Abstract
Background
Cryptococcosis is the most common opportunistic fungal infection. High morbidity and mortality are frequently observed among hospitalized HIV/AIDS patients, particularly having CD4 count ≤100 cells/μl. Therefore, this study aimed to determine the prevalence of cryptococcal antigenemia and associated factors among HIV/AIDS patients.
Methods
A hospital-based cross-sectional study was conducted among 140 HIV/AIDS patients. A cryptococcal antigen test was performed for all patients along with medical chart and laboratory registration book review. Cryptococcal antigen was detected from serum by using Remel Cryptococcal Antigen Test Kit. Data related to possible associated factors were extracted from patients' charts and laboratory registration book. Data were coded, entered, and analyzed using SPSS version 20. Logistic regression analysis was done to see the association between dependent and independent variables. A P value <0.05 was considered statistically significant. Finally, data were presented in the form of texts, figures, and tables.
Result
Among 140 serum cryptococcal antigenemia-tested study subjects, 16 (11.43%) were positive for serum cryptococcal antigen. Of them, 43.8% (7/16) were pulmonary tuberculosis coinfected, 31.2% (5/16) were extrapulmonary tuberculosis positive, and 25% (4/16) had bacterial bloodstream infections. In addition, 68.7% (11/16) had CD4 count less than 100 cells/μl, 18.7% (3/16) had CD4 count 100–150 cells/μl, 50% (8/16) were antiretroviral therapy defaulters, and 31.3% (5/16) were naïve. In this study, the majority, 75% (12/16), of the serum cryptococcal antigen-positive subjects were clinical stage IV. Of the assessed associated factors, tuberculosis coinfection (AOR: 0.04; 95% CI [0.005–0.25]) and antiretroviral therapy status (AOR: 0.02; 95% CI [0.001–0.5]) were significantly associated factors enhancing serum cryptococcal antigenemia.
Conclusion
In this study, the high rate of cryptococcal antigenemia was observed among hospitalized HIV/AIDS patients, and it is alarming and highlights the need for improving CD4 status, expanding serum cryptococcal antigen screening, and strengthening regular cryptococcal antigenemia surveillance systems.
1. Introduction
1.1. Background
Cryptococcosis is one of the most common opportunistic systemic fungal infections among human immune-deficiency virus/acquired immune-deficiency syndrome (HIV/AIDS) patients and other immune-suppressed individuals resulted from cancer chemotherapy, diabetes mellitus, sarcoidosis, steroid therapy, renal transplantation, or other genetically related diseases. Cryptococcus neoformans and Cryptococcus gattii are the most common causative agents of cryptococcosis [1].
Cryptococcosis is an important contributor to morbidity and mortality among HIV/AIDS patients [2,3]. Cryptococcus meningitis is an opportunistic fungal infection of the central nervous system (CNS). HIV is one of the most important factors for the development of cryptococcal infection [4]. Cryptococcal infections are the major cause of death among HIV/AIDS patients accounting for 20–25% of HIV/AIDS-related mortality in Africa [5]. Therefore, screening of serum cryptococcal antigenemia and early antifungal treatment are important for reducing deaths by cryptococcosis [6, 7]. Serum cryptococcal antigen is detectable in blood weeks to months before the development of clinical symptoms. This prolonged subclinical period of asymptomatic infection presents an opportunity to identify persons with asymptomatic or early treatment [8]. Insufficient antiretroviral treatment of HIV/AIDS patients can increase the rate of cryptococcosis infection among them [9].
Serum cryptococcal antigen-positive HIV/AIDS patients taking no ART treatment are at high risk of developing symptomatic cryptococcal meningitis [9, 10]. The majority of the deaths that occur among AIDS patients in Ethiopia are in the first four months of ART [11]. Different studies from different corners of the world showed an increased prevalence of cryptococcal infections. However, the mortality and morbidity rate of cryptococcosis and its associated factors among HIV/AIDS patients in Ethiopia are poorly documented. Therefore, this study aimed to screen serum cryptococcal antigen and its associated factors among HIV/AIDS patients which will help health policymakers in the development of strategies for early diagnosis and proper treatment of suspected patients.
