Abstract
Objective
Tranexamic acid (TXA) is increasingly used in orthopedic surgery to reduce blood loss; however, there are concerns about the risk of venous thromboembolic (VTE) complications. The aim of this study was to evaluate TXA safety in patients undergoing lower limb orthopedic surgical procedures.
Design
A meta-analysis was performed on the PubMed, Web of Science, and Cochrane Library databases in January 2020 using the following string (Tranexamic acid) AND ((knee) OR (hip) OR (ankle) OR (lower limb)) to identify RCTs about TXA use in patients undergoing every kind of lower limb surgical orthopedic procedures, with IV, IA, or oral administration, and compared with a control arm to quantify the VTE complication rates.
Results
A total of 140 articles documenting 9,067 patients receiving TXA were identified. Specifically, 82 studies focused on TKA, 41 on THA, and 17 on other surgeries, including anterior cruciate ligament reconstruction, intertrochanteric fractures, and meniscectomies. The intravenous TXA administration protocol was studied in 111 articles, the intra-articular in 45, and the oral one in 7 articles. No differences in terms of thromboembolic complications were detected between the TXA and control groups neither in the overall population (2.4% and 2.8%, respectively) nor in any subgroup based on the surgical procedure and TXA administration route.
Conclusions
There is an increasing interest in TXA use, which has been recently broadened from the most common joint replacement procedures to the other types of surgeries. Overall, TXA did not increase the risk of VTE complications, regardless of the administration route, thus supporting the safety of using TXA for lower limb orthopedic surgical procedures.
1. Introduction
Lower limb procedures represent the majority of orthopedic surgeries, including joint arthroplasties, sport medicine treatments, and fracture osteosynthesis, with a high rate of over 500 per 100,000 population every year, increasing over time [1, 2]. Among these, total hip arthroplasty (THA) and total knee arthroplasty (TKA) are the most commonly performed. Albeit being successful procedures routinely performed in the clinical practice, they are frequently encumbered by complications [3]. In particular, joint arthroplasties are often associated with a significant amount of postoperative blood loss, ranging from 800 to 1,800 ml in primary TKA or THA, with up to 25% and 37% of patients, respectively, requiring blood transfusion for postoperative anemia [4, 5]. Allogenic blood transfusions are financial burdens, and even more importantly, they are associated with an unneglectable risk of serious complications, including infection, immunosuppression, and cardiovascular dysfunction [6], resulting in potentially life-threatening effects on patients [7]. Various strategies have been attempted to minimize blood loss and the need for blood transfusion [8], and to this aim, the use of hemostatic agents, in particular of tranexamic acid (TXA), has recently widely increased in orthopedic lower limb surgery.
TXA is a synthetic antifibrinolytic agent that competitively blocks the lysine binding sites on plasminogen, thereby slowing the conversion of plasminogen to plasmin, thus preventing fibrin clot degradation [9, 10]. A large amount of randomized controlled trials (RCTs) and high-level evidence [11–14] converges in showing that TXA, applied either through systemic or local administration, is effective in reducing blood loss and subsequent transfusions in replacement procedures, as well as in other kinds of lower limb surgeries, including sport medicine procedures and fracture treatment [15, 16]. However, there are still concerns about the risk of increasing venous thromboembolic (VTE) complications, such as deep venous thrombosis (DVT) or pulmonary embolisms (PE) [17, 18]. In this light, it is of paramount importance to understand if the scientific high-level literature evidence supports the safety of TXA for the different orthopedic applications.
Thus, the aim of this study was to evaluate, through a meta-analysis of RCTs, the safety of TXA used in patients undergoing lower limb orthopedic surgical procedures, including joint arthroplasty, fracture treatment, and sport injuries.
2. Material and Methods
2.1. Search Strategy and Article Selection
A meta-analysis was performed based on the literature evidence about the thromboembolic complications of TXA in patients undergoing orthopedic lower limb surgery. The search was conducted on the PubMed, Web of Science, and Cochrane Library databases on January 9, 2020, using the following parameters: (Tranexamic acid) AND ((knee) OR (hip) OR (ankle) OR (lower limb)). The guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were used [19]. A flowchart of the study selection for the quantitative data synthesis is reported in Figure 1.
Figure 1.
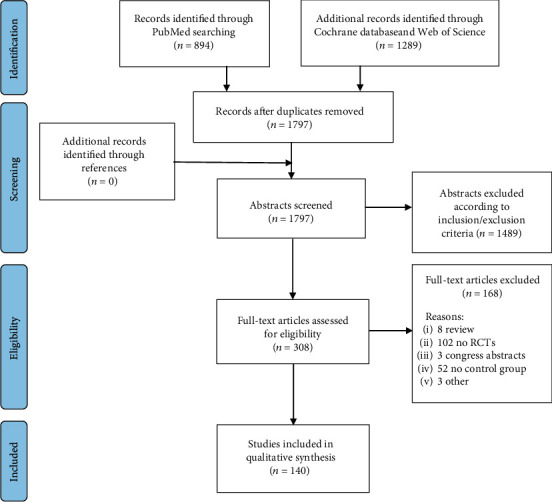
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the study selection process.
The screening process and analysis were conducted separately by 2 independent observers (DR and SG). In the first step, the articles were screened by title and abstract. The following inclusion criteria for relevant articles were used during the initial screening of titles and abstracts: RCTs, written in the English language, and about TXA use in patients undergoing every kind of lower limb surgical orthopedic procedure. Exclusion criteria were articles written in other languages, preclinical studies, studies of a different design than RCT, and reviews. No exclusions were made regarding the administration route (intra-articular (IA), intravenous (IV), and oral), dose (dosage amount and bolus versus continuous infusion), and timing of administration (preoperative, intraoperative, or postoperative).
In the second step, the full texts of the selected articles were retrieved and screened, with the further exclusion of RCTs without at least one treatment group receiving TXA and a control group, either receiving placebo or not. For example, surveys that had multiple treatment arms with other antifibrinolytic agents such as epsilon aminocaproic acid (EACA) were eligible, but only if they also analyzed separately one treatment arm with TXA and one control arm, with only TXA and control groups being included in the analysis. Moreover, studies not addressing the occurrence of VTE complications were excluded. No limitations were set based on the postoperative VTE chemoprophylaxis or on the method of screening or diagnosis for VTE used in each study. Reference lists from the selected papers were also screened, and all selected studies were included in the quantitative data synthesis.
2.2. Data Extraction, Outcome Measurement, and Statistical Analysis
The following information on trial methodology and included patients were extracted and collected in a database with the consensus of the two observers: publication year, study design, number of patients included, type of surgery, route of administration of TXA, and number of VTE complications to the purpose of this study. Accordingly, a meta-analysis on the VTE complication rate of the studies included was performed.
The statistical analysis and the forest plot were carried out according to Neyeloff et al. [20] using Microsoft Excel. The dichotomous outcome was a rare event, and most of the articles compared groups of similar dimensions and showed zero events, so Peto's method (Yusuf 1985 and Bradburn 2007) was used to provide a pooled odds ratio; the reason for the choice was that Peto's method does not need the corrections for zero cell counts. The statistical significance was based on the z statistics of Peto's estimation of the pooled odds ratio. p value of 0.05 was used as the level of statistical significance.
