Abstract
Objective
To identify the clinical characteristics, magnetic resonance imaging (MRI) results, and prognostic factors of neuropsychiatric (NP) systemic lupus erythematosus (SLE; NPSLE) in a relatively large patient series in China.
Methods
Data of patients with NPSLE at Peking Union Medical College Hospital (PUMCH) were collected retrospectively from June 2012 to June 2016. NPSLE patients were compared with 220 non-NPSLE patients. Survival rates were evaluated using the Kaplan-Meier curves, log-rank test, and Cox proportional hazards modeling. Cranial MRI results were also studied.
Results
Of the 194 included patients, sixteen subtypes of NPSLE were identified, and the most common manifestations were seizure (36.6%), acute confusional state (25.3%), and cerebral vascular disease (15.5%). Compared with the non-NPSLE group, NPSLE patients were significantly more likely to have typical lupus symptoms, higher Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) scores (P = 0.002), and positive rate of anti-ribosomal P protein antibodies (P = 0.008). Patients with seizure were more likely to have higher SLEDAI-2K scores and positive anti-β2GP1 than non-NPSLE patients. Sixteen patients died during follow-up. The most common cause of death was infection (37.5%). NPSLE significantly decreased survival rates of SLE patients. Patients with elevated serum creatinine (P = 0.001), hypocomplementemia (P = 0.031), and SLEDAI − 2K scores ≥ 15 (P = 0.014) had shorter survival periods. Eighty-two patients underwent detailed cranial MRI analysis; of these, 50 (61.0%) had abnormal results. Small vessel disease was the most common abnormal finding, followed by inflammatory-like lesions and large vessel disease.
Conclusions
High disease activity and positive rate of anti-ribosomal P protein antibodies may be risk factors for NPSLE. NPSLE decreases survival rates of SLE patients. Renal insufficiency and high disease activity are predictive of poor prognoses for NPSLE patients.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple organ systems. Neuropsychiatric (NP) involvement is one of the most serious disorders in SLE and is usually associated with a poor prognosis [1]. The incidence of neuropsychiatric systemic lupus erythematosus (NPSLE) ranges from 12.2% to 94.7% for SLE patients [2, 3]. This wide range is probably due to the high variability of NP presentations and differences in study designs. The diversity and heterogeneity of NP presentations suggest that multiple pathogenetic mechanisms are involved in NPSLE. Previous studies showed that high disease activity is likely associated with diffuse central nervous system (CNS) NP manifestations [4, 5], and antiphospholipid antibody positivity is believed to be associated with cerebrovascular events [6]. Currently, research efforts are focusing on the identification of pathways involved in NPSLE development, including antibodies, cell-related inflammation, cytokine-related inflammation, and complement activity [7]. Seizure is one of the common subtypes of NPSLE. The underlying pathogenesis of seizure may be multifactorial and may include infarction, inflammatory mediators, and autoantibodies [8]. A previous study suggested that seizure is predictive of poor prognoses for SLE. Cranial magnetic resonance imaging (MRI) is still the most commonly used imaging technique for the detection and evaluation of NPSLE in clinical practice [9]. Whether abnormal or specific cranial MRI results can indicate the prognosis for NPSLE is questionable. There are many areas of NPSLE that have not yet been clarified. Furthermore, NPSLE leads to a decline in the quality of life and can be life-threatening. Therefore, NPSLE requires further study.
Because there have been few extensive studies of NPSLE in China, this study comprehensively analyzed the risk factors and short- to midterm outcomes of NPSLE in a large dataset of NPSLE patients in China. Clinical features of seizure and the relationship between cranial MRI and the prognosis for NPSLE were also evaluated.
2. Methods
2.1. Patient Recruitment
NPSLE inpatients and outpatients treated at Peking Union Medical College Hospital between June 2012 and June 2016 were considered. SLE was diagnosed when the SLE classification criteria recommended by the American College of Rheumatology (ACR) in 1997 [10] or those of the Systemic Lupus International Collaborating Clinics (SLICC) in 2012 [2] and the diagnostic criteria for NPSLE in SLE proposed by the ACR in 1999 were fulfilled [11]. The ACR defined 19 neurological syndromes (12 central and 7 peripheral NP), of which diffuse CNS disease included anxiety disorder, psychosis, mood disorder, cognitive dysfunction, and acute confusional states [11]. In the ACR case definitions [11], headache is defined using the International Headache Society (IHS) classification [12], and mood disorders are determined by clinical judgment based on the Diagnostic and Statistical Manual-IV (DSM-IV) criteria [13]. NP events caused by primary central nervous system diseases, central nervous system infections, metabolic abnormalities, electrolyte imbalance, trauma, degenerative disease, neoplasm, toxic exposure, major substance abuse, and primary psychiatric diseases were excluded. NP events were new or worsened at baseline. The control group was comprised of 220 SLE patients without NPSLE treated at Peking Union Medical College Hospital at the same time. The control group was matched for sex, age, and disease duration.
2.2. Data Collection
Clinical data were retrospectively collected, including demographic data (sex, age at enrollment, SLE duration, and NP duration), systemic manifestations, physical signs (including neurological signs), and laboratory examination results, including complete blood count, blood biochemical examination, immunoglobulin, complement, and anti-nuclear antibody (ANA) spectrum, including anti-ribosomal P protein antibody and antiphospholipid (aPL) antibody results consisting of anticardiolipin (aCL), anti-β2 glycoprotein 1, and lupus anticoagulant (LA). Dilute Russell viper venom time (dRVVT) was used to test LA. Cerebrospinal fluid (CSF) was collected and biochemical analyses and cranial MRI were performed. Data regarding the treatment and prognosis were also compiled. All the above manifestations and laboratory test results were recorded at the time of the NP event for NPSLE patients and at enrollment for the non-NPSLE patients. The SLE disease activity index (SLEDAI) was used to evaluate lupus activity [14]. SLEDAI ≥ 15 points indicates severe SLE activity [14]. The follow-up period was identified as the time of enrollment until the time of death or last follow-up examination. The survival time of NPSLE patients was identified as the period from the new onset of NP manifestations to the end of follow-up. The worsened patients were excluded from this analysis. Causes of death were determined by reviewing the case records and discussions with the attending physicians [15].
