Highlights
-
•
Heightened inflammation and acute lung injury in major burn and COVID-19.
-
•
Baseline comorbidities and possible worsening consequence.
-
•
Medical management in concomitant burn and SARS-CoV-2 infection.
-
•
Multidisciplinary approaches to manage coexisting diseases.
Keywords: Burn, Comorbidity, COVID-19, SARS-CoV-2, Acute respiratory failure
Abstract
Background
The recently emerged severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) disease (COVID-19) has become a human pandemic. Heightened inflammation, vascular hyperpermeability, acute lung injury, coagulopathy, and cardiovascular abnormalities are among the SARS-CoV-2 infection-related complications. Major burn is also associated with metabolic derangements, vascular leak, and hemodynamic instability. Burn patients are at high risk for infections and developing sepsis. COVID-19 in burn victims might worsen the clinical outcome and make their medical management challenging.
Result
Here, we present four cases of concomitant burn and COVID-19 with different degrees of complications. They had no (three out of four) or multiple (one out of four) baseline comorbidities and all were admitted to hospital for further management. Three out of four cases demonstrated acute respiratory failure and were intubated (no longer than 7 days). It seems that one of them had COVID-19 on arrival, the other apparently contracted at outside hospital, and the last two infected during the index hospitalization.
Conclusion
Concomitant severe burn and COVID-19 might complicate the clinical presentation and hospital course. Such combination was associated with poor outcome in a case with baseline comorbidities, beyond what was expected from the severity of burn injury. However, a more comprehensive study with larger sample size is required to make a valid conclusion. With an ongoing COVID-19 global pandemic, SARS-CoV-2 infection might be a concurrent disease with other illnesses or traumas such as burn. This dictate multidisciplinary approaches to risk stratify, screen, assess, and manage coexisting diseases. Additionally, appropriate preparations and careful precautions need to be executed in burn units to prevent COVID-19 exposure and transmission to limit potential adverse outcomes.
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) disease (COVID-19) was primarily reported at Wuhan China in late December 2019. The disease was declared as pandemic by the World Health Organization (WHO) before long on 11 March 2020 [1].
SARS-CoV-2 virus enters cells through binding to the angiotensin converting enzyme 2 (ACE2) cell-surface protein which is widely expressed in different organs such as lungs, heart, blood vessels, brain, gut, and skin. ACE2 acts as a negative regulator of the renin-angiotensin system (RAS), amino acid transporter, catalyst, and cellular receptor associating COVID-19 to immune response, inflammation, and multi-organ system dysfunction [2]. In other words, the maladies associated with COVID-19 derives from its multiorgan involvement owing to the ubiquity and functional versatility of the viral receptor. While the COVID-mediated loss of ACE2-related cell support causes severe, downstream impairments of SARS-CoV-2 infection including heightened inflammation, vascular leak, acute lung injury, systemic and pulmonary hypertension, coagulopathy, cardiovascular and gastrointestinal complications [2], [3], comorbidities with other preexisting diseases or traumas such as burn injuries might potentially aggravate the clinical outcome. Such possible detrimental combinations make their medical management challenging, suggest constant and thorough multidisciplinary care.
Multiorgan failure is also common in individuals with major burn injuries. This is most likely instigated by their hypermetabolic/catabolic state and microvascular hyperpermeability which leads to hemodynamic instability and immune system dysfunction. Accordingly, such patients become more susceptible to infections, sepsis, subsequent perpetuating systemic damage, worsening clinical manifestation and outcome [4].
SARS-CoV-2 screening and prevention strategies need to be implemented at burn care centers, both outpatient and inpatient settings during the current COVID-19 pandemic. This is due to the substantial vulnerability of burn casualties to infection and the ease of transmission among them.
In Iran, the first confirmed COVID-19 was announced in mid-February 2020. Soon after, we admitted four burn patients with PCR-proven SARS-CoV-2 infection. Herein, we present the first case series, to the best of our knowledge, of patients with concomitant burn injuries and Covid-19 infection with absence or different degrees of associated multiorgan failure at Zare Hospital.