3. Results
The database search retrieved 1,797 records; out of these, 140 articles [11, 12, 15, 16, 21–157] were eligible for the meta-analysis of VTE (Figure 1). The publication trend showed that the majority of these RCTs were published in the last five years: 46% from 1996 to 2015 and 54% after 2015 (Figure 2).
Figure 2.
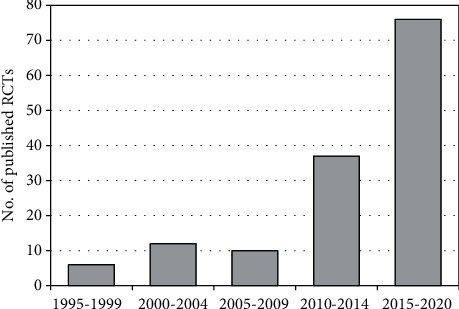
Publications' trend of RCTs about tranexamic acid in orthopedic lower limb surgery from 1995 to 2020.
Overall, 9,067 patients received TXA while 6,592 were included in the control groups. With regard to the type of surgery, 82 studies [21–24, 26–28, 30, 31, 34–38, 40, 45, 47, 49–51, 55–58, 60, 61, 63, 65, 66, 70, 72, 73, 75, 76, 78–82, 84, 85, 87, 88, 90, 92, 93, 96, 98, 99, 104–111, 113, 114, 117–119, 121, 123, 124, 126–128, 130, 134–137, 140–142, 146, 147, 151, 153, 154, 156] (5,267 cases and 3,498 controls) were focused on TKA, 41 studies [11, 12, 25, 32, 33, 42–44, 48, 52–54, 62, 64, 67–69, 74, 77, 79, 83, 88, 89, 94, 101–103, 116, 122, 125, 129, 131–133, 138, 139, 143, 144, 148, 150, 152] (2,655 cases and 1,941 controls) on THA, and 17 studies [15, 16, 39, 41, 46, 59, 71, 86, 91, 95, 112, 115, 120, 145, 149, 155, 157] (1,025 cases and 1,035 controls) on other lower limb surgeries. Four articles analyzed both TKA and THA: two of them [79, 88] reported separately VTE complications for TKA and THA and thus were considered in each type of surgery group; conversely, the other two [97, 100] did not report separately VTE complications and thus were not allocated in any group. The administration protocol of TXA included 3 types of administration routes: IV (111 studies, 5,599 cases and 4,980 controls), IA (45 studies, 2,756 cases and 2,495 controls), and oral (7 studies, 712 cases and 362 controls). Studies applying a combination of modalities were included only in the largest group according to the following order: IV>IA>oral. All modalities (dosage amount and bolus versus continuous infusion) and timing (preoperative, intraoperative, or postoperative) of TXA administration were included without further selection.
The quantitative analysis on VTE was first performed and reported on the total of the studies included, then separately for TKA, THA, and other orthopedic surgical procedures of the lower limb. Moreover, a subanalysis for the different administration routes (IV, IA, and oral) with enough available studies reporting VTE was performed.
3.1. Total Lower Limb Orthopedic Surgical Procedures
Among 140 high-level RCTs, including 9,067 patients receiving TXA and 6,592 controls, no significant difference in the occurrence of thromboembolic complications between the two groups was detected, with a total VTE rate of 2.4% and 2.8% for TXA and controls, respectively (OR 0.91 [95% CI 0.76-1.09]; p = 0.291). With regard to the administration route, the 111 RCTs [12, 15, 16, 21, 22, 26, 30–35, 37–40, 42–45, 47–55, 57, 59–64, 66–68, 70–87, 89–95, 97–100, 103, 105–150, 152–157] comparing IV TXA versus controls, on 5,599 and 4,980 patients, respectively, reported a rate of VTE equal to 2.7% and 2.6%, respectively (OR 0.96 [95% CI 0.77-1.20]; p = 0.709). The 45 RCTs [11, 21, 24, 25, 27, 28, 36, 41, 45–47, 56, 58, 65, 69, 75, 79–81, 88, 96, 99, 101, 102, 104–108, 117, 118, 123, 126, 127, 129–132, 134–137, 139, 140, 144] comparing IA TXA administration (2,756 patients) and controls (2,495 patients) reported a rate of VTE of 2.3% and 2.8%, respectively (OR 0.84 [95% CI 0.60-1.17]; p = 0.307). Only 7 studies [23, 124, 125, 136, 143, 147, 151] compared VTE events between oral TXA (712 patients) and controls (362 patients), reporting a rate of 1.0% and 2.8%, respectively (OR 0.66 [95% CI 0.28-1.58]; p = 0.391).
3.2. Total Knee Arthroplasty
The analysis of the 82 RCTs on TKA showed a total of 5,267 patients belonging to the TXA group with 164 VTE and 3,498 patients of the control group with 135 VTE, without any significant difference in terms of VTE complications, being 3.1% and 3.9%, respectively (OR 0.86 [95% CI 0.70-1.07]; p = 0.189). Among 62 RCTs comparing 3,122 TXA administered IV to 2,687 controls, the number of VTE complications was 105 and 90, respectively, with a corresponding equal VTE rate of 3.4% (OR 0.92 [95% CI 0.70-1.21]; p = 0.549). Analogously, the meta-analysis of the 33 studies about IA TXA administration showed no significant difference for the VTE rate (1,713 out of 3,178 patients) compared to the control group (1,465 out of 3,178 patients), being 3.0% and 4.1%, respectively (OR 0.81 [95% CI 0.56-1.17]; p = 0.268) (Figure 3).
Figure 3.
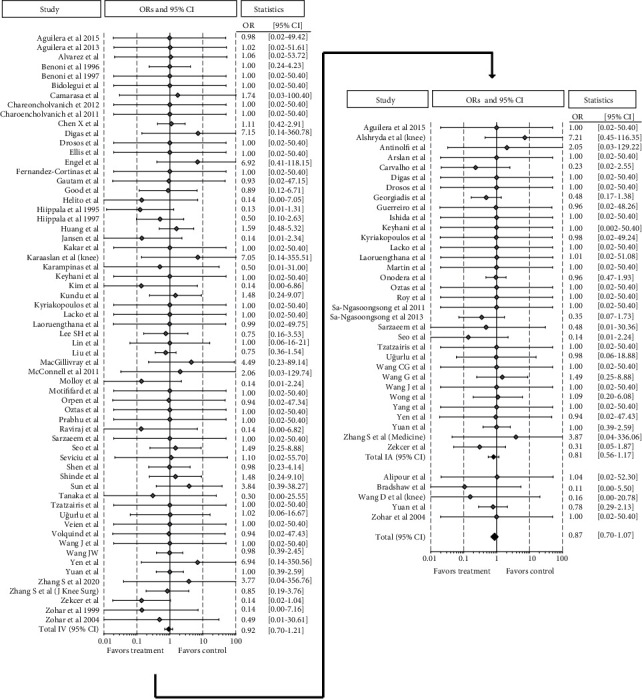
Forest plot of the 82 studies comparing the VTE rate between the TXA group and the control group for TKA. Point estimates of the weighted odds ratios for each study are represented by squares, and the 95% CIs are represented by horizontal bars. The summary odds ratio is represented by a black diamond.