2.3. Cranial MRI Data Collection
The MRI results of all patients included T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), fluid-attenuated inversion recovery (FLAIR) pulse sequence, diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC). Some patients underwent perfusion-weighted imaging and enhanced T1WI. All MRI results were read and reported by two neuroradiologists who did not know the clinical manifestations, examination results, and prognosis information of the patients. Abnormal cranial MRI results were divided into inflammatory lesions, large vessel disease (LVD), and small vessel disease (SVD). Inflammatory lesions, defined as high signal on T1WI/FLAIR, can involve gray matter or white matter, are medium in size, have unclear boundaries and nonvascular distribution, and may have an occupying effect. LVD, defined as cerebral infarction in the great artery area, was described according to the number of lesions (single/multiple), related artery, and status (acute/chronic). According to the standards for reporting vascular changes observed on neuroimaging (stream), SVD was divided into white matter hyperintensity (WMH; including the basal ganglia and subtentorial area), short-term subcutaneous small infarcts, luminal infarction, microbleeding, and brain atrophy [16].
2.4. Statistical Analysis
SPSS 23.0 software and GraphPad Prism 8.0 were used for statistical analyses. Continuous data are expressed as mean ± standard deviation (SD). An independent sample t-test was used to compare variables between the two groups. For non-normally distributed data, the Mann–Whitney U test was used. Categorical data are expressed as numbers or percentages. The chi-square test or the Fisher's exact test was used to analyze the relationship between categorical variables. P < 0.05 indicated statistical significance. A multivariate logistic stepwise regression was performed with variables with a P value less than 0.05 in univariate analysis. The Kaplan-Meier method was used for survival analyses, and the log-rank test was used to compare survival rates. The Cox proportional hazard model was adopted to analyze predicting factors for mortality.
3. Results
3.1. Clinical Characteristics of NPSLE Patients and NPSLE Subtypes
Data from a total of 194 NPSLE patients were collected; 180 (92.8%) patients were female, and the average age was 29.9 ± 10.8 years. The median duration of SLE was 16 months (range, 0-361 months). The median duration of NP was 0.5 months (range, 0-191 months). NP involvement was the first symptom of 92 (47.4%) patients. Of all NPSLE patients, 184 (94.8%) had central nervous system (CNS) involvement, 21 (10.8%) had peripheral nervous system (PNS) involvement, and 11 (5.7%) had both CNS and PNS involvement. There were 16 subtypes of NPSLE. Seventy-one patients (36.6%) had more than one NP subtype. The most common subtypes are seizure, acute confusional state (ACS), cerebrovascular disease, headache, and psychosis (Table 1). Regarding laboratory examination results, 153 patients (78.9%) had hypocomplementemia during the course of the disease, 192 (99%) patients had positive antinuclear antibody (ANA), 97 patients (50.0%) had positive anti-dsDNA antibody, 81 patients (41.8%) had positive antiribosomal P protein antibody, and 58 patients (29.9%) had positive antiphospholipid antibody (aPL). The average SLEDAI score of NPSLE patients was 20.3 ± 9.1 points (range, 0-44 points); after removing the nervous system score, the average SLEDAI score was 12.3 ± 6.6 points (range 0-28 points). Lumbar puncture and CSF examinations were completed for all patients; 73 patients (37.6%) had elevated CSF pressure, and 78 patients (40.2%) had elevated CSF protein levels. Steroid pulse therapy (intravenous drip of methylprednisolone 1000 mg daily for three consecutive days) was administered to 146 patients (75.3%), which continued with oral prednisone (1 mg/kg·d) for 4 weeks; then, the steroid was tapered down. Immunosuppressive therapy was administered to 191 patients (98.5%); of these, 179 (92.3%) received cyclophosphamide (CTX) alone, 9 (4.6%) received mycophenolate mofetil (MMF) alone (Table 1), and 6 (3.1%) were administered combined immunosuppressive agents. Furthermore, 18 (9.3%) patients were on anticoagulation or antiplatelet treatment, 13 (6.7%) patients were on antiepileptic drugs, and 26 (13.4%) patients were on antipsychotics.
Table 1.
Baseline characteristics of the 194 patients with NPSLE.
| Characteristics | Value (n = 194) (%) |
|---|---|
| Female | 180 (92.8) |
| Age (years) | 29.8 ± 10.8 |
| SLE duration (years), mean ± SD | 3.09 ± 4.40 |
| NP duration (years), mean ± SD | 2.78 ± 4.16 |
| >1 NP subtype | 71 (36.6) |
| Subtypes of NPSLE | |
| Central nervous system involvement | 184 (94.8) |
| Seizure | 71 (36.6) |
| Acute confusional state | 49 (25.3) |
| Cerebrovascular disease | 30 (15.5) |
| Headache | 27 (13.9) |
| Psychosis | 22 (11.3) |
| Cognitive impairment | 19 (9.8) |
| Mood disorder | 17 (8.8) |
| Demyelination | 6 (3.1) |
| Dyskinesia | 1 (0.5) |
| Myelitis | 5 (2.6) |
| Aseptic meningitis | 4 (2.1) |
| Anxiety disorder | 3 (1.5) |
| Peripheral nervous system involvement | 21 (10.8) |
| Cranial neuropathy | 8 (15.5) |
| AIDP | 3 (1.5) |
| Single/multiplex mononeuropathy | 5 (3.6) |
| Polyneuropathy | 5 (4.1) |
| Increased CSF pressure | 73 (37.6) |
| Increased CSF protein | 78 (40.2) |
| Treatment | |
| Steroid pulse | 146 (75.3) |
| CTX | 179 (92.3) |
| MMF | 9 (4.6) |
NPSLE: neuropsychiatric systemic lupus erythematosus; AIDP: acute inflammatory demyelinating polyradiculoneuropathy; CSF: cerebrospinal fluid; CTX: cyclophosphamide; MMF: mycophenolate mofetil.