2. Case 1
On 27 March 2020, a 16 years old female, BMI 26, with no past medical history (PMH) was brought to Zare Burn Center for further evaluation of her burn injuries due to gas explosion. Patient was found to have 37% total body surface area (TBSA) burns in her back, trunk, lower extremities, left upper extremity, and mild facial burn and edema, but her lung exam was unremarkable. On arrival, patient reported having three days of sore throat, malaise, and fever which started one week prior to the admission. Labs done at outside hospital (post burn) showed white blood cells (WBC) 20.5 (x10 (3)/mcL) with 67% granulocytes and elevated CRP (++). She reported that her significant other had coughs, sore throat, malaise, fevers, and chills and was hospitalized two weeks prior. Patient’s initial vital signs were unremarkable. Repeat WBC were 4.6 (x10 (3)/mcL) with 50% granulocytes and 40% lymphocytes. Two days after admission, patient developed severe dry cough and was transferred to the operation room for debridement of second and third-degree burns. However, the surgery was canceled due to severe respiratory distress and hypoxia with oxygen saturation down to 70%; patient got intubated and was transferred to burn critical care unit (ICU). Fig. 1 A shows chest x-ray (CXR) of the patient when coronavirus infection was highly suspected. She was then started on Hydroxychloroquine and Kaletra (Lopinavir and Ritonavir). Patient was found to have temperature of 38.8° with markedly elevated CRP (+++), WBC at 3.6 (x10(3)/mcL) with %70 polymorphonuclear cells and %21 lymphocytes; imaging studies compatible with acute respiratory distress syndrome (ARDS) pattern on day eight of admission. She then received IVIG and interferon beta. Blood cultures remained negative. However, sputum cultures six days later showed Acinetobacter MDR sensitive to Polymyxin E. Patient was switched from Ciprofloxacin to Polymyxin E then. Eventually her coronavirus PCR came back positive. On the Day 19 of admission, with negative repeat SARS-CoV-2 PCR, patient underwent reconstruction with graft placement for her burns, then discharged home in stable condition 24 days after admission.
Fig. 1.
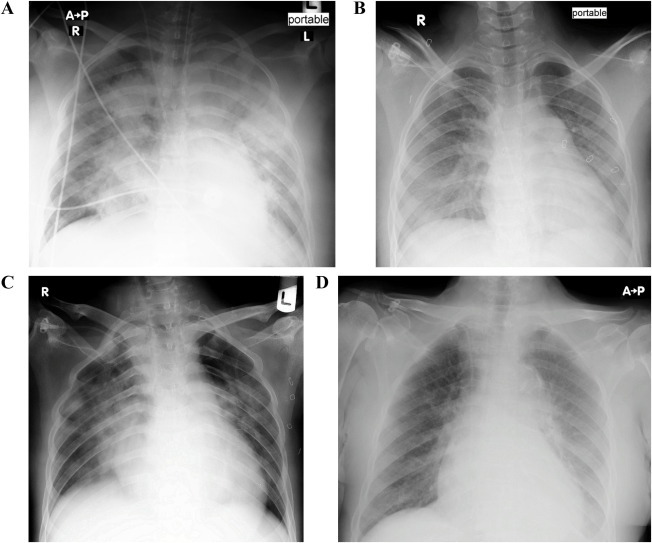
Chest X-Ray of all cases at the time COVID-19 was suspected. Varying degrees of interstitial and/or patchy airspace opacities noted. The pattern compatible with the COVID-19 pneumonia. 1A belongs to Case 1, 1B Case 2, 1C Case 3, and 1D Case 4. Cases 1, 2 and 4 developed acute respiratory failure.