3.3. Total Hip Arthroplasty
Regarding THA, a total of 41 studies with 2,655 patients belonging to the TXA group and 1,941 patients to the control group reported 30 and 21 VTE, respectively, without any significant difference in terms of VTE complications, being 1.1% in both groups (OR 1.07 [95% CI 0.67-1.70]; p = 0.769). Among the 33 RCTs comparing TXA (1,583 patients) administered IV to controls (1,389 patients), the rates of VTE were 1.4% and 1.2%, respectively (OR 1.09 [95% CI 0.63-1.89]; p = 0.749). Similarly, the meta-analysis of 12 studies on IA TXA showed no significant difference in the VTE rate compared to controls, being 1.0% and 0.9%, respectively (OR 1.09 [95% CI 0.44-2.68]; p = 0.857) (Figure 4).
Figure 4.
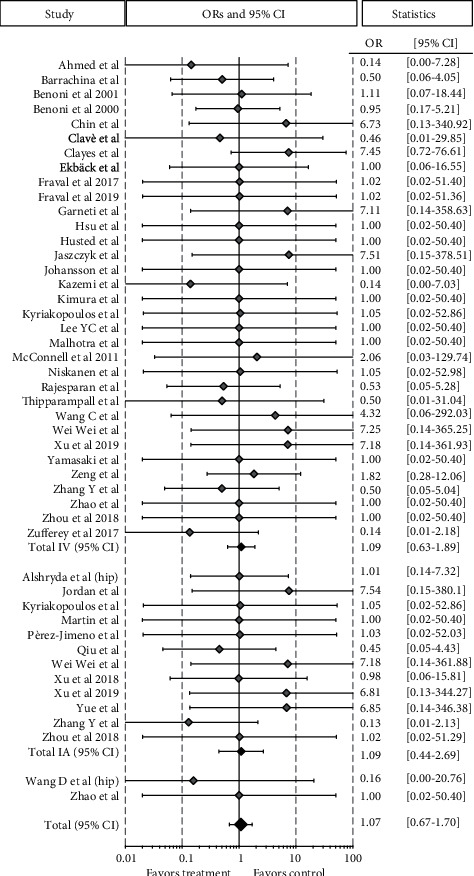
Forest plot of the 41 studies comparing the VTE rate between the TXA group and the control group for THA. Point estimates of the weighted odds ratios for each study are represented by squares, and the 95% CIs are represented by horizontal bars. The summary odds ratio is represented by a black diamond.
3.4. Other Lower Limb Orthopedic Surgical Procedures
Among the 17 RCTs on the patients undergoing other lower limb surgical procedures, including intertrochanteric fractures (13 studies, 763 cases and 771 controls), meniscectomies (1 study, 18 cases and 23 controls), and ACL reconstructions (3 studies, 244 cases and 241 controls), 1,025 patients were included in the TXA group and 1,035 in the control group, reporting a similar total rate of VTE of 2.6% and 2.4%, respectively (OR 1.06 [95% CI 0.63-1.77]; p = 0.827). Regarding the IV administration group, the meta-analysis of 15 studies had a similar rate of VTE in the 774 patients with TXA compared to 786 controls, being 3.1% and 2.7%, respectively (OR 1.11 [95% CI 0.64-1.93]; p = 0.712) (Figure 5).
Figure 5.
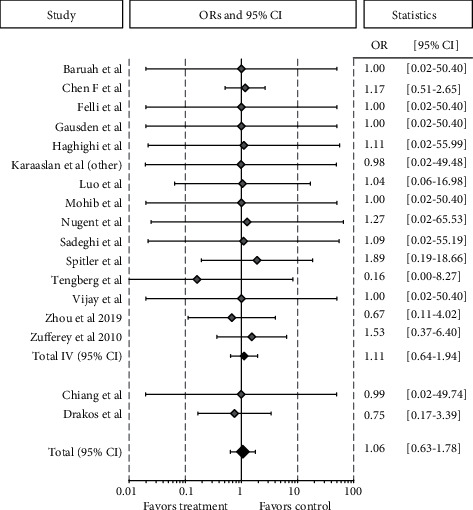
Forest plot of the 17 studies comparing the VTE rate between the TXA group and the control group for other orthopedic lower limb surgical procedures. Point estimates of the weighted odds ratios for each study are represented by squares, and the 95% CIs are represented by horizontal bars. The summary odds ratio is represented by a black diamond.
4. Discussion
The main finding of this RCT meta-analysis was that TXA did not increase the risk of VTE complications in patients undergoing lower limb orthopedic surgery. This finding remained consistent for patients undergoing both THA and TKA and other lower limb surgical procedures, as well as for different TXA administration routes (IV, IA, and oral).
TXA was introduced in the clinical practice as a synthetic antifibrinolytic agent aiming at reducing intra- and postoperative blood loss. This represents one of the most common complications associated with major orthopedic surgeries resulting in increased impairment of functional ability, longer hospitalization, and increased morbidity and mortality [158]. Allogenic transfusions are often used to address postoperative anemia and its detrimental consequences, but they are not free from serious adverse events. Accordingly, TXA has been increasingly employed in recent years in replacement surgery of the lower limb joints, and now plenty of high-level studies regarding RCTs and meta-analyses have been published, demonstrating its effectiveness in reducing blood loss and consequently the need for postoperative transfusions [159, 160]. Among these, in 2015, Wu et al. [159] published a meta-analysis on 34 studies and reported that IV or IA use of TXA significantly reduced the postoperative blood loss, hemoglobin decrease, and transfusion rate after primary TKA, though without reducing intraoperative blood loss. Similarly, in the same year, Wei and Liu [160] analyzed 39 trials, confirming that the administration of TXA significantly reduced blood loss and the need for allogeneic blood transfusion. In light of such promising results, the use of TXA has been recently extended also to other lower limb procedures, such as ACLR and meniscectomies, where the aim of TXA, in addition to reducing perioperative blood loss, is to improve early functional recovery. Promising results have been shown also for these indications, with a significant reduction of hemarthrosis and the amount of suction drainage blood volume, as well as improved ROM and quadriceps strength and a lower rate of fever during the first 2 weeks after surgery [15]. Despite the positive findings of these recent attempts to broaden the application of TXA in orthopedic surgery, there are still concerns about its use among orthopedic surgeons and anesthesiologists regarding its safety, in particular the possible increased risk of VTE [17, 18].
In the current study, a meta-analysis was performed to quantify the complication rate reported for TXA compared to controls in high-level trials. In these studies, TXA was used for the intra- and postoperative blood loss management in TKA and THA and also in other lower limb surgical procedures, such as ACLR, meniscectomies, and intertrochanteric fractures. Based on this meta-analysis of 140 RCTs, including 15,659 patients, no significant differences were found in the occurrence of VTE between TXA and controls, with a total rate of 2.4% and 2.8%, respectively. Furthermore, included patients were grouped according to the type of surgery (TKA, THA, and other procedures) and to the method of administration (IV, IA, and oral) in order to investigate possible differences in the complication risk in the different subgroups. The subanalysis confirmed no significant differences in terms of the VTE rate, demonstrating the safety of TXA administration for all these surgical procedures regardless of the administration route, either IV, IA, or oral.