3.2. Comparison of Clinical Characteristics of the NPSLE Group and Non-NPSLE Group
Compared to the control group, NPSLE patients had a significantly higher likelihood of also having malar rash (P < 0.001), oral ulcer (P < 0.001), alopecia (P = 0.001), arthritis (P = 0.004), serositis (P < 0.001), renal disorder (P = 0.001), and fever (P < 0.001) than non-NPSLE patients. Leukopenia (P < 0.001), thrombocytopenia (P < 0.001), and hypocomplementemia (P < 0.001) significantly more frequently occurred in NPSLE patients than in the control group. The SLEDAI-2K score after removing the NP scores of the NPSLE group was significantly higher than that of non-NPSLE group (12.3 ± 6.8 vs. 8.7 ± 6.7, P < 0.001). Furthermore, the erythrocyte sedimentation rate (ESR) (P < 0.001) and CRP (P < 0.001) levels of the NPSLE group were significantly higher than those of the non-NPSLE group. The positive rate of anti-ribosomal P protein antibodies in the NPSLE group was significantly higher than that in the non-NPSLE group (41.8% vs. 21.4%, P < 0.001), but the positive rates of the anti-Sm antibodies and anti-SSB antibodies in the NPSLE group were significantly lower (35.6% vs. 51.8%, P = 0.001 and 17.5% vs. 32.7%, P = 0.001, respectively) than those of the non-NPSLE group. There was no difference in the positive aPL rates of the two groups (Table 2). In the logistic regression analysis, compared with the non-NPSLE group, the NPSLE group had more frequent malar rash (OR = 3.30, 95% CI 1.91–5.68, P < 0.001), serositis (OR = 3.98, 95% CI 2.12–7.45, P < 0.001), thrombocytopenia (OR = 2.10, 95% CI 1.17–3.78, P = 0.013), SLEDAI scores (excluding NP manifestations) ≥ 15 (OR = 2.97, 95% CI 1.50–5.90, P = 0.002), and positive rate of anti-ribosomal P protein antibodies (OR = 2.01, 95% CI 1.20–3.37, P = 0.008).
Table 2.
Comparison of clinical characteristics of the NPSLE and non-NPSLE groups.
| NPSLE group (n = 194) (%) | Non-NPSLE group (n = 220) (%) | P value | |
|---|---|---|---|
| Female | 180 (92.8) | 202 (91.8) | 0.714 |
| Age (years), mean ± SD | 29.86 ± 10.78 | 31.78 ± 11.25 | 0.397 |
| SLE duration (years), mean ± SD | 3.09 ± 4.40 | 2.81 ± 4.57 | 0.535 |
| Malar rash | 136 (70.1) | 79 (35.9) | <0.001 |
| Oral ulcer | 39 (20.1) | 17 (7.7) | <0.001 |
| Alopecia | 75 (38.7) | 52 (23.6) | 0.001 |
| Arthritis | 60 (30.9) | 41 (18.6) | 0.004 |
| Vasculitis | 12 (6.2) | 20 (9.1) | 0.269 |
| Serositis | 84 (43.3) | 21 (9.5) | <0.001 |
| Renal involvement | 103 (53.1) | 82 (37.3) | 0.001 |
| Fever | 79 (40.7) | 35 (15.9) | <0.001 |
| Leukopenia | 97 (50.0) | 40 (18.2) | <0.001 |
| Thrombocytopenia | 84 (43.3) | 31 (14.1) | <0.001 |
| ESR (mm/h) | 45.73 ± 32.79 | 33.29 ± 28.84 | <0.001 |
| CRP (mg/L) | 16.72 ± 31.57 | 6.56 ± 10.02 | <0.001 |
| Hypocomplementemia | 153 (78.9) | 138 (62.7) | <0.001 |
| Anti-dsDNA positive | 97 (50.0) | 116 (52.7) | 0.580 |
| SLEDAI | 20.3 ± 9.1 | 8.6 ± 6.7 | <0.001 |
| SLEDAI-removed NP scores | 12.3 ± 6.6 | 8.6 ± 6.7 | <0.001 |
| Immunologic disorder at enrollment | |||
| Anti-Sm positive | 69 (35.6) | 114 (51.8) | 0.001 |
| Anti-RNP positive | 101 (52.1) | 121 (55.0) | 0.489 |
| Anti-SSA positive | 126 (64.9) | 151 (68.6) | 0.436 |
| Anti-SSB positive | 34 (17.5) | 72 (32.7) | 0.001 |
| Anti-ribosomal P protein positive | 81 (41.8) | 47 (21.4) | <0.001 |
| Anticardiolipin positive | 31 (16.6) | 19 (11.7) | 0.189 |
| Lupus anticoagulant positive | 38 (22.2) | 44 (24.9) | 0.562 |
| Anti-β2 glycoprotein 1 positive | 26 (14.3) | 18 (9.6) | 0.167 |
NPSLE: neuropsychiatric systemic lupus erythematosus; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; dsDNA: double-stranded deoxyribonucleic acid; SLEDAI: systemic lupus erythematosus disease activity index; NP: neuropsychiatric; RNP: ribonucleoprotein; Sm: Smith; SSA: Sjogren's syndrome antigen A; SSB: Sjogren's syndrome antigen B. Significant P values are shown in bold typeface.
3.3. Prognostic Analysis of Clinical Characteristics of NPSLE Patients
The average survival time of NPSLE patients was 35.5 ± 23.5 months. Among the 194 patients, 16 died (1 male and 15 female patients). The overall 1-, 2-, 3-, and 4-year survival rates of NPSLE patients were 94.1% (95% confidence interval (CI), 92.5%-95.7%), 93.0% (95% CI, 91.3%-94.7%), 92.2% (95% CI, 90.0%-94.4%), and 85.6% (95% CI 81.2%-90.0%), respectively (Figure 1). The main causes of death were infection (6 patients; 37.5%), SLE-related causes (5 patients; 31.25%: pulmonary hypertension for 2 patients; nervous system involvement for 2 patients, and sudden cardiac death for 1 patient), and unknown cause (5 patients; 31.25%). The average follow-up time from enrolment to the endpoint for non-NP SLE patients was 50.2 ± 11.7 months. Eight patients died in the non-NPSLE group. The survival rate of the NPSLE group was significantly lower than that of the non-NPSLE group (P = 0.001) (Figure 1). NPSLE patients with a SLEDAI score ≥ 15 (P = 0.014), proteinuria (P = 0.035), elevated serum creatinine (Scr) (P = 0.001), and hypocomplementemia (P = 0.031) had shorter survival periods (Table 3; Figure 1). On multivariate analysis, elevated Scr (P = 0.029) was an independent prognostic factor of death (Table 4).
Figure 1.