3. Case 2
A 34-year-old male, BMI 24, with no PMH except for opioid addiction, presented with 35% TBSA burn. He was found to have wounds in his extremities, face, and head with facial edema with concern for inhalation injury. Three days after admission, patient was noted to have high temperature at 39.5° with purulent discharges of the wounds; as result of that, he was started on ciprofloxacin. Later, he developed respiratory failure and was intubated. Patient had procalcitonin of 10.2 (ng/mL) and his WBC was 5.4 (×10(3)/mcL). Patient underwent escharectomy of the hands and arms nine days after admission and was extubated on the day 10th of admission. He had more debridement four days later with escharectomy and graft placement. Later, during his hospital course, patient developed some shortness of breath. CXR (Fig. 1B) and Computed Tomography (CT) scan of the lungs was obtained that showed patchy ground-glass opacity, sings of multifocal pneumonia with mild pleural effusion on the right side. COVID-19 was suspected, and the result came back positive. Patient noted to have markedly elevated CRP (+++) with white blood cell count low at 1.9 with 8% lymphocytes and 90% granulocytes. He received Hydroxychloroquine as soon as COVID-19 was suspected. Throughout his hospital course, he underwent more surgical interventions with escharectomy and graft placement of the head, arms, and legs. He also received Tetracycline and polymyxin E for treatment of Acinetobacter sepsis. Patient was discharged 43 days after admission in stable condition.
4. Case 3
Patient was a 12 years old female, BMI 23, with no PMH who presented with scald burn secondary to boiling water with 22% TBSA burn. Patient had second and third-degree burns in her lower extremities bilaterally. She was transferred from outside medical center to our hospital four days after burn. On arrival, she was found to have temperature of 38.8° with cough and shortness of breath. CT scan of chest was obtained that showed ground-glass opacities with possible coronavirus pattern. Two days after admission, patient felt better however she was still febrile with temperature of 38.2°, heart rate of 95 and had loss of appetite. Her respiratory exam appeared to be normal with no wheezing, rhonchi, or crackles. Patient underwent escharectomy and debridement one day after admission. She received prophylactic antibiotic (Cefazolin). Labs were remarkable for elevated CRP (+++) and subsequently coronavirus PCR came back positive. Except supportive care, no Hydroxychloroquine or antivirals were used for treatment of her COVID-19. Patient’s wounds improved gradually after grafting. She was then discharged in stable condition 11 days after admission.
5. Case 4
An 87-year-old female, BMI 27, with PMH of hypertension, mild-to-moderate mitral and tricuspid valve regurgitation and Alzheimer dementia brought to burn center for further management of chemical burns from acid. Patient was found to have 17% TBSA with third and fourth degrees burning. Her hospital course was complicated with blood per rectum along with lower GI bleed. Patient enoxaparin was discontinued; however, she could not undergo colonoscopy due to poor preparation. Also, patient was found to have encephalopathy with altered mental status worse than her baseline. Cultures were obtained, and patient was started on broad-spectrum antibiotics with Vancomycin, Colomycin and Meropenem. Patient appeared to be more obtunded and developed hypoxemia with increase in creatinine. Vancomycin was switched to linezolid due to acute kidney injury. CXR (Fig. 1D) and CT scan of the chest was obtained which showed ground-glass opacity with patchy infiltration. Coronavirus was suspected and patient was started on Kaletra and Hydroxychloroquine. Patient’s WBCs noted to be at 18 (x10 (3)/mcL) with 86% granulocytes and 10% lymphocytes. Two days later, she noted to have worsening respiratory failure, acute renal failure, and increased WBC to 21 (granulocytes rose to 95%, lymphocytes dropped to 4%). She was intubated and transferred to burn ICU for further management. Patient then started on intravenous immunoglobulin (IVIG) and Hydrocortisone considering worsening inflammatory markers and clinical status. She also started on dopamine and norepinephrine due to shock status. Later, her condition deteriorated, and patient expired 41 days after admission (5 days after transferring to burn ICU) due to multiple organ failure.
6. Discussion
The coexistence of COVID-19 and other diseases or traumas have been encountered during the present pandemic. The current study, to the best of our knowledge, is the first case series of patients with concomitant burn injuries and COVID-19 who were admitted to a specialized burn center in the North of Iran.