Interestingly, the rate of VTE observed in THA, close to 1%, was lower compared to the 3% reported in TKA, and this difference was consistent in both the TXA and control groups. A possible explanation of this lower VTE rate in THA compared to TKA could be traced back to different rehabilitation protocols with earlier weight bearing and the different applications of cement and tourniquet [161–163]. Nevertheless, a strong statistical methodology could be applied to the selected papers only to detect any possible differences between TXA and controls in the different procedures, while it was not possible to perform an indirect comparative analysis of different subgroups according to the specific surgery. Similarly, it was not possible to compare subgroups differing in terms of administration routes within TXA and control groups. However, this aspect has been already investigated in other studies, which have suggested superior safety of topical application of TXA compared with IV application of TXA, based on the finding that topically applied TXA results in a 90% reduction in plasma concentration compared to IV [164]. What was instead proven with robust statistical analysis of high-level trials, all directly comparing TXA and control groups, was that both the IV administration and the IA administration of TXA did not increase the risk of thromboembolic events compared to controls, regardless of the joint or the surgery considered.
While many literature analysis attempts were focused on the effects of TXA in terms of blood loss reduction, only a few studies specifically addressed the possible complications [165, 166]. Fillingham et al. [165] performed a meta-analysis on 78 studies focusing only on joint replacement procedures, including patients exclusively undergoing joint arthroplasty, published up to 2017, reporting that TXA was not associated with an increased risk of VTE also in “high-risk” patients ASA ≥ 3 undergoing TKA. Likewise, in the same year, Franchini et al. [166] concluded that TXA is a safe pharmacological treatment after a meta-analysis on 73 RCTs focused only on IV administration in patients undergoing joint replacements or hip fracture management.
The current analysis included the latest high-level evidence quantifying the VTE risks considering all types of lower limb orthopedic surgeries and all TXA administration routes, not considered in the previous studies, which allowed to further corroborate the TXA safety and extend the interpretation of existing data to a broader scenario of clinical applications. This is of particular importance since the complications of each application should be weighted with the respective expected benefits. In fact, while joint replacement procedures entail high risks of blood loss, which justifies the potential risk of using drugs to reduce blood loss, an increased VTE risk would be less justified in other procedures, where the expected benefit is mainly in terms of faster recovery. In this light, the results of the meta-analysis about patients undergoing ACLR, intramedullary nailing for femoral fractures and meniscectomies, are of importance. In particular, the results confirm no difference in terms of the VTE rate for patients treated with TXA compared to controls, thus supporting its use also for these new indications, which are increasingly explored in both the literature and the clinical practice.
The large number of high-level trials included in this meta-analysis assures the most reliable methodology to synthesize the scientific evidence about complications related to the use of TXA in lower limb orthopedic surgeries. However, this study still presents several limitations. First, it must be underlined that the included studies are heterogeneous with regard to TXA dosing and timing of administration. Moreover, different VTE screening methods were applied in the different studies, and also antithrombotic prophylaxis regimens were not consistent among the included studies. On the other side, the same methods were used within each RCT. Moreover, other studies demonstrated no effect on the risk of VTE in patients under antithrombotic treatment who received TXA during surgery, regardless of the type of antithrombotic prophylaxis, thus not affecting the conclusions of this study [130, 165]. Another limitation may be represented by the analysis only of VTE complications, although this focus was necessary due to the available data. In fact, albeit including a large number of patients, the low frequency of other complications, such as infections, made it not possible to evaluate and compare them. Nevertheless, previous studies did not suggest a higher infection risk when TXA is applied, and in fact, TXA could rather reduce this kind of complication since it was proved that the infection risk is directly correlated with the number of allogeneic blood transfusions administered [167, 168]. Further studies and large registry analysis should investigate deeper these aspects. Finally, the validity of the results regarding the safety of TXA is limited to the patient categories defined by the inclusion and exclusion criteria used in the published literature. In fact, due to concerns about the administration of TXA in high-risk patients, such as those with a history of thromboembolic and ischemic events as well as vascular stents, these types of patients were commonly excluded from RCTs. However, a recent study [165] suggested that patients with ASA ≥ 3 do not carry an increased risk of VTE with TXA administration, demonstrating the possibility to apply it also in such challenging conditions.
Overall, this study not only confirms the safety of TXA but also underlines the need to standardize the administration protocol (dosage, timing, and duration of administration) since great variability exists in the literature. This is particularly important also in consideration of the heterogeneous applications documented in this meta-analysis, which suggests the possibility to broaden the TXA indication also to other surgeries than just joint arthroplasties, including fractures and sport medicine procedures, supporting the safety of using TXA for all lower limb orthopedic surgical procedures.
5. Conclusions
This meta-analysis of TXA for lower limb orthopedic surgical procedures showed an increasing interest over time, with most of the articles published in the last 5 years. Moreover, besides the most common applications for joint replacement procedures, TXA use has been recently broadened to other types of surgery. Overall, TXA did not increase the risk of VTE complications. This finding remained consistent for patients undergoing THA, TKA, and other lower limb surgical procedures, regardless of the administration route, thus supporting the safety of using TXA for lower limb orthopedic surgical procedures.
Acknowledgments
The authors acknowledge E. Pignotti for the statistical analysis.
Data Availability
The data generated during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Only Stefano Zaffagnini has a potential conflict of interest. He has received institutional support from Fidia Farmaceutici, Cartiheal, IGEA Clinical Biophysics, Biomet, and Kensey Nash. He has received grant support from I+ and royalties from Springer. Other authors declare that they have no potential conflict of interest.
Authors' Contributions
Giuseppe Filardo and Stefano Zaffagnini shared the co-senior authorship.