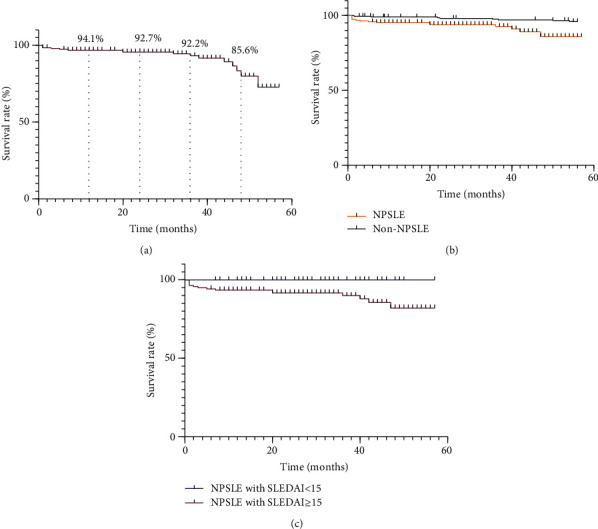
(a) The overall 1-, 2-, 3-, and 4-year survival rates of NPSLE patients. (b) Survival rates of NPSLE patients and non-NPSLE patients. The survival rate of NPSLE patients is significantly lower than that of non-NPSLE patients (P = 0.001). (c) Survival rates of NPSLE patients with SLEDAI score < 15 and SLEDAI score ≥ 15. The survival rate of NPSLE patients with SLEDAI score < 15 was significantly higher than that of patients with SLEDAI score ≥ 15 (P = 0.014).
Table 3.
Prognostic analysis of clinical characteristics of NPSLE patients.
| Risk factors | Dead group (N = 16), n (%) | Survival group (N = 178), n (%) | χ 2 | P value |
|---|---|---|---|---|
| Female | 15 (93.8) | 165 (92.7) | 0.049 | 0.824 |
| Age older than 25 years | 12 (75.0) | 111 (62.4) | 1.394 | 0.238 |
| Seizure | 9 (56.3) | 62 (34.8) | 2.945 | 0.086 |
| Acute confusional state | 5 (31.3) | 44 (24.7) | 0.345 | 0.557 |
| Cerebrovascular disease | 4 (25.0) | 27 (15.2) | 0.823 | 0.364 |
| Psychosis | 0 (0) | 22 (12.4) | 2.229 | 0.135 |
| Rash | 11 (68.8) | 15 (70.2) | 0.002 | 0.964 |
| Oral ulcer | 3 (18.8) | 36 (20.2) | 0.002 | 0.963 |
| Vasculitis | 0 (0) | 12 (6.7) | 1.099 | 0.294 |
| Arthritis | 8 (50.0) | 52 (29.2) | 2.795 | 0.095 |
| Serositis | 9 (56.3) | 75 (42.1) | 0.880 | 0.348 |
| Respiratory involvement | 5 (31.3) | 28 (15.7) | 2.422 | 0.120 |
| Cardiac involvement | 6 (37.5) | 68 (38.2) | 0.001 | 0.970 |
| Renal involvement | 12 (75.0) | 90 (50.6) | 3.108 | 0.078 |
| Proteinuria | 13 (81.3) | 94 (52.8) | 4.436 | 0.035 |
| Elevated serum creatinine | 6 (37.5) | 19 (10.7) | 10.584 | 0.001 |
| Hematological involvement | 10 (62.5) | 103 (57.9) | 0.156 | 0.693 |
| Hypocomplementemia | 16 (100) | 137 (80.0) | 4.666 | 0.031 |
| Anti-dsDNA positive | 9 (56.3) | 88 (49.4) | 0.273 | 0.602 |
| Anti-Sm positive | 6 (37.5) | 64 (36.0) | 0.031 | 0.861 |
| Anti-RNP positive | 8 (16.0) | 93 (52.2) | 0.045 | 0.831 |
| Anti-SSA positive | 10 (62.5) | 116 (65.2) | 0.004 | 0.953 |
| Anti-SSB positive | 2 (12.5) | 32 (18.0) | 0.296 | 0.587 |
| Anti-ribosomal P protein positive | 9 (56.3) | 72 (40.4) | 1.397 | 0.237 |
| Anticardiolipin positive | 1 (6.3) | 30 (16.9) | 1.042 | 0.307 |
| Anti-β2 glycoprotein 1 positive | 2 (12.5) | 24 (13.5) | 0.035 | 0.852 |
| Lupus anticoagulant positive | 1 (6.3) | 37 (20.8) | 1.616 | 0.204 |
| SLEDAI ≥ 15 | 16 (100) | 122 (68.5) | 6.076 | 0.014 |
| Elevated ESR | 12 (75.0) | 126 (70.8) | 0.097 | 0.755 |
| Elevated CRP | 10 (93.8) | 109 (61.2) | 0.029 | 0.866 |
| Increased CSF pressure | 5 (31.3) | 68 (38.2) | 0.228 | 0.633 |
| Increased CSF protein | 5 (31.3) | 73 (41.0) | 0.358 | 0.550 |
| Steroid pulse | 13 (81.3) | 133 (74.7) | 0.363 | 0.547 |
NPSLE: neuropsychiatric systemic lupus erythematosus; dsDNA: double-stranded deoxyribonucleic acid; RNP: ribonucleoprotein; Sm: Smith; SSA: Sjogren's syndrome antigen A; SSB: Sjogren's syndrome antigen B; SLEDAI: systemic lupus erythematosus disease activity index; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; CSF: cerebrospinal fluid. Significant P values are shown in bold typeface.
Table 4.
Cox's regression modeling of predictors of mortality.
| Risk factor | HR (95% CI) | P value |
|---|---|---|
| Proteinuria | 2.16 (0.54-8.59) | 0.276 |
| Elevated serum creatinine | 3.27 (1.13-9.45) | 0.029 |
| SLEDAI ≥ 15 | 1.71 (0.57 -5.14) | 0.340 |
HR: hazard ratio; CI: confidence interval; SLEDAI: systemic lupus erythematosus disease activity index. A multivariate Cox proportional hazard model was employed to identify independent predictors of survival. Significant P values are shown in bold typeface.