The first presented case was a teenage female with no significant medical history who most likely contracted COVID-19 prior to the admission. She had mild upper respiratory tract infection (URTI) symptoms prior to the presentation. She developed ARDS likely due to a combination of inhalation injury and COVID-19 pneumonia. However, she was fully recovered and discharged home in stable condition 24 days after admission. The second case was a male with no PMH who was intubated due to inhalation injuries. COVID-19 was diagnosed later during his long course of hospitalization (post extubation) and after multiple surgical interventions with escharectomy and graft placement. He was discharged home in stable condition. The third case, an adolescent who was transferred from other medical facility, presented with URTI signs and symptoms, and with CXR indicative of COVID-19 pneumonia pattern on arrival (Fig. 1C). She indeed had no complications, labs were unremarkable, and she was discharged in stable condition soon (compared with the other cases) after admission. The last case was an overweight, elderly, patient with dementia, long history of hypertension, valvular heart disease, underlying both physical and psychosocial deconditioning, who contracted COVID-19 during the index hospitalization. She subsequently demonstrated the most severe complications with associated multiorgan failure, beyond what is usually expected from only 17% TBSA burn injury, most likely due to concomitant COVID-19 and chemical burn injuries.
Although the size and severity of the burn injuries are independently associated with worsening outcome [4] and are considered as confounders, the developed ARDS in our very young patient (Case one) was most likely due to the simultaneous COVID-19 infection, although fully recovered due to the lack of other comorbidities or risk factors at baseline and with proper management. In addition, the concomitant COVID-19 infection presumably contributed to the poor outcome in Case four who also had preexisting comorbidities and multiple cardiovascular risk factors.
The pathophysiology SARS-CoV-2 has not been clearly elucidated yet. Current findings in part demonstrate similar features with SARS-CoV [3]. The viral transmission occurs by droplets and contact. They attach to the nasopharyngeal mucosa, begin to replicate, then, move into the lungs and subsequently cause lower respiratory tract infection. Viremia can also happen through circulatory access from lung with subsequent involvement of various target organs (e.g., liver, heart, renal, gastrointestinal tract, central nervous system) where viral receptor, ACE2, has been expressed [2], [5]. ACE2 is a key negative regulator of the renin-angiotensin system, has different roles including catalytic activities, functional receptor for SARS-CoV, and amino acid transporter [1], linking COVID-19 to immunity, inflammation, and multi-organ system disease.
The critical role of ACE2 in RAS and its abundance in several organs causes marked systemic disturbances following its depletion at the cell surface due to endocytosis of the viral-receptor complex, viral-induced downregulation of the receptor and shedding of its active domain. Subsequent loss of ACE2-associated cell support considers as a serious, downstream complications of SARS-CoV-2 infection ranging from increased inflammation, acute lung injury, systemic and pulmonary hypertension, cardiovascular problems, and gastrointestinal abnormalities [2], [3]. Moreover, epithelial and endothelial apoptosis, impaired vascular integrity, and release of profuse pro-inflammatory mediators might be the deleterious consequence of the rapid viral replication.
A large number of case series from both China and New York City exhibit the worse clinical outcomes among individuals with coexisting comorbidities (e.g., hypertension, obesity, and diabetes) [6], [7].
Severe burn is considered as one of the most dramatic forms of trauma. Such patients demonstrate physiological alterations from baseline including hypermetabolism with drastic metabolic shift towards catabolism, hyperdynamic circulatory state and heightened inflammation, subsequently linked to multiorgan failure and poor outcome [4], [8].
Patient’s age, percent of total body surface area with burn, and inhalation injury have been among the clinical factors that predict prognosis of burns [9]. Additionally, the subsequent organ dysfunction has also been associated with poor prognosis and considered as independent prognosticator of mortality [4], [10]. In absence of other concomitant diseases, respiratory failure, hypotension, and thrombocytopenia reported to be among the most common described organ derangements in burn casualties [4].