References
- 1.Vaishya R., Lal H. Three common orthopaedic surgical procedures of the lower limb. Journal of Clinical Orthopaedics and Trauma. 2018;9(2):101–102. doi: 10.1016/j.jcot.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healthcare Cost and Utilization Project (HCUP) Statistical brief #186. 2014. May 2020, https://www.hcup-us.ahrq.gov/reports/statbriefs/sb186-Operating-Room-Procedures-United-States-2012.jsp. [PubMed]
- 3.George J., Chughtai M., Khlopas A., et al. Readmission, reoperation, and complications: total hip vs total knee arthroplasty. The Journal of Arthroplasty. 2018;33(3):655–660. doi: 10.1016/j.arth.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 4.Ye W., Liu Y., Liu W. F., Li X. L., Fei Y., Gao X. Comparison of efficacy and safety between oral and intravenous administration of tranexamic acid for primary total knee/hip replacement: a meta-analysis of randomized controlled trial. Journal of Orthopaedic Surgery and Research. 2020;15(1):p. 21. doi: 10.1186/s13018-019-1528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C., Park S. S., Davey J. R. Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: current evidence. Journal of Blood Medicine. 2015;6:239–244. doi: 10.2147/JBM.S61915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjdpour C., Spahn D. R. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. British Journal of Anaesthesia. 2005;95(1):33–42. doi: 10.1093/bja/aeh290. [DOI] [PubMed] [Google Scholar]
- 7.Bilgili M. G., Ercin E., Peker G., et al. Efficiency and cost analysis of cell saver auto transfusion system in total knee arthroplasty. Balkan Medical Journal. 2014;31(2):149–153. doi: 10.5152/balkanmedj.2014.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conteduca F., Massai F., Iorio R., Zanzotto E., Luzon D., Ferretti A. Blood loss in computer-assisted mobile bearing total knee arthroplasty. A comparison of computer-assisted surgery with a conventional technique. International Orthopaedics. 2009;33(6):1609–1613. doi: 10.1007/s00264-008-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannucci P. M. Hemostatic drugs. The New England Journal of Medicine. 1998;339(4):245–253. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M., Mannucci P. M. Adjunct agents for bleeding. Current Opinion in Hematology. 2014;21(6):503–508. doi: 10.1097/MOH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 11.Yue C., Kang P., Yang P., Xie J., Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. The Journal of Arthroplasty. 2014;29(12):2452–2456. doi: 10.1016/j.arth.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Barrachina B., Lopez-Picado A., Remon M., et al. Tranexamic acid compared with placebo for reducing total blood loss in hip replacement surgery: a randomized clinical trial. Anesthesia and Analgesia. 2016;122(4):986–995. doi: 10.1213/ANE.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 13.Moskal J. T., Capps S. G. Meta-analysis of intravenous tranexamic acid in primary total hip arthroplasty. Orthopedics. 2016;39(5):e883–e892. doi: 10.3928/01477447-20160526-02. [DOI] [PubMed] [Google Scholar]
- 14.Chen S., Wu K., Kong G., Feng W., Deng Z., Wang H. The efficacy of topical tranexamic acid in total hip arthroplasty: a meta-analysis. BMC Musculoskeletal Disorders. 2016;17(1):p. 81. doi: 10.1186/s12891-016-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felli L., Revello S., Burastero G., et al. Single intravenous administration of tranexamic acid in anterior cruciate ligament reconstruction to reduce postoperative hemarthrosis and increase functional outcomes in the early phase of postoperative rehabilitation: a randomized controlled trial. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2019;35(1):149–157. doi: 10.1016/j.arthro.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Gausden E. B., Garner M. R., Warner S. J., et al. Tranexamic acid in hip fracture patients: a protocol for a randomised, placebo controlled trial on the efficacy of tranexamic acid in reducing blood loss in hip fracture patients. BMJ Open. 2016;6(6, article e010676) doi: 10.1136/bmjopen-2015-010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham I. D., Alvarez G., Tetroe J., McAuley L., Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Canadian Journal of Surgery. 2002;45(2):132–140. [PMC free article] [PubMed] [Google Scholar]
- 18.Haas S. B., Barrack R. L., Westrich G., Lachiewicz P. F. Venous thromboembolic disease after total hip and knee arthroplasty. The Journal of bone and joint surgery American volume. 2008;90(12):2764–2780. [PubMed] [Google Scholar]
- 19.Shamseer L., Moher D., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350, article g7647 doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 20.Neyeloff J. L., Fuchs S. C., Moreira L. B. Meta-analyses and forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Research Notes. 2012;5(1):p. 52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguilera X., Martínez-Zapata M. J., Hinarejos P., et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Archives of Orthopaedic and Trauma Surgery. 2015;135(7):1017–1025. doi: 10.1007/s00402-015-2232-8. [DOI] [PubMed] [Google Scholar]
- 22.Aguilera X., Martinez-Zapata M. J., Bosch A., et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. The Journal of Bone and Joint Surgery American Volume. 2013;95(22):2001–2007. doi: 10.2106/JBJS.L.01182. [DOI] [PubMed] [Google Scholar]
- 23.Alipour M., Tabari M., Keramati M., Zarmehri A. M., Makhmalbaf H. Effectiveness of oral tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfusion and Apheresis Science. 2013;49(3):574–577. doi: 10.1016/j.transci.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Alshryda S., Mason J., Vaghela M., et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K) The Journal of bone and joint surgery American volume. 2013;95(21):1961–1968. doi: 10.2106/JBJS.L.00907. [DOI] [PubMed] [Google Scholar]
- 25.Alshryda S., Mason J., Sarda P., et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H) The Journal of bone and joint surgery American Volume. 2013;95(21):1969–1974. doi: 10.2106/JBJS.L.00908. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez J. C., Santiveri F. X., Ramos I., Vela E., Puig L., Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48(3):519–525. doi: 10.1111/j.1537-2995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 27.Antinolfi P., Innocenti B., Caraffa A., Peretti G., Cerulli G. Post-operative blood loss in total knee arthroplasty: knee flexion versus pharmacological techniques. Knee surgery, sports traumatology, arthroscopy. 2014;22(11):2756–2762. doi: 10.1007/s00167-013-2674-x. [DOI] [PubMed] [Google Scholar]
- 28.Arslan A., Gormeli G. Using intra-articular tranexamic acid in total knee replacement surgery with and without bleeding control: a prospective randomized double blind study. European review for medical and pharmacological sciences. 2018;22(18):6127–6132. doi: 10.26355/eurrev_201809_15952. [DOI] [PubMed] [Google Scholar]
- 29.Rosen A., Miller V., Parker G. Standards of care for area mental health services. The Australian and New Zealand journal of psychiatry. 1989;23(3):379–395. doi: 10.1177/000486748902300325. [DOI] [PubMed] [Google Scholar]
- 30.Benoni G., Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. The Journal of bone and joint surgery British volume. 1996;78(3):434–440. [PubMed] [Google Scholar]
- 31.Benoni G., Lethagen S., Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thrombosis Research. 1997;85(3):195–206. doi: 10.1016/S0049-3848(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 32.Benoni G., Fredin H., Knebel R., Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta orthopaedica Scandinavica. 2009;72(5):442–448. doi: 10.1080/000164701753532754. [DOI] [PubMed] [Google Scholar]
- 33.Benoni G., Lethagen S., Nilsson P., Fredin H. Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta orthopaedica Scandinavica. 2000;71(3):250–254. doi: 10.1080/000164700317411834. [DOI] [PubMed] [Google Scholar]