3.4. Comparison of Clinical Characteristics and Prognosis for NPSLE Patients with Seizure and Non-NPSLE Patients and NPSLE Patients without Seizure
Malar rash (P < 0.001), oral ulcer (P = 0.001), serositis (P < 0.001), renal involvement (P = 0.009), fever (P < 0.001), leukopenia (P < 0.001), and thrombocytopenia (P < 0.001) occurred significantly more frequently in NPSLE patients with seizure than in non-NPSLE patients. The SLEDAI-2K score, after the removal of NP scores, of NPSLE patients with seizure was significantly higher than that of non-NPSLE patients (13.3 ± 7.0 vs. 8.6 ± 6.7, P < 0.001). The positive rate of anti-ribosomal P protein antibodies for seizure patients was significantly higher than that for non-NPSLE patients (P = 0.001). Although there was no significant difference, the positive rate of anti-β2GP1 antibodies for seizure patients was higher than that for non-NPSLE patients (P = 0.058) (Table 5). This study also analyzed the clinical characteristics of NPSLE patients with seizure and those without seizure. Serositis (57.7% vs. 35.0%, P = 0.002) and thrombocytopenia (45.1% vs. 30.9%, P = 0.048) occurred significantly more frequently in NPSLE patients with seizure than in those without seizure. The SLEDAI-2K score of NPSLE patients with seizure was significantly higher than that of NPSLE patients without seizure (25.3 ± 8.8 vs 17.4 ± 8.0, P < 0.001) (Table 5). Among 71 seizure patients, 35 patients had diffuse NPSLE. There was no difference between seizure patients with diffuse NPSLE and those without diffuse NPSLE in the positivity of anti-ribosomal P protein antibodies (40.0% vs 42.9%, P = 0.808). Seventy-one NPSLE patients with seizure were followed up; of them, nine died. Thirteen of the 62 surviving patients were still using antiepileptic drugs at the last follow-up examination, and three patients had recurrent seizure symptoms within 6 months after the last follow-up examination.
Table 5.
Comparison of clinical characteristics of NPSLE patients with seizure, NPSLE patients without seizure, and non-NPSLE patients.
| NPSLE with seizure, (n = 71) (%) | Non-NPSLE (n = 220) (%) | P value | NPSLE without seizure, (n = 123) (%) | P value | |
|---|---|---|---|---|---|
| Female | 66 (93.0) | 202 (91.8) | 0.757 | 114 (92.7) | 0.943 |
| Age (years) | 26.20 ± 8.41 | 30.78 ± 11.25 | 0.002 | 32.0 ± 11.4 | <0.001 |
| SLE duration (years) | 2.66 ± 3.71 | 2.81 ± 4.57 | 0.796 | 3.3 ± 4.7 | 0.304 |
| Malar rash | 51 (71.8) | 79 (35.9) | <0.001 | 85 (69.1) | 0.690 |
| Oral ulcer | 16 (22.5) | 17 (7.7) | 0.001 | 23 (18.7) | 0.521 |
| Arthritis | 21 (29.6) | 41 (18.6) | 0.050 | 39 (31.7) | 0.757 |
| Serositis | 41 (57.7) | 21 (9.5) | <0.001 | 43 (35.0) | 0.002 |
| Vasculitis | 5 (7.0) | 20 (9.1) | 0.592 | 7 (5.7) | 0.707 |
| Renal involvement | 39 (54.9) | 82 (37.3) | 0.009 | 64 (52.0) | 0.697 |
| Fever | 30 (42.3) | 35 (15.9) | <0.001 | 49 (39.8) | 0.741 |
| Leukopenia | 37 (52.1) | 46 (20.9) | <0.001 | 48 (39.0) | 0.077 |
| Thrombocytopenia | 32 (45.1) | 33 (15.0) | <0.001 | 38 (30.9) | 0.048 |
| ESR (mm/h) | 49.15 ± 33.20 | 33.29 ± 28.84 | <0.001 | 43.72 ± 32.52 | 0.296 |
| CRP (mg/L) | 18.55 ± 36.83 | 6.56 ± 10.02 | <0.001 | 15.65 ± 28.17 | 0.540 |
| Hypocomplementemia | 57 (80.3) | 138 (62.7) | 0.006 | 96 (78.0) | 0.714 |
| Anti-dsDNA positive | 33 (46.5) | 116 (52.7) | 0.360 | 58 (47.2) | 0.506 |
| Anti-Sm positive | 27 (38.6) | 114 (51.8) | 0.043 | 43 (35.0) | 0.616 |
| Anti-RNP positive | 38 (54.3) | 121 (55.0) | 0.210 | 63 (51.2) | 0.628 |
| Anti-SSA positive | 42 (60.0) | 151 (68.6) | 0.087 | 84 (68.3) | 0.245 |
| Anti-SSB positive | 11 (15.7) | 72 (32.7) | 0.005 | 23 (18.7) | 0.601 |
| Anti-RibP positive | 29 (40.8) | 47 (21.4) | 0.001 | 52 (42.6) | 0.872 |
| aCL positive | 11/67 (16.4) | 19/163 (11.7) | 0.330 | 20/120 (16.7) | 0.965 |
| LA positive | 13/62 (21.0) | 44 (24.9) | 0.536 | 25/109 (22.9) | 0.766 |
| Anti-β2GP1 positive | 12/65 (18.5) | 18/187 (9.6) | 0.058 | 14/117 (12.0) | 0.230 |
| SLEDAI | 25.3 ± 8.8 | 8.6 ± 6.7 | <0.001 | 17.4 ± 8.0 | <0.001 |
| SLEDAI without NP scores | 13.3 ± 7.0 | 8.6 ± 6.7 | <0.001 | 11.7 ± 6.6 | 0.105 |
NPSLE: neuropsychiatric systemic lupus erythematosus; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; dsDNA: double-stranded deoxyribonucleic acid; RNP: ribonucleoprotein; Sm: Smith; SSA: Sjogren's syndrome antigen A; SSB: Sjogren's syndrome antigen B; RibP: ribosomal P protein; aCL: anticardiolipin; LA: lupus anticoagulant; β2GP1: β2 glycoprotein 1; SLEDAI: systemic lupus erythematosus disease activity index. Significant P values are shown in bold typeface.
3.5. Analysis of Cranial MRI of NPSLE Patients
Among 194 patients, cranial MRI data of 82 patients were finally collected and reviewed in detail by two experienced neuroradiologists. Among 82 patients, 50 (61.0%) had abnormal MRI results, including 44 (88.0%) SVD, 3 (6.0%) LVD, and 3 (6.0%) inflammatory disease. Thirty-nine (78.0%) patients had white matter hyperintensities (WMH); these were mainly focal and most often involved the frontal lobe. Three patients (6.0%) had lumen infarction, 7 (14.0%) had subcutaneous infarction, 6 (12.0%) had microhemorrhage on MRI, and 19 (38.0%) had brain atrophy. At the univariate analysis, patients with inflammatory lesions (P = 0.019) and LVD (P = 0.034) had shorter survival periods (Table 6). At multivariate analysis using Cox regression analysis, inflammatory lesions (P = 0.021), LVD (P = 0.038), proteinuria (P = 0.033), and elevated serum creatinine (P < 0.001) were independent prognostic factors of death.
Table 6.