System failure in burn injuries seems to be multifactorial. The microvascular hyperpermeability mediated by polymorphonuclear activation presumed to be the key mechanistic underpinning [4]. Moreover, immunosuppression which has been reported in major burn injuries [12] can prone the casualties to the downstream superimposed infection, sepsis and ensued multiorgan dysfunction. Animal study demonstrated that the burn injuries with polymicrobial septic complications aggravated activation of inflammatory cells, worsened microvascular permeability, and caused hemodynamic instability and metabolic imbalances more than the isolated burn or infection [11].
Burn casualties are in acute conditions and need to be evaluated and managed even before being screened or ruled out for COVID-19. They are also more prone to infections with increased transmissibility among them. Moreover, obtaining intravenous access, endotracheal intubation, tracheostomy, wound care, and related interventions such of escharectomy, debridement or grafting raise their infection exposure [13], [14]. Additionally, SARS-CoV-2 has a high degree of infectivity, various routes of transmission with a mounting dissemination rate.
The potential detrimental consequences of concomitant burn and COVID-19 suggest mandating extra precautions and sophisticated strategies which need to be implemented in burn units. Such policies help prevent infection, recognize different types of exposure, establish detailed and systematic protocols on proper diagnosis and management. The key steps include the fast and careful patient screening for COVID-19 on arrival, frequent screening of hospital staff, obtaining detailed history on travel risk factors in two weeks prior to the admission, assess for fever or other respiratory signs and symptoms before or during hospitalization with continuous clinical surveillance, personal protective equipment (PPE) in all areas with proper social distancing, provision of disinfectants and sanitation equipment, and staff travel restrictions [15], [16].
7. Current evolving strategies
Patients are tested for COVID-19 on arrival to emergency department at Zare Burn Center. Individuals with clinical suspicion for SARS-CoV-2 infection and pending test are maintained in isolation room. The confirmed COVID-19 patients are then admitted to COVID-19 unit and taken care in separate airborne infection isolation room. Infectious disease physicians are consulted early with active participation in patient care. All staff are provided with full PPE. In confirmed COVID-19 or highly suspected cases, surgical interventions are minimized when possible, limited to essential debridements in case of wound infection. There are no changes in wound care or management of skin grafts.
COVID-19 treatment guidelines are continuously updating based on revising, legitimate national and international guidelines for the optimal management. Antivirals such as Kaletra, Favipiravir and Remdesivir have been used by infectious disease consultants but are not part of our COVID-19 treatment guidelines yet. Oseltamivir or Ribavirin are not used any longer. Hydroxychloroquine has not been used any more in treatment of COVID-19 patients. Interferon-Beta and/or IVIG have been given in case of excessive inflammatory responses, mostly in our burn critical care COVID-19 patients. Corticosteroids such as Dexamethasone and Methylprednisolone have recently been added to our COVID-19 treatment protocols in the setting of severe inflammatory responses and/or hypoxemia (blood oxygen saturation < 90%). Convalescent plasma has also been added to the therapeutic regimen of deteriorating burn critical care cases. All our adult patients (ward or ICU, with or without COVID-19) have been receiving pharmacological thromboprophylaxis (low molecular weight Heparin, standard-dose unfractionated Heparin), unless there were contraindications. Frequent use of antibiotics without clear indications is not anymore recommended. However, they have often been used in burn critical care cases with worsening clinical conditions. All our patients receive usual dose of daily vitamin C, per standard burn treatment protocols. Our current COVID-19 guidelines have not yet recommended high dose vitamin C.
In case of acute hypoxemic respiratory failure with higher oxygen need, our COVID-19 respiratory protocols have recently changed. They suggest superior use of high flow nasal cannula over other noninvasive ventilation modalities and preventing early invasive intubation. COVID-19 tracheostomy precautions are not mentioned here as none of our cases had prolonged intubation.