- 34.Bidolegui F., Arce G., Lugones A., Pereira S., Vindver G. Tranexamic acid reduces blood loss and transfusion in patients undergoing total knee arthroplasty without tourniquet: a prospective randomized controlled trial. The Open Orthopaedics Journal. 2014;8(1):250–254. doi: 10.2174/1874325001408010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camarasa M. A., Ollé G., Serra-Prat M., et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. British Journal of Anaesthesia. 2006;96(5):576–582. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho L. H., Jr., Frois Temponi E., Machado Soares L. F., Gonçalves M. B. J., Paiva Costa L., Tavares de Souza M. L. Bleeding reduction after topical application of tranexamic acid together with Betadine solution in total knee arthroplasty. A randomised controlled study. Orthopaedics & Traumatology, Surgery & Research. 2015;101(1):83–87. doi: 10.1016/j.otsr.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Chareancholvanich K., Siriwattanasakul P., Narkbunnam R., Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13(1):p. 124. doi: 10.1186/1471-2474-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charoencholvanich K., Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clinical orthopaedics and related research. 2011;469(10):2874–2880. doi: 10.1007/s11999-011-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F., Jiang Z., Li M., Zhu X. Efficacy and safety of perioperative tranexamic acid in elderly patients undergoing trochanteric fracture surgery: a randomised controlled trial. Hong Kong Medical Journal=Xianggang yi xue za zhi. 2019;25(2):120–126. doi: 10.12809/hkmj187570. [DOI] [PubMed] [Google Scholar]
- 40.Chen X., Cao X., Yang C., Guo K., Zhu Q., Zhu J. Effectiveness and safety of fixed-dose tranexamic acid in simultaneous bilateral total knee arthroplasty: a randomized double-blind controlled trial. The Journal of Arthroplasty. 2016;31(11):2471–2475. doi: 10.1016/j.arth.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Chiang E. R., Chen K. H., Wang S. T., et al. Intra-articular injection of tranexamic acid reduced postoperative hemarthrosis in arthroscopic anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. 2019;35(7):2127–2132. doi: 10.1016/j.arthro.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Chin J., Blackett J., Kieser D. C., Frampton C., Hooper G. The value of routine intravenous tranexamic acid in total hip arthroplasty: a preliminary study. Advances in Orthopedics. 2020;2020:5. doi: 10.1155/2020/2943827.2943827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clavé A., Gérard R., Lacroix J., et al. A randomized, double-blind, placebo-controlled trial on the efficacy of tranexamic acid combined with rivaroxaban thromboprophylaxis in reducing blood loss after primary cementless total hip arthroplasty. The Bone & joint journal. 2019;101-B(2):207–212. doi: 10.1302/0301-620x.101b2.bjj-2018-0898.r1. [DOI] [PubMed] [Google Scholar]
- 44.Claeys M. A., Vermeersch N., Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta chirurgica Belgica. 2016;107(4):397–401. doi: 10.1080/00015458.2007.11680081. [DOI] [PubMed] [Google Scholar]
- 45.Digas G., Koutsogiannis I., Meletiadis G., Antonopoulou E., Karamoulas V., Bikos C. Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. European journal of orthopaedic surgery & traumatology. 2015;25(7):1181–1188. doi: 10.1007/s00590-015-1664-8. [DOI] [PubMed] [Google Scholar]
- 46.Drakos A., Raoulis V., Karatzios K., et al. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. Journal of orthopaedic trauma. 2016;30(8):409–414. doi: 10.1097/BOT.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 47.Drosos G. I., Ververidis A., Valkanis C., et al. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. Journal of Orthopaedics. 2016;13(3):127–131. doi: 10.1016/j.jor.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekbäck G., Axelsson K., Ryttberg L., et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesthesia and analgesia. 2000;91(5):1124–1130. doi: 10.1213/00000539-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Ellis M. H., Fredman B., Zohar E., Ifrach N., Jedeikin R. The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. Journal of clinical anesthesia. 2001;13(7):509–513. doi: 10.1016/S0952-8180(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 50.Engel J. M., Hohaus T., Ruwoldt R., Menges T., Jurgensen I., Hempelmann G. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesthesia and analgesia. 2001;92(3):775–780. doi: 10.1213/00000539-200103000-00041. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Cortinas A. B., Quintans-Vazquez J. M., Gomez-Suarez F., Murillo O. S., Sanchez-Lopez B. R., Pena-Gracia J. M. Effect of tranexamic acid administration on bleeding in primary total hip arthroplasty. Revista espanola de cirugia ortopedica y traumatologia. 2017;61(5):289–295. doi: 10.1016/j.recot.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Fraval A., Effeney P., Fiddelaers L., Smith B., Towell B., Tran P. OBTAIN A: Outcome benefits of tranexamic acid in hip arthroplasty. A randomized double-blinded controlled trial. The Journal of Arthroplasty. 2017;32(5):1516–1519. doi: 10.1016/j.arth.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 53.Fraval A., Duncan S., Murray T., Duggan J., Tirosh O., Tran P. OBTAIN E: outcome benefits of tranexamic acid in hip arthroplasty with enoxaparin: a randomised double-blinded controlled trial. Hip international. 2019;29(3):239–244. doi: 10.1177/1120700018780125. [DOI] [PubMed] [Google Scholar]
- 54.Garneti N., Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid 1. The Journal of Arthroplasty. 2004;19(4):488–492. doi: 10.1016/j.arth.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 55.Gautam V. K., Sambandam B., Singh S., Gupta P., Gupta R., Maini L. The role of tranexamic acid in reducing blood loss in total knee replacement. Journal of clinical orthopaedics and trauma. 2013;4(1):36–39. doi: 10.1016/j.jcot.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georgiadis A. G., Muh S. J., Silverton C. D., Weir R. M., Laker M. W. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. The Journal of arthroplasty. 2013;28(8):78–82. doi: 10.1016/j.arth.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 57.Good L., Peterson E., Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. British journal of anaesthesia. 2003;90(5):596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 58.Guerreiro J. P. F., Badaro B. S., Balbino J. R. M., Danieli M. V., Queiroz A. O., Cataneo D. C. Application of tranexamic acid in total knee arthroplasty - prospective randomized trial. The open orthopaedics journal. 2017;11(1):1049–1057. doi: 10.2174/1874325001711011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haghighi M., Ettehad H., Mardani-Kivi M., et al. Does tranexamic acid reduce bleeding during femoral fracture operation? The archives of bone and joint surgery. 2017;5(2):103–108. [PMC free article] [PubMed] [Google Scholar]
- 60.Helito C. P., Bonadio M. B., Sobrado M. F., et al. Comparison of Floseal® and tranexamic acid for bleeding control after total knee arthroplasty: a prospective randomized study. Clinics. 2019;74, article e1186 doi: 10.6061/clinics/2019/e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiippala S. T., Strid L. J., Wennerstrand M. I., et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesthesia and Analgesia. 1997;84(4):839–844. doi: 10.1213/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 62.Hsu C. H., Lin P. C., Kuo F. C., Wang J. W. A regime of two intravenous injections of tranexamic acid reduces blood loss in minimally invasive total hip arthroplasty: a prospective randomised double-blind study. The bone & joint journal. 2015;97-B(7):905–910. doi: 10.1302/0301-620X.97B7.35029. [DOI] [PubMed] [Google Scholar]