2. Materials and Methods
2.1. Study Setting
This study was conducted at Felege-Hiwot Referral Hospital, Bahir Dar, Ethiopia. Bahir Dar city is located to the northwest and 540 km away from Addis Ababa, the capital city of Ethiopia. Felege-Hiwot Referral Hospital was purposefully selected with the fact that it is one of the biggest tertiary level referral hospitals in the region visited by around seven million people per year from the surrounding zones and nearby regions. Felege-Hiwot Referral Hospital has different departments including a diagnostic laboratory and around 400 beds, nine operating tables, one ART clinic with an ART laboratory, and one ART pharmacy.
2.2. Study Design and Period
A hospital-based cross-sectional study was conducted to determine serum cryptococcal antigen and associated factors among hospitalized HIV/AIDS patients from July 15, 2019, to December 15, 2019.
2.3. Study Subjects
All HIV/AIDS patients who were suspected of having cryptococcosis and having laboratory diagnosis for serum cryptococcal antigen during the study period were included as study subjects.
2.4. Inclusion Criteria
All HIV/AIDS patients who were suspected of having cryptococcosis during the study period were included.
2.5. Exclusion Criteria
Patients receiving antifungal treatment and patients less than 15 years were excluded from this study.
2.6. Sample Size
During the five months of the study period, a total of 140 cryptococcal infection-suspected HIV/AIDS patients visited Felege-Hiwot Referral Hospital and included in this study.
2.7. Data Collection and Laboratory Methods
A pretested, structured questionnaire was used to collect sociodemographic information. Data related to associated factors such as recent CD4 count, ART treatment, clinical stage, and current history of tuberculosis (pulmonary tuberculosis or extrapulmonary tuberculosis) were collected from patients' charts and laboratory registration books.
2.7.1. Sample Collection and Laboratory Procedures
In the Felege-Hiwot Referral Hospital laboratory, different clinical specimens are being processed with respective standard procedures. For instance, during this study, screening of pulmonary and extrapulmonary tuberculosis was done by gene expert and fluorescent microscopy techniques, and clinical specimens like sputum, gastric lavage, and cerebrospinal fluid were used for diagnosis. Venous blood has been also used for the diagnosis of blood stream infections. Blood for screening serum cryptococcal antigen was collected under strict aseptic conditions using the venipuncture technique. Before blood collection, careful skin cleaning using 2% iodine tincture and 70% alcohol was done to prevent contamination. Serum samples were prepared through centrifugation and tested with Remel Cryptococcal Antigen Test Kit (Remel, London, UK). The serum was treated with protease enzyme, heated at 100°C for five minutes, cooled to room temperature, and then mixed with latex reagent. Clumping within 10 minutes of rotation was interpreted as the specimen is positive for cryptococcal antigen. Bloodstream infection of the study participants was screened by manual blood culture.
2.7.2. Data Analysis and Interpretation
All the data were coded and entered into Epi data 3.1 and exported into SPSS 20 version statistical software for analysis. During analysis, cross-tabulations and odds ratios were used to compare frequencies. Descriptive statistics were computed to describe the study subjects in relation to relevant variables. Logistic regression analyses were done to determine factors associated with cryptococcal antigenemia. A variable with a P value <0.2 in bivariate logistic regression was included in multivariate analysis. Crude and adjusted odds ratios were calculated to quantify the strength of association between the rate of cryptococcal infection and associated factors. The 95% confidence interval was used, and associated factors with a P value <0.05 in multivariate analyses were considered statistically significant. Finally, data were presented in the form of texts, tables, and graphs.
2.8. Ethical Consideration
Ethical clearance was obtained from the Departmental Research and Ethics Review Committee (DRERC) of Addis Ababa University. Then, permission was obtained from the Felege-Hiwot Referral Hospital to access data from the study population. Written consent was obtained from all eligible subjects and informed about the purpose of the study and their willingness to take part in the study. All information got from the study participants was coded to maintain confidentiality.
3. Results
3.1. Sociodemographic Characteristics
A total of 140 HIV/AIDS hospital-admitted patients were enrolled in this study, of whom 59 (42%) and 81 (58%) were males and females, respectively. All study subjects were in the age range of 15–60 years (mean ± SD = 35 ± 11.8 years). 62 (44%) of them were married, 100 (71.4%) were literates, and over 70% of them came from urban areas (Table 1).
Table 1.