Prognostic analysis of neuroimaging results of NPSLE patients.
| Risk factor | Dead group (n = 5), n (%) | Survival group (n = 77), n (%) | χ 2 | P |
|---|---|---|---|---|
| Abnormal MRI | 3 (60.0%) | 47 (61.0%) | 0.006 | 0.936 |
| Inflammatory lesions | 1 (20.0%) | 2 (2.6%) | 5.516 | 0.019 |
| Large vessel disease | 1 (20.0%) | 2 (2.6%) | 4.512 | 0.034 |
| Small vessel disease | 1 (20.0%) | 43 (55.8%) | 2.453 | 0.117 |
| White matter hyperintensities | 2 (40.0%) | 37 (48.1%) | 0.129 | 0.720 |
| Lacunes | 0 (0) | 3 (3.9%) | 0.196 | 0.658 |
| Subcutaneous infarction | 1 (20.0%) | 6 (7.8%) | 0.919 | 0.338 |
| Microbleeds | 0 (0) | 6 (7.8%) | 0.408 | 0.523 |
| Brain atrophy | 1 (20.0%) | 18 (23.4%) | 0.040 | 0.841 |
NPSLE: neuropsychiatric systemic lupus erythematosus; MRI: magnetic resonance imaging. Significant P values are shown in bold typeface.
4. Discussion
In this study, we analyzed the risk factors for the development of NP manifestations and prognosis factors for NPSLE in Chinese SLE patients. Higher disease activity and positive anti-ribosomal P antibody levels increased the risk of NPSLE development. NPSLE patients with renal insufficiency and higher SLEDAI scores had worse prognoses. We also analyzed the cranial MRI results of many patients. Patients with inflammatory lesions and LVD on MRI might have poor prognoses.
Nervous system involvement is common in SLE and is a major cause of morbidity and mortality [17]. The majority of patients with NPSLE were female in this study and in previous studies; this is because SLE more commonly occurs in women. The mean age of our patients at the onset of NPSLE was similar to the median age of 27.5 to 28 years reported in other studies [18]. In this study, NP syndromes usually occurred within 3 years after onset of SLE, which is consistent with other studies [19]. Some studies showed that NP syndromes can occur during the early stage of SLE [20, 21]. NPSLE commonly occurs in young and middle-aged patients, and it often occurs during the early stage of SLE. Therefore, treatment is urgently necessary during these times.
This study showed that NPSLE patients were more likely to also have other typical lupus symptoms, and that a combination of multiple symptoms indicates high disease activity [22]. Furthermore, our results showed that the SLEDAI-2K score of NPSLE patients is significantly higher than that of non-NPSLE patients, even after removing the scores related to NP symptoms. Patients are prone to NP symptoms during high lupus activity, and this result was consistent with that of previous studies [23, 24]. It has been reported that the presence of active disease and the presence of circulating autoantibodies are major risk factors for NP events [25]. In this study, leukopenia and thrombocytopenia were more likely to occur in NPSLE patients. Early observation suggested that thrombocytopenia was significantly and strikingly correlated with NP involvement in SLE patients [22]. Furthermore, it is widely accepted that antiribosomal P is related to NP involvement [26], which was confirmed by our results. Anti-RibP is more specifically associated with specific NPSLE manifestations, CNS involvement, depression, and psychosis [27]. Other antibodies, such as autoantibodies to NMDA receptor subunit NR2 (anti-NR2) [28] and anti-glucose-regulated protein 78 (anti-GRP78) [29], have been associated with the development of diffuse NPSLE. Also, the presence of anti-NMDA in cerebrospinal fluid but not in serum is associated significantly with overwhelming CNS abnormalities, suggesting the importance of direct access of autoantibodies to brain dysfunction [30]. However, it remains controversial whether the antibodies discussed above are important biomarkers for NPSLE; it may be advantageous to have more sensitive antigens to allow for earlier detection and treatment.
Previous studies reported that SLE patients are at higher risk for seizures than the general population [21, 31]. In this study, seizure was the most common subtype of NPSLE, which is consistent with the results of a SLICC study [32]. The pathogenesis of seizure in SLE is not clear; systemic inflammation, focal microvascular brain injuries, direct autoantibody effects on neuronal networks, and/or APS may all have a role [33, 34]. Other studies indicated that risk factors for seizures in SLE include disease activity, female sex, race, aPL level, and younger age [33, 35–37]. This study also confirmed that higher disease activity of lupus, positive anti-β2GP1, and younger age were risk factors for seizure in lupus.
It has been reported that NPSLE is a poor prognostic factor for SLE patients [21, 38, 39], which is consistent with our results. It has also been reported that ACS, seizure, and CVD in NPSLE are risk factors that affect survival [39]. Our results showed that NPSLE patients with high disease activity had decreased survival rates. Strong immunosuppressive treatments were used for NPSLE patients. However, aggressive treatment, including high doses of corticosteroids and strong immunosuppressants such as cyclophosphamide and mycophenolate mofetil may increase the risk of infection. Previous studies confirmed that the risk of opportunistic infection is higher in systemic lupus erythematosus patients treated with steroids than those not treated with steroids. Medium and high doses were associated with a higher risk of opportunistic infection compared with low doses [40, 41]. The most common cause of death in this study was infection, which is consistent with a previous study [19]. Therefore, research efforts are aggressively pursuing better treatment that can effectively control NPSLE and poses a relatively small risk of infection. Several cytokines have been preliminarily proven to be related to NPSLE. Agents for these specific cytokines are currently being explored, including type I IFN receptor inhibition [42], macrophage colony-stimulating factor 1 receptor (CSF1R) [43], and Bruton tyrosine kinase (BTK) inhibitor [44]. Furthermore, our result showed renal involvement, especially elevated Scr had decreased the survival rate of NPSLE patients, which was consistent with a previous study [19]. It was reported that lupus nephritis is a major risk factor for overall morbidity and mortality in SLE [45, 46], which could explain why elevated Scr is the risk factor of mortality in NPSLE.
Similar to a previous study, more than 30% of NPSLE patients had normal cranial MRI results [47]. Nearly half of the abnormal MRI results showed WMH, mainly involving the frontal lobe and parietooccipital lobe, which was also similar to other study results [48]. Inflammatory lesions occurred in 5.9% of patients who underwent MRI in this study. Other studies showed that the prevalence of inflammatory lesions ranged from 0% to 38.1% [23, 24]. A previous study showed that inflammatory lesions may be related to cerebral vasculitis in SLE patients, which can be diffuse or have focal distribution, and the large and small arteries can be involved [49]. A correlation between inflammatory lesions and clinical features was not found in this study, but the survival rate of patients with inflammatory lesions on MRI was significantly reduced. No similar study has focused on the relationship between inflammatory lesions and the prognosis for NPSLE. Another study has shown that abnormal cranial MRI results are poor prognostic factors for patients with diffuse NPSLE [50]; however, such results were not found in our study. Because a small number of patients underwent detailed MRI in this study, the results need to be verified by larger-scale research.