- 63.Huang Z., Xie X., Li L., et al. Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: a prospective, randomized controlled trial. The Journal of bone and joint surgery American volume. 2017;99(24):2053–2061. doi: 10.2106/JBJS.16.01525. [DOI] [PubMed] [Google Scholar]
- 64.Husted H., Blond L., Sonne-Holm S., Holm G., Jacobsen T. W., Gebuhr P. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta orthopaedica Scandinavica. 2009;74(6):665–669. doi: 10.1080/00016470310018171. [DOI] [PubMed] [Google Scholar]
- 65.Ishida K., Tsumura N., Kitagawa A., et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. International Orthopaedics. 2011;35(11):1639–1645. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jansen A. J., Andreica S., Claeys M., D'Haese J., Camu F., Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. British journal of Anaesthesia. 1999;83(4):596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 67.Jaszczyk M., Kozerawski D., Kolodziej L., Kazimierczak A., Sarnecki P., Sieczka L. Effect of single preoperative dose of tranexamic acid on blood loss and transfusion in hip arthroplasty. Ortopedia, Traumatologia, Rehabilitacja. 2015;17(3):265–273. doi: 10.5604/15093492.1162426. [DOI] [PubMed] [Google Scholar]
- 68.Johansson T., Pettersson L. G., Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta orthopaedica. 2009;76(3):314–319. [PubMed] [Google Scholar]
- 69.the TRANEXFER Group, Jordan M., Aguilera X., et al. Prevention of postoperative bleeding in hip fractures treated with prosthetic replacement: efficacy and safety of fibrin sealant and tranexamic acid. A randomised controlled clinical trial (TRANEXFER study) Archives of orthopaedic and trauma surgery. 2019;139(5):597–604. doi: 10.1007/s00402-018-3089-4. [DOI] [PubMed] [Google Scholar]
- 70.Kakar P. N., Gupta N., Govil P., Shah V. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Indian journal of anaesthesia. 2009;53(6):667–671. [PMC free article] [PubMed] [Google Scholar]
- 71.Karaaslan F., Karaoglu S., Yurdakul E. Reducing intra-articular hemarthrosis after arthroscopic anterior cruciate ligament reconstruction by the administration of intravenous tranexamic acid: a prospective, randomized controlled trial. The American journal of sports medicine. 2015;43(11):2720–2726. doi: 10.1177/0363546515599629. [DOI] [PubMed] [Google Scholar]
- 72.Karaaslan F., Karaoglu S., Mermerkaya M. U., Baktir A. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous-intra-articular tranexamic acid administration. A prospective randomized controlled trial. The Knee. 2015;22(2):131–135. doi: 10.1016/j.knee.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Karampinas P. K., Megaloikonomos P. D., Lampropoulou-Adamidou K., et al. Similar thromboprophylaxis with rivaroxaban and low molecular weight heparin but fewer hemorrhagic complications with combined intra-articular and intravenous tranexamic acid in total knee arthroplasty. European journal of orthopaedic surgery & traumatology. 2019;29(2):455–460. doi: 10.1007/s00590-018-2307-7. [DOI] [PubMed] [Google Scholar]
- 74.Kazemi S. M., Mosaffa F., Eajazi A., et al. The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics. 2010;33(1):17–22. doi: 10.3928/01477447-20091124-30. [DOI] [PubMed] [Google Scholar]
- 75.Keyhani S., Esmailiejah A. A., Abbasian M. R., Safdari F. Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? The archives of bone and joint surgery. 2016;4(1):65–69. [PMC free article] [PubMed] [Google Scholar]
- 76.Kim T. K., Chang C. B., Kang Y. G., et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee surgery, sports traumatology, arthroscopy. 2014;22(8):1870–1878. doi: 10.1007/s00167-013-2492-1. [DOI] [PubMed] [Google Scholar]
- 77.Kimura O. S., Freitas E. H., Duarte M. E., Cavalcanti A. S., Fernandes M. B. Tranexamic acid use in high-risk blood transfusion patients undergoing total hip replacement: a randomised controlled trial. Hip international. 2019;9 doi: 10.1177/1120700019889947. [DOI] [PubMed] [Google Scholar]
- 78.Kundu R., Das A., Basunia S. R., Bhattacharyya T., Chattopadhyay S., Mukherjee A. Does a single loading dose of tranexamic acid reduce perioperative blood loss and transfusion requirements after total knee replacement surgery? A randomized, controlled trial. Journal of natural science, biology, and medicine. 2015;6(1):94–99. doi: 10.4103/0976-9668.149099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kyriakopoulos G., Oikonomou L., Panagopoulos A., et al. Transfusion rate, hospital stay and cost-effectiveness of intravenous or local administration of tranexamic acid in total hip and knee arthroplasty: a single-center randomized controlled clinical study. Orthopedic Reviews. 2019;11(2):p. 7866. doi: 10.4081/or.2019.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lacko M., Cellar R., Schreierova D., Vasko G. Comparison of intravenous and intra-articular tranexamic acid in reducing blood loss in primary total knee replacement. Eklem hastaliklari ve cerrahisi=Joint diseases & related surgery. 2017;28(2):64–71. doi: 10.5606/ehc.2017.54914. [DOI] [PubMed] [Google Scholar]
- 81.Laoruengthana A., Rattanaprichavej P., Rasamimongkol S., Galassi M., Weerakul S., Pongpirul K. Intra-articular tranexamic acid mitigates blood loss and morphine use after total knee arthroplasty. a randomized controlled trial. The Journal of arthroplasty. 2019;34(5):877–881. doi: 10.1016/j.arth.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 82.Lee S. H., Cho K. Y., Khurana S., Kim K. I. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee surgery, sports traumatology, arthroscopy. 2013;21(11):2611–2617. doi: 10.1007/s00167-012-2213-1. [DOI] [PubMed] [Google Scholar]
- 83.Lee Y. C., Park S. J., Kim J. S., Cho C. H. Effect of tranexamic acid on reducing postoperative blood loss in combined hypotensive epidural anesthesia and general anesthesia for total hip replacement. Journal of clinical anesthesia. 2013;25(5):393–398. doi: 10.1016/j.jclinane.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Lin P. C., Hsu C. H., Chen W. S., Wang J. W. Does tranexamic acid save blood in minimally invasive total knee arthroplasty? Clinical orthopaedics and related research. 2011;469(7):1995–2002. doi: 10.1007/s11999-011-1789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu W., Yang C., Huang X., Liu R. Tranexamic acid reduces occult blood loss, blood transfusion, and improves recovery of knee function after total knee arthroplasty: a comparative study. The journal of knee surgery. 2018;31(3):239–246. doi: 10.1055/s-0037-1602248. [DOI] [PubMed] [Google Scholar]
- 86.Luo X., He S., Lin Z., Li Z., Huang C., Li Q. Efficacy and safety of tranexamic acid for controlling bleeding during surgical treatment of intertrochanteric fragility fracture with proximal femoral nail anti-rotation: a randomized controlled trial. Indian journal of orthopaedics. 2019;53(2):263–269. doi: 10.4103/ortho.IJOrtho_401_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacGillivray R. G., Tarabichi S. B., Hawari M. F., Raoof N. T. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. The Journal of arthroplasty. 2011;26(1):24–28. doi: 10.1016/j.arth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Martin J. G., Cassatt K. B., Kincaid-Cinnamon K. A., Westendorf D. S., Garton A. S., Lemke J. H. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. The Journal of arthroplasty. 2014;29(5):889–894. doi: 10.1016/j.arth.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 89.McConnell J. S., Shewale S., Munro N. A., Shah K., Deakin A. H., Kinninmonth A. W. Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. Acta orthopaedica. 2011;82(6):660–663. doi: 10.3109/17453674.2011.623568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McConnell J. S., Shewale S., Munro N. A., Shah K., Deakin A. H., Kinninmonth A. W. Reducing blood loss in primary knee arthroplasty: a prospective randomised controlled trial of tranexamic acid and fibrin spray. The Knee. 2012;19(4):295–298. doi: 10.1016/j.knee.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Mohib Y., Rashid R. H., Ali M., Zubairi A. J., Umer M. Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized control trial. The Journal of the Pakistan Medical Association. 2015;65(11) Supplement 3:S17–S20. [PubMed] [Google Scholar]