Sociodemographic characteristics of HIV/AIDS patients screened for serum cryptococcal antigen in Felege-Hiwot referral hospital, Bahir Dar, Ethiopia.
| Characteristics | Pos., N (%) | Neg., N (%) | Total, N (%) |
|---|---|---|---|
| Sex | |||
| Male | 7 (44) | 52 (42) | 59 (42) |
| Female | 9 (56) | 72 (58) | 81 (58) |
|
| |||
| Age | |||
| 15–24 | 3 (19) | 16 (13) | 19 (14) |
| 25–44 | 11 (69) | 76 (63) | 87 (63.5) |
| >45 | 2 (12) | 29 (24) | 31 (22.6) |
|
| |||
| Residence | |||
| Urban | 9 (56) | 91 (73.4) | 100 (71) |
| Rural | 7 (44) | 33 (26.6) | 40 (29) |
|
| |||
| Marital status | |||
| Unmarried | 6 (37.5) | 33 (26.6) | 39 (28) |
| Married | 5 (31.2) | 57 (46) | 62 (44) |
| Othersa∗ | 5 (31.2) | 34 (27.4) | 39 (28) |
|
| |||
| Educational level | |||
| Illiterate | 4 (25) | 36 (29) | 40 (28.6) |
| Literate | 12 (75) | 88 (71) | 100 (71.4) |
|
| |||
| Occupation | |||
| Student | 1 (6.2) | 17 (14) | 18 (12.8) |
| House wife | 2 (12.5) | 20 (16) | 22 (15.7) |
| Employed | 11 (68.8) | 71 (57) | 82 (58.6) |
| Others | 2 (12.5) | 16 (13) | 18 (12.8) |
|
| |||
| CD4+ (cells/ml) | |||
| <100 | 11 (68.7) | 7 (5.6) | 18 (12.9) |
| 100–150 | 3 (18.7) | 31 (25) | 34 (24.3) |
| 150–200 | 1 (6.3) | 14 (11.3) | 15 (10.7) |
| >200 | 1 (6.3) | 72 (57.2) | 73 (52.1) |
|
| |||
| Antiretroviral therapy status | |||
| On ART | 3 (18.8) | 67 (54) | 70 (50) |
| ART naïve | 5 (31.2) | 38 (30.6) | 43 (30.7) |
| ART defaulter | 8 (50) | 19 (15.3) | 27 (19.3) |
|
| |||
| Bacterial infection status (n = 79) | |||
| Pulmonary TB | 7 (43.8) | 7 (11.1) | 14 (17.7) |
| Ex pulmonary TB | 5 (31.2) | 6 (9.5) | 11 (13.9) |
| BSI | 4 (25) | 50 (79.4) | 54 (68.4) |
|
| |||
| WHO clinical stage | |||
| Stages I and II | 1 (6) | 105 (84.7) | 106 (75.7) |
| Stage III | 3 (19) | 17 (13.7) | 20 (14.3) |
| Stage IV | 12 (75) | 2 (1.6) | 14 (10) |
Note. WHO, World Health Organization; others, widow and divorced; BSI, bacterial infection isolated by blood culture; employed, government and self-employed; others, jobless and children.
3.2. Prevalence of Cryptococcal Antigenemia and Coinfections
In the current study, the prevalence of cryptococcal antigenemia was 11.43% (16). Of this, 43.8% (7/16) were pulmonary tuberculosis positive, 31.2% (5/16) were extrapulmonary tuberculosis positive, and 25% (4/16) had bacterial bloodstream infections. The majority (68.7%) of serum cryptococcal antigen-positive participants had CD4 count less than 100 cells/μl, and they were ART defaulters (Table 1).
3.3. Factors Associated with Cryptococcal Antigenemia
Of the assessed associated factors, tuberculosis coinfection (AOR: 0.04; 95% CI [0.005–0.25]) and antiretroviral therapy status (AOR: 0.02; 95% CI [0.001–0.5]) were significantly associated factors enhancing serum cryptococcal antigenemia (Table 2).
Table 2.
Bivariate and multivariate analysis of factors associated with serum cryptococcal antigenemia among HIV/AIDS patients in Felege-Hiwot Referral Hospital, Bahir Dar, Ethiopia.