There were several limitations to this study. This was a retrospective study and the control group was relatively small, which could have affected the outcome and conclusions. Using hospitalized NPSLE patients may have led to a higher proportion of patients with severe cases. This study also had a short follow-up period. Longer-term studies are needed to investigate late mortality and organ damage of NPSLE patients. Furthermore, this was a single-center study; therefore, there was minimal ethnic variation.
5. Conclusions
High disease activity and positive rate of anti-ribosomal P protein antibodies may be risk factors for NPSLE. NPSLE decreases survival rates of SLE patients. Renal insufficiency and high disease activity are predictive of poor prognoses for NPSLE patients.
Acknowledgments
This study was supported by the Chinese National Key Technology R&D Program, Chinese Ministry of Science and Technology (2017YFC0907601, 2017YFC0907602, and 2017YFC0907603) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-2-008).
Contributor Information
Mengtao Li, Email: mengtao.li@cstar.org.cn.
Xiaofeng Zeng, Email: zengxfpumc@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Authors' Contributions
Shangzhu Zhang and Meng Li contributed equally to this work.
References
- 1.Ahn G. Y., Kim D., Won S., et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus. 2018;27(8):1338–1347. doi: 10.1177/0961203318772021. [DOI] [PubMed] [Google Scholar]
- 2.Petri M., Orbai A. M., Alarcón G. S., et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis & Rheumatism. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unterman A., Nolte J. E., Boaz M., Abady M., Shoenfeld Y., Zandman-Goddard G. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Seminars in Arthritis and Rheumatism. 2011;41(1):1–11. doi: 10.1016/j.semarthrit.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Hanly J. G., Su L., Urowitz M. B., et al. Mood disorders in systemic lupus erythematosus: results from an international inception cohort study. Arthritis & Rheumatology. 2015;67(7):1837–1847. doi: 10.1002/art.39111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanly J. G., Harrison M. J. Management of neuropsychiatric lupus. Best Practice & Research Clinical Rheumatology. 2005;19(5):799–821. doi: 10.1016/j.berh.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Bortoluzzi A., Scirè C. A., Bombardieri S., et al. Development and validation of a new algorithm for attribution of neuropsychiatric events in systemic lupus erythematosus. Rheumatology. 2015;54(5):891–898. doi: 10.1093/rheumatology/keu384. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz N., Stock A. D., Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nature Reviews Rheumatology. 2019;15(3):137–152. doi: 10.1038/s41584-018-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeltsch-David H., Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nature Reviews Neurology. 2014;10(10):579–596. doi: 10.1038/nrneurol.2014.148. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso C. R., Signorelli F. V., Papi J. A., Salles G. F. Initial and accrued damage as predictors of mortality in Brazilian patients with systemic lupus erythematosus: a cohort study. Lupus. 2008;17(11):1042–1048. doi: 10.1177/0961203308093829. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40(9) doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis & Rheumatism. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::aid-anr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8(Supplement 7):1–96. [PubMed] [Google Scholar]
- 13.Gmitrowicz A., Kucharska A. Developmental disorders in the fourth edition of the American classification: diagnostic and statistical manual of mental disorders (DSM IV -- optional book) Psychiatria polska. 1994;28(5):509–521. [PubMed] [Google Scholar]
- 14.Bombardier C., Gladman D. D., Urowitz M. B., et al. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis & Rheumatism. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 15.Mok C. C. Epidemiology and survival of systemic lupus erythematosus in Hong Kong Chinese. Lupus. 2011;20(7):767–771. doi: 10.1177/0961203310388447. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw J. M., Smith E. E., Biessels G. J., et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neill S., Cervera R. Systemic lupus erythematosus. Best Practice & Research Clinical Rheumatology. 2010;24(6):841–855. doi: 10.1016/j.berh.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Steup-Beekman G. M., Zirkzee E. J., Cohen D., et al. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: epidemiology and radiology pointing to an immune-mediated cause. Annals of the rheumatic diseases. 2013;72(supplement 2):ii76–ii79. doi: 10.1136/annrheumdis-2012-202369. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Xiang X., Sun J., et al. Prevalence, outcome and prognostic factors of neuropsychiatric systemic lupus erythematosus: a real world single center study. Modern rheumatology. 2020;30(2):321–326. doi: 10.1080/14397595.2019.1589912. [DOI] [PubMed] [Google Scholar]
- 20.Yu H.-H., Lee J.-H., Wang L.-C., Yang Y.-H., Chiang B.-L. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus: a 20-year study. Lupus. 2016;15(10):651–657. doi: 10.1177/0961203306070990. [DOI] [PubMed] [Google Scholar]
- 21.Hanly J. G., Urowitz M. B., Su L., et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2010;69(3):529–535. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karassa F. B., Ioannidis J. P., Touloumi G., Boki K. A., Moutsopoulos H. M. Risk factors for central nervous system involvement in systemic lupus erythematosus. QJM: An International Journal of Medicine. 2000;93(3):169–174. doi: 10.1093/qjmed/93.3.169. [DOI] [PubMed] [Google Scholar]
- 23.Toledano P., Sarbu N., Espinosa G., Bargalló N., Cervera R. Neuropsychiatric systemic lupus erythematosus: magnetic resonance imaging findings and correlation with clinical and immunological features. Autoimmunity Reviews. 2013;12(12):1166–1170. doi: 10.1016/j.autrev.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Tan Z., Zhou Y., Li X., et al. Brain magnetic resonance imaging, cerebrospinal fluid, and autoantibody profile in 118 patients with neuropsychiatric lupus. Clinical Rheumatology. 2018;37(1):227–233. doi: 10.1007/s10067-017-3891-3. [DOI] [PubMed] [Google Scholar]
