.S., Lim T.C., Wang L. Nipah virus infection. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.01875-17. e01875-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lo M.K., Feldmann F., Gary J.M., Jordan R., Bannister R., Cronin J., Patel N.R., Klena J.D., Nichol S.T., Cihlar T. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau9242. eaau9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt M.E., Varga S.M. Modulation of the host immune response by respiratory syncytial virus proteins. J. Microbiol. 2017;55:161–171. doi: 10.1007/s12275-017-7045-8. [DOI] [PubMed] [Google Scholar]
- 103.Martínez-Sobrido L., De la Torre J.C. Reporter-expressing, replicating-competent recombinant arenaviruses. Viruses. 2016;8:197. doi: 10.3390/v8070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bahar F.G., Ohura K., Ogihara T., Imai T. Species difference of esterase expression and hydrolase activity in plasma. J. Pharmaceut. Sci. 2012;101:3979–3988. doi: 10.1002/jps.23258. [DOI] [PubMed] [Google Scholar]
- 105.WHO . World Health Organization. 2018. WHO R&D Blueprint – ad-hoc expert consultation on clinical trials for Ebola therapeutics.https://www.who.int/ebola/drc-2018/treatments-approved-for-compassionate-use-update/en/ accessed 20 April 2020. [Google Scholar]
- 106.Kujawski S.A., Wong K.K., Collins J.P., Epstein L., Killerby M.E., Midgley C.M., Abedi G.R., Ahmed N.S., Almendares O., Alvarez F.N., Anderson K.N., Balter S., Barry V., Bartlett K., Beer K., Ben-Aderet M.A., Benowitz I., Biggs H., Binder A.M., Black S.R., Bonin B., Brown C.M., Bruce H., Bryant-Genevier J., Budd A., Buell D., Bystritsky R., Cates J., Charles E.M., Chatham-Stephens K., Chea N., Chiou H., Christiansen D., Chu V., Cody S., Cohen M., Conners E., Curns A., Dasari V., Dawson P., DeSalvo T., Diaz G., Donahue M., Donovan S., Duca L.M., Erickson K., Esona M.D., Evans S., Falk J., Feldstein L.R., Fenstersheib M., Fischer M., Fisher R., Foo C., Fricchione M.J., Friedman O., Fry A.M., Galang R.R., Garcia M.M., Gerber S.I., Gerrard G., Ghinai I., Gounder P., Grein J., Grigg C., Gunzenhauser J.D., Gutkin G.I., Haddix M., Hall A.J., Han G., Harcourt J., Harriman K., Haupt T., Haynes A., Holshue M., Hoover C., Hunter J.C., Jacobs M.W., Jarashow C., Jhung M.A., Joshi K., Kamali T., Kamili S., Kim L., Kim M., King J., Kirking H.L., Kita-Yarbro A., Klos R., Kobayashi M., Kocharian A., Komatsu K.K., Koppaka R., Layden J.E., Li Y., Lindquist S., Lindstrom S., Link-Gelles R., Lively J., Livingston M., Lo K., Lo J., Lu X., Lynch B., Madoff L., Malapati L., Marks G., Marlow M., Mathisen G.E., McClung N., McGovern O., McPherson T.D., Mehta M., Meier A., Mello L., Moon S.-s, Morgan M., Moro R.N., Murray J., Murthy R., Novosad S., Oliver S.E., O’Shea J., Pacilli M., Paden C.R., Pallansch M.A., Patel M., Patel S., Pedraza I., Pillai S.K., Pindyck T., Pray I., Queen K., Quick N., Reese H., Rha B., Rhodes H., Robinson S., Robinson P., Rolfes M., Routh J., Rubin R., Rudman S.L., Sakthivel S.K., Scott S., Shepherd C., Shetty V., Smith E.A., Smith S., Stierman B., Stoecker W., Sunenshine R., Sy-Santos R., Tamin A., Tao Y., Terashita D., Thornburg N.J., Tong S., Traub E., Tural A., Uehara A., Uyeki T.M., Vahey G., Verani J.R., Villarino E., Wallace M., Wang L., Watson J.T., Westercamp M., Whitaker B., Wilkerson S., Woodruff R.C., Wortham J.M., Wu T., Xie A., Yousaf A., Zahn M., Zhang J. 2020. First 12 Patients with Coronavirus Disease 2019 (COVID-19) in the United States. medRxiv. Published online 12 March 2020. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]