| Variable | Pos., N (%) | Neg., N (%) | P value | COR (95% CI) | P value | AOR (95% CI) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 7 (44) | 52 (42) | 1 | 1 | ||
| Female | 9 (56) | 72 (58) | 0.890 | 1 (0.377–3) | 0.329 | 0.4 (0.05–2.7) |
|
| ||||||
| Age | ||||||
| 15–24 | 3 (19) | 16 (13) | 0.300 | 0.4 (0.056–2.4) | 0.268 | 0.2 (0.007–3.9) |
| 25–44 | 11 (69) | 76 (63) | 0.354 | 0.5 (0.1–2.3) | 0.586 | 0.6 (0.07–4.6) |
| >45 | 2 (12) | 29 (24) | 1 | |||
|
| ||||||
| Marital status | ||||||
| Unmarried | 6 (37.5) | 33 (26.6) | 0.745 | 0.8 (0.23–2.9) | 0.637 | 1.7 (0.2–13) |
| Married | 5 (31.2) | 57 (46) | 0.440 | 1.7 (0.45–6.2) | 0.846 | 0.8 (0.1–6.3) |
| Others | 5 (31.2) | 34 (27.4) | 1 | |||
|
| ||||||
| Educational status | ||||||
| Illiterate | 4 (25) | 36 (29) | 1 | 1 | ||
| Literate | 12 (75) | 88 (71) | 0.737 | 0.82 (025–2.7) | 0.366 | 0.4 (0.04–3.2) |
|
| ||||||
| Occupation | ||||||
| Student | 1 (6.2) | 17 (14) | 1 | 1 | ||
| House wife | 2 (12.5) | 20 (16) | 0.676 | 0.6 (0.05–7) | 0.580 | 0.4 (0.012–12) |
| Employed | 11 (68.8) | 71 (57) | 0.369 | 0.4 (0.05–3) | 0.185 | 0.14 (0.008–2.6) |
| Jobless | 2 (12.5) | 16 (13) | 0.554 | 0.5 (0.04–5.7) | 0.340 | 0.19 (0.006–5.9) |
|
| ||||||
| Residence | ||||||
| Urban | 9 (56) | 91 (73.4) | 0.160 | 2 (0.74–6) | 0.831 | 1.3 (0.2–10) |
| Rural | 7 (44) | 33 (26.6) | 1 | |||
|
| ||||||
| Bacterial status (n = 79) | ||||||
| Pulmonary | 7 (43.8) | 7 (11.1) | 0.001 | 0.08 (0.02–0.35) | 0.001 | 0.04 (0.005–0.25) |
| Ex pulmonary | 5 (31.2) | 6 (9.5) | 0.003 | 0.1 (0.02–0.46) | 0.006 | 0.07 (0.01–0.47) |
| BSI | 4 (25) | 50 (79.4) | 1 | |||
|
| ||||||
| CD4 status | ||||||
| <100 | 11 (68.7) | 7 (5.6) | 0.007 | 0.05 (0.005–0.4) | 0.108 | 0.04 (0.001–2) |
| 100–150 | 3 (18.7) | 31 (25) | 0.800 | 0.74 (0.07–7.7) | 0.343 | 4.6 (0.2–110) |
| 151–200 | 1 (6.3) | 14 (11.3) | 1 | |||
| >200 | 1 (6.3) | 72 (59.4) | 0.257 | 5 (0.3–87) | 0.096 | 31 (0.54–1865) |
|
| ||||||
| ART status | ||||||
| On ART | 3 (18.8) | 67 (54) | 1 | |||
| ART naïve | 5 (31.2) | 38 (30.6) | 0.155 | 0.34 (0.07–1.5) | 0.161 | 0.09 (0.004–2.5) |
| ART defaulter | 8 (50) | 19 (15.3) | 0.002 | 0.1 (0.026–0.44) | 0.019 | 0.02 (0.001–0.5) |
Note. 1 = as a reference; others, widow and divorced; BSI, bacterial infection isolated by blood culture; AOR, adjusted odds ratio; OR, crude odds ratio; CI, confidence interval.
In the current study, the prevalence of cryptococcal antigenemia was more frequently detected among ART defaulters, 50% (8/16). More than 31% of the cryptococcal antigen-positive participants had CD4 count less than 100 cells/μl, and they were on ART (Figure 1).
Figure 1.
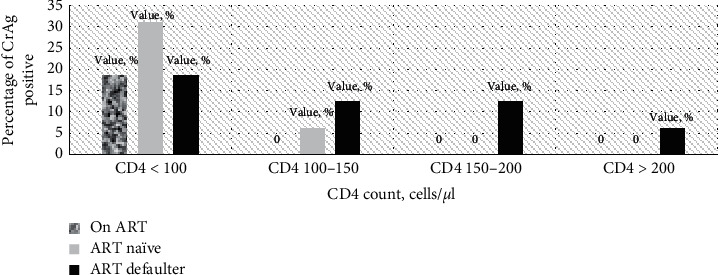
Percentage of cryptococcal antigen positive HIV/AIDS patients associated with CD4 count and ART use.