- 25.de Amorim J. C., Frittoli R. B., Pereira D., et al. Epidemiology, characterization, and diagnosis of neuropsychiatric events in systemic lupus erythematosus. Expert Review of Clinical Immunology. 2019;15(4):407–416. doi: 10.1080/1744666X.2019.1564040. [DOI] [PubMed] [Google Scholar]
- 26.Briani C., Lucchetta M., Ghirardello A., et al. Neurolupus is associated with anti-ribosomal P protein antibodies: an inception cohort study. Journal of Autoimmunity. 2009;32(2):79–84. doi: 10.1016/j.jaut.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Choi M. Y., FitzPatrick R. D., Buhler K., Mahler M., Fritzler M. J. A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmunity Reviews. 2020;19(3, article 102463) doi: 10.1016/j.autrev.2020.102463. [DOI] [PubMed] [Google Scholar]
- 28.Arinuma Y., Yanagida T., Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis & Rheumatism. 2008;58(4):1130–1135. doi: 10.1002/art.23399. [DOI] [PubMed] [Google Scholar]
- 29.Matsueda Y., Arinuma Y., Nagai T., Hirohata S. Elevation of serum anti-glucose-regulated protein 78 antibodies in neuropsychiatric systemic lupus erythematosus. Lupus Science & Medicine. 2018;5(1, article e000281) doi: 10.1136/lupus-2018-000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arinuma Y. Antibodies and the brain: anti-N-methyl-D-aspartate receptor antibody and the clinical effects in patients with systemic lupus erythematosus. Current Opinion in Neurology. 2018;31(3):294–299. doi: 10.1097/WCO.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 31.Watad A., Tiosano S., Bragazzi N. L., et al. Epilepsy among systemic lupus erythematosus patients: insights from a large database analysis. Neuroepidemiology. 2018;50(1-2):1–6. doi: 10.1159/000485136. [DOI] [PubMed] [Google Scholar]
- 32.Hanly J. G., Urowitz M. B., Su L., et al. Seizure disorders in systemic lupus erythematosus results from an international, prospective, inception cohort study. Annals of the Rheumatic Diseases. 2012;71(9):1502–1509. doi: 10.1136/annrheumdis-2011-201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appenzeller S., Cendes F., Costallat L. T. Epileptic seizures in systemic lupus erythematosus. Neurology. 2004;63(10):1808–1812. doi: 10.1212/01.wnl.0000144178.32208.4f. [DOI] [PubMed] [Google Scholar]
- 34.Rosati A., Guerrini R., Cimaz R. Lupus, antiphospholipid syndrome and epilepsy: an update. Lupus. 2016;26(1):3–5. doi: 10.1177/0961203316666961. [DOI] [PubMed] [Google Scholar]
- 35.Cieslinski J. Z., Skare T. L., Nisihara R., de Messias-Reason I. J., Utiyama S. R. R. Mannose-binding lectin serum levels in patients with systemic lupus erythematosus: association with thrombocytopaenia and seizure. Lupus. 2018;27(3):372–379. doi: 10.1177/0961203317722846. [DOI] [PubMed] [Google Scholar]
- 36.Huang X., Magder L. S., Petri M. Predictors of incident seizure in systemic lupus erythematosus. The Journal of Rheumatology. 2016;43(3):565–575. doi: 10.3899/jrheum.150135. [DOI] [PubMed] [Google Scholar]
- 37.Hawro T., Bogucki A., Krupińska-Kun M., Maurer M., Woźniacka A. Intractable headaches, ischemic stroke, and seizures are linked to the presence of anti-β2GPI antibodies in patients with systemic lupus erythematosus. PLoS One. 2015;10(3, article e0119911) doi: 10.1371/journal.pone.0119911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zirkzee E. J. M., Huizinga T. W. J., Bollen E. L. E. M., et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE) Lupus. 2013;23(1):31–38. doi: 10.1177/0961203313512540. [DOI] [PubMed] [Google Scholar]
- 39.Tokano Y., Morimoto S., Amano H., et al. The relationship between initial clinical manifestation and long-term prognosis of patients with systemic lupus erythematosus. Modern Rheumatology. 2005;15(4):275–282. doi: 10.1007/s10165-005-0411-0. [DOI] [PubMed] [Google Scholar]
- 40.Yang S. C., Lai Y. Y., Huang M. C., Tsai C. S., Wang J. L. Corticosteroid dose and the risk of opportunistic infection in a national systemic lupus erythematosus cohort. Lupus. 2018;27(11):1819–1827. doi: 10.1177/0961203318792352. [DOI] [PubMed] [Google Scholar]
- 41.Pimentel-Quiroz V. R., Ugarte-Gil M. F., Harvey G. B., et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus. 2019;28(9):1101–1110. doi: 10.1177/0961203319860579. [DOI] [PubMed] [Google Scholar]
- 42.Bialas A. R., Presumey J., Das A., et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546(7659):539–543. doi: 10.1038/nature22821. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers S. A., Wen J., Shum J., Doerner J., Herlitz L., Putterman C. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clinical Immunology. 2017;185:100–108. doi: 10.1016/j.clim.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmers S. A., Wen J., Doerner J., et al. Highly selective inhibition of Bruton’s tyrosine kinase attenuates skin and brain disease in murine lupus. Arthritis Research & Therapy. 2018;20(1) doi: 10.1186/s13075-017-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng M., Lv J., Fu S., et al. Clinical features and mortality in Chinese with lupus nephritis and neuropsychiatric lupus: a 124-patient study. Journal of Research in Medical Sciences. 2014;19(5):414–419. [PMC free article] [PubMed] [Google Scholar]
- 46.Almaani S., Meara A., Rovin B. H. Update on lupus nephritis. Clinical Journal of the American Society of Nephrology. 2017;12(5):825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Postal M., Lapa A. T., Reis F., Rittner L., Appenzeller S. Magnetic resonance imaging in neuropsychiatric systemic lupus erythematosus: current state of the art and novel approaches. Lupus. 2017;26(5):517–521. doi: 10.1177/0961203317691373. [DOI] [PubMed] [Google Scholar]
- 48.Sarbu N., Alobeidi F., Toledano P., et al. Brain abnormalities in newly diagnosed neuropsychiatric lupus: systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort. Autoimmunity Reviews. 2015;14(2):153–159. doi: 10.1016/j.autrev.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Jennekens F. G., Kater L. The central nervous system in systemic lupus erythematosus. Part 2. Pathogenetic mechanisms of clinical syndromes: a literature investigation. Rheumatology. 2002;41(6):619–630. doi: 10.1093/rheumatology/41.6.619. [DOI] [PubMed] [Google Scholar]
- 50.Abe G., Kikuchi H., Arinuma Y., Hirohata S. Brain MRI in patients with acute confusional state of diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Modern Rheumatology. 2017;27(2):278–283. doi: 10.1080/14397595.2016.1193966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.