8. Conclusion
Concomitant major burn injuries and COVID-19 might complicate the clinical presentation and hospital course. Such combination was associated with poor outcome in one of our cases with multiple chronic illnesses, beyond what was anticipated from the severity of burn injury. However, a more comprehensive study with larger sample size is needed to make a valid conclusion. With an ongoing COVID-19 pandemic, SARS-CoV-2 infection might be a preceding, concomitant, or subsequent disease in addition to other various medical problems or traumas such as burn. Thorough risk stratification and multidisciplinary approaches to strategic management of comorbid conditions are paramount to prevent possible worsening outcomes.
9. Declarations
Ethics approval: The patients have given permission and informed consent for the publication of this case series. The subjects' consent was obtained according to the Declaration of Helsinki.
Availability of the data: The presented data in this case series are available upon reasonable request.
Disclosures: The authors have no financial relationships to disclose.
Funding: No external funding was utilized to support this study.
Authors Contribution: All authors have read and approved the revised manuscript and have taken care
Acknowledgments
We thank all the staff members form Zare Hospital’s Burn Unit and Burn Intensive Care Unit for their ceaseless efforts and assistance.
References
- 1.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J.-Y., Chien J.-Y., Kao K.-C., Tsai C.-L., Hung F.M., Lin F.-M., Hu H.-C., Huang K.-L., Yu C.-J., Yang K.-Y. Predictors of early onset multiple organ dysfunction in major burn patients with ventilator support: experience from a mass casualty explosion. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-29158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-J., Liang W.-H., Zhao Y.i., Liang H.-R., Chen Z.-S., Li Y.-M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020.Shareable1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herndon D.N., Tompkins R.G. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith D.L., Cairns B.A., Ramadan F., Dalston J.S., Fakhry S.M., Rutledge R., Meyer A.A., Peterson H.D. Effect of inhalation injury, burn size, and age on mortality: a study of 1447 consecutive burn patients. J Trauma. 1994;37(4):655–659. doi: 10.1097/00005373-199410000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Lorente J.A., Vallejo A., Galeiras R., Tómicic V., Zamora J., Cerdá E., de la Cal M.A., Esteban A. Organ dysfunction as estimated by the sequential organ failure assessment score is related to outcome in critically ill burn patients. Shock. 2009;31(2):125–131. doi: 10.1097/SHK.0b013e31817fc3ef. [DOI] [PubMed] [Google Scholar]
- 11.Goto M., Samonte V., Ravindranath T., Sayeed M.M., Gamelli R.L. Burn injury exacerbates hemodynamic and metabolic responses in rats with polymicrobial sepsis. J Burn Care Res. 2006;27(1):50–59. doi: 10.1097/01.bcr.0000192568.77001.b1. [DOI] [PubMed] [Google Scholar]
- 12.Accardo-Palumbo A., D’Amelio L., Pileri D., D’Arpa N., Mogavero R., Amato G. Reduction of plasma granzyme A correlates with severity of sepsis in burn patients. Burns. 2010;36(6):811–818. doi: 10.1016/j.burns.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z., Zhuang D., Xiong B., Deng D.X., Li H., Lai W. Occupational exposure to SARS-CoV-2 in burns treatment during the COVID-19 epidemic: Specific diagnosis and treatment protocol. Biomed Pharmacother. 2020;127:110176. doi: 10.1016/j.biopha.2020.110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha S., Kumar A., Dash S., Singhal M. Managing burns during COVID-19 outbreak. J Burn Care Res. 2020 doi: 10.1093/jbcr/iraa086. iraa086. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barret J.P., Chong S.J., Depetris N., Fisher M.D., Luo G., Moiemen N., Pham T., Qiao L., Wibbenmeyer L., Matsumura H. Burn center function during the COVID-19 pandemic: An international multi-center report of strategy and experience. Burns. 2020;46(5):1021–1035. doi: 10.1016/j.burns.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S., Yuan Z., Peng Y., Chen J., Li H., Luo Q., Song H., Xiang F., Tan J., Zhou J., Ning L.i., Hu G., Luo G. Experience and suggestion of medical practices for burns during the outbreak of COVID-19. Burns. 2020;46(4):749–755. doi: 10.1016/j.burns.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