4. Discussion
HIV-infected people are at high risk of developing different opportunistic infections, including fungal and bacterial infections. Late presentations to care of HIV-infected people will put them at risk for the development of the cryptococcal disease. In resource-constrained settings, continuous surveillance of the cryptococcal infection in HIV‐infected people and associated factors is quite limited because of lack of cheap diagnostic tools, but it is important to support the national treatment program. For these resource-constrained countries, Remel Cryptococcal Antigen Test Kit might be a good possible option for diagnosing cryptococcal antigenemia among HIV/AIDS patients because according to the manufacture's instruction, this test kit has 100 percent specificity and sensitivity. In the current study, the prevalence of cryptococcal antigenemia was 11.43%. However, this percentage was significantly lower than previous reports in India (33.33%) and in China (21%) [12]. Another similar study was conducted among hospitalized AIDS patients on the continent level, and 69%, 80%, and 80% cryptococcal antigenemia was reported in Africa, Europe, and the USA, respectively [12]. This magnitude was significantly higher than the study done in Ethiopia among HIV/AIDS patients receiving antiretroviral therapy, which is 8.8% and 10.2% [8, 13].
In this study, cryptococcal antigenemia was more prevalent among females (56%) than male patients (44%). This percentage was almost similar to previous reports from Addis Ababa, Ethiopia (47% females and 52.9% males) [14]. The difference in prevalence between females and males might be resulted due to the difference of exposure rather than the difference in host susceptibility and environmental exposure, hormonal, and genetic predisposition [15–17]. Over 69% of the serum CrAg-positive participants were in the age group of 25–44 years. Similarly, different studies from many parts of the world showed that the prevalence of cryptococcosis among HIV-infected patients was higher in a similar age group [18, 19].
The screening of serum cryptococcal antigen was also done among HIV/AIDS patients having tuberculosis coinfection. In this study, the majority of the serum CrAg-positive individuals had a clinical history of tuberculosis. Cryptococcal antigenemia positivity rate was higher among patients with a history of both pulmonary (7/16 (43.8%) P=0.001) and extrapulmonary tuberculosis coinfection (5/16 (31.2%), P=0.006) compared to patients with other blood stream infections, 4/16 (25%). In this study, the association between cryptococcal antigenemia positivity rate and antiretroviral therapy status was also statistically significant (AOR: 0.02; 95% CI [0.001–0.5]). Our result was consistent with a result reported in another Ethiopian study [8]. Therefore, antiretroviral therapy with good adherence is an important consideration for AIDS patients for reducing the prevalence of cryptococcal antigenemia [8, 10, 20].
4.1. Limitation
We, the authors, could not perform other laboratory procedures like fungal blood culture, chest radiography, and determination of liver enzyme levels because of the resource limitation.
5. Conclusions
In this study, we found a previously unreported high prevalence of serum cryptococcal antigen among HIV/AIDS patients. Particularly, it was quite high among patients having CD4 counts less than 100 cells/μl, and it is alarming and highlights the need for improving CD4 status, expanding serum cryptococcal antigen screening, and strengthening regular cryptococcal antigenemia surveillance systems.
Acknowledgments
The authors acknowledge the University of Gondar for its support and encouragement until the end of this work. The authors are also thankful to the Felege-Hiwot Referral Hospital and Amhara Regional Reference Laboratory and their laboratory professionals for their unlimited support during the laboratory work. Finally, the authors would like to thank all the study participants for their collaboration.
Data Availability
Data and supporting material associated with this study will be shared upon request.
Ethical Approval
Ethical clearance was obtained from the Departmental Research and Ethics Review Committee (DRERC) of Addis Ababa University. Permission was obtained from the Felege-Hiwot Referral Hospital to access data from the study population. All information obtained from the study participants was coded to maintain confidentiality.
Consent
Written consent was obtained from all eligible subjects and informed about the purpose of the study and their willingness to take part in.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
M.J., T.B., T.D., and Y.A. were involved in designing the study, data collection, data entry, and clean up. All authors have also participated in data analysis and interpretation of the results and development of the manuscript. All authors read and approved the final manuscript and provided consent to publish.
References
- 1.Phangreichon Lungran A. V. D., Waikhom Shashi Singh S. D., Mate H., Golmei A. Cryptococcosis: its prevalence and clinical presentation among HIV positive and negative patients in Rims, Manipur. Journal of Dental and Medical Sciences (IOSR-JDMS) 2014;13:38–41. doi: 10.9790/0853-13743841. [DOI] [Google Scholar]
- 2.Jarvis J. N. M. G., Williams A., Brown Y., Crede T., et al. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infectious Diseases. 2010;10:p. 67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen D. B., Zijlstra E. E., Mukaka M., et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Tropical Medicine & International Health. 2010;15(8):910–917. doi: 10.1111/j.1365-3156.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- 4.Alemayehu T., Ayalew S., Buzayehu T., Daka D. Magnitude of cryptococcosis among HIV patients in sub‐saharan Africa countries: a systematic review and meta‐analysis. African Health Sciences. 2020;20(1):114–121. doi: 10.4314/ahs.v20i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/qad.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 6.Rajasingham R., Meya D. B., Boulware D. R. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. (JAIDS) Journal of Acquired Immune Deficiency Syndromes. 2012;59(5):e85–e91. doi: 10.1097/qai.0b013e31824c837e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pongsai P., Atamasirikul K., Sungkanuparph S. The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell counts. Journal of Infection. 2010;60(6):474–477. doi: 10.1016/j.jinf.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Beyene T. W. Y., Asrat D., Ayana G., Boulware D. R. Comparison of cryptococcal antigenemia between anti-retroviral Naïve and antiretroviral experienced HIV positive patients at two hospitals in Ethiopia. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meya D. B., Manabe Y. C., Castelnuovo B., et al. Cost‐effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV‐infected persons with a CD4+ cell count ≤100 cells/μl who start HIV therapy in resource‐limited settings. Clinical Infectious Diseases. 2010;51(4):448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis J. N., Lawn S. D., Vogt M., Bangani N., Wood R., Harrison T. S. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clinical Infectious Diseases. 2009;48(7):856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alemu A. W., Sebastián M. S. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Global Health Action. 2010;3(1):p. 5398. doi: 10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L., Li Y., Cao H., Huang S., Long M. Epidemiology and clinical characteristic of cryptococcal meningitis in China (1981–2013): a review of the literature. Medical Mycology. 2017;3:p. 1. [Google Scholar]
- 13.Alemu A. S., Kempker R. R., Tenna A., et al. High prevalence of cryptococcal antigenemia among HIV-infected patients receiving antiretroviral therapy in Ethiopia. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058377.e58377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitew A., Getachew T., Fentaw S. Prevalence of crytpococcal infection in patients clinically diagnosed to have meningitis in Ethiopia. Clinical Medicine Research. 2016;5(4):73–76. doi: 10.11648/j.cmr.20160504.12. [DOI] [Google Scholar]
- 15.Lindenberg A. D. S. C., Chang M. R., Paniago A. M. M., et al. Clinical and epidemiological features of 123 cases of cryptococcosis in Mato Grosso do Sul, Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 2008;50(2):75–78. doi: 10.1590/s0036-46652008000200002. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S. W. A., Wanchu A., Chakrabarti A., Sharma A., Bambery P., Singh S. Cryptococcal meningitis in HIV infected experience from a North Indian tertiary center. Neurology India. 2008;56:444–449. [PubMed] [Google Scholar]
- 17.Tay S. T. R. M., Soo Hoo T. S., Hamimah H. Epidemiology of cryptococcosis in Malaysia. Mycoses. 2010;53:509–514. doi: 10.1111/j.1439-0507.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- 18.Tintelnot K., Lemmer K., Losert H., Schar G., Polak A. Follow-up of epidemiological data of cryptococcosis in Austria, Germany and Switzerland with special focus on the characterization of clinical isolates. Mycoses. 2004;47(11-12):455–464. doi: 10.1111/j.1439-0507.2004.01072.x. [DOI] [PubMed] [Google Scholar]
- 19.Dzoyem J. P. K. F., Ngaba G. P., Lunga P. K., Lohoue P. J. Prevalence of cryptococcosis among HIV-infected patients in Yaounde, Cameroon. African Health Sciences. 2012;12:129–133. doi: 10.4314/ahs.v12i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liechty C. A., Solberg P., Were W., et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Tropical Medicine & International Health. 2007;12(8):929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and supporting material associated with this study will be shared upon request.