- 92.Molloy D. O., Archbold H. A., Ogonda L., McConway J., Wilson R. K., Beverland D. E. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. The Journal of bone and joint surgery British volume. 2007;89(3):306–309. doi: 10.1302/0301-620X.89B3.17565. [DOI] [PubMed] [Google Scholar]
- 93.Motififard M., Tahririan M. A., Saneie M., Badiei S., Nemati A. Low dose perioperative intravenous tranexamic acid in patients undergoing total knee arthroplasty: a double-blind randomized placebo controlled clinical trial. Journal of Blood Transfusion. 2015;2015 doi: 10.1155/2015/948304.948304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niskanen R. O., Korkala O. L. Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double-blind study of 39 patients with osteoarthritis. Acta orthopaedica. 2009;76(6):829–832. doi: 10.1080/17453670510045444. [DOI] [PubMed] [Google Scholar]
- 95.Nugent M., May J. H., Parker J. D., et al. Does tranexamic acid reduce knee swelling and improve early function following arthroscopic meniscectomy? A double-blind randomized controlled trial. Orthopaedic journal of sports medicine. 2019;7(8):p. 2325967119866122. doi: 10.1177/2325967119866122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Onodera T., Majima T., Sawaguchi N., Kasahara Y., Ishigaki T., Minami A. Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. The Journal of arthroplasty. 2012;27(1):105–108. doi: 10.1016/j.arth.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Oremus K., Sostaric S., Trkulja V., Haspl M. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014;54(1):31–41. doi: 10.1111/trf.12224. [DOI] [PubMed] [Google Scholar]
- 98.Orpen N. M., Little C., Walker G., Crawfurd E. J. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. The Knee. 2006;13(2):106–110. doi: 10.1016/j.knee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Oztas S., Ozturk A., Akalin Y., Sahin N., Ozkan Y., Otuzbir A. The effect of local and systemic application of tranexamic acid on the amount of blood loss and allogeneic blood transfusion after total knee replacement. Acta Orthopaedica Belgica. 2015;81(4):698–707. [PubMed] [Google Scholar]
- 100.Painter T. W., Daly D. J., Kluger R., et al. Intravenous tranexamic acid and lower limb arthroplasty-a randomised controlled feasibility study. Anaesthesia and Intensive Care. 2018;46(4):386–395. doi: 10.1177/0310057X1804600407. [DOI] [PubMed] [Google Scholar]
- 101.Perez-Jimeno N., Munoz M., Mateo J., Mayoral A. P., Herrera A. Efficacy of topical tranexamic acid within a blood-saving programme for primary total hip arthroplasty: a pragmatic, open-label randomised study. Blood Transfusion. 2018;16(6):490–497. doi: 10.2450/2018.0133-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiu J., Sun X., Zhang W., Ke X., Yang G., Zhang L. Effect of topical tranexamic acid in total hip arthroplasty patients who receive continuous aspirin for prevention of cardiovascular or cerebrovascular events: a prospective randomized study. Orthopaedics & Traumatology, Surgery & Research. 2019;105(7):1327–1332. doi: 10.1016/j.otsr.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 103.Rajesparan K., Biant L. C., Ahmad M., Field R. E. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. The Journal of Bone and Joint Surgery British Volume. 2009;91(6):776–783. doi: 10.1302/0301-620X.91B6.22393. [DOI] [PubMed] [Google Scholar]
- 104.Roy S. P., Tanki U. F., Dutta A., Jain S. K., Nagi O. N. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy. 2012;20(12):2494–2501. doi: 10.1007/s00167-012-1942-5. [DOI] [PubMed] [Google Scholar]
- 105.Sa-ngasoongsong P., Wongsak S., Chanplakorn P., et al. Efficacy of low-dose intra-articular tranexamic acid in total knee replacement; a prospective triple-blinded randomized controlled trial. BMC Musculoskeletal Disorders. 2013;14(1):p. ???. doi: 10.1186/1471-2474-14-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sa-Ngasoongsong P., Channoom T., Kawinwonggowit V., et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthopedic Reviews. 2011;3(2, article e12) doi: 10.4081/or.2011.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarzaeem M. M., Razi M., Kazemian G., Moghaddam M. E., Rasi A. M., Karimi M. Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. The Journal of Arthroplasty. 2014;29(8):1521–1524. doi: 10.1016/j.arth.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 108.Seo J. G., Moon Y. W., Park S. H., Kim S. M., Ko K. R. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy. 2013;21(8):1869–1874. doi: 10.1007/s00167-012-2079-2. [DOI] [PubMed] [Google Scholar]
- 109.Seviciu A., Gross I., Fathima S., Walsh S. M. Effects of tranexamic acid and bipolar sealer alone or in combination in primary total knee arthroplasty: a prospective, randomized, controlled trial. Arthroplasty Today. 2016;2(2):77–82. doi: 10.1016/j.artd.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen P. F., Hou W. L., Chen J. B., Wang B., Qu Y. X. Effectiveness and safety of tranexamic acid for total knee arthroplasty: a prospective randomized controlled trial. Medical Science Monitor. 2015;21:576–581. doi: 10.12659/MSM.892768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shinde A., Sobti A., Maniar S., Mishra A., Gite R., Shetty V. Tranexamic acid reduces blood loss and need of blood transfusion in total knee arthroplasty: a prospective, randomized, double-blind study in Indian population. Asian Journal of Transfusion Science. 2015;9(2):168–172. doi: 10.4103/0973-6247.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spitler C. A., Row E. R., Gardner W. E., 2nd, et al. Tranexamic acid use in open reduction and internal fixation of fractures of the pelvis, acetabulum, and proximal femur: a randomized controlled trial. Journal of Orthopaedic Trauma. 2019;33(8):371–376. doi: 10.1097/BOT.0000000000001480. [DOI] [PubMed] [Google Scholar]
- 113.Sun Q., Yu X., Wu J., Ge W., Cai M., Li S. Efficacy of a single dose and an additional dose of tranexamic acid in reduction of blood loss in total knee arthroplasty. The Journal of Arthroplasty. 2017;32(7):2108–2112. doi: 10.1016/j.arth.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 114.Tanaka N., Sakahashi H., Sato E., Hirose K., Ishima T., Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. The Journal of Bone and Joint Surgery British Volume. 2001;83-B(5):702–705. doi: 10.1302/0301-620X.83B5.0830702. [DOI] [PubMed] [Google Scholar]
- 115.Tengberg P. T., Foss N. B., Palm H., Kallemose T., Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: results of a randomised controlled trial. The bone & joint journal. 2016;98-B(6):747–753. doi: 10.1302/0301-620X.98B6.36645. [DOI] [PubMed] [Google Scholar]
- 116.Thipparampall A. K., Gurajala I., Gopinath R. The effect of different dose regimens of tranexamic acid in reducing blood loss during hip surgery. Indian Journal of Anaesthesia. 2017;61(3):235–239. doi: 10.4103/ija.IJA_495_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tzatzairis T. K., Drosos G. I., Kotsios S. E., Ververidis A. N., Vogiatzaki T. D., Kazakos K. I. Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: a randomized controlled study. The Journal of Arthroplasty. 2016;31(11):2465–2470. doi: 10.1016/j.arth.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 118.Ugurlu M., Aksekili M. A., Caglar C., Yuksel K., Sahin E., Akyol M. Effect of topical and intravenously applied tranexamic acid compared to control group on bleeding in primary unilateral total knee arthroplasty. The Journal of Knee Surgery. 2017;30(2):152–157. doi: 10.1055/s-0036-1583270. [DOI] [PubMed] [Google Scholar]
- 119.Veien M., Sorensen J. V., Madsen F., Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiologica Scandinavica. 2002;46(10):1206–1211. doi: 10.1034/j.1399-6576.2002.461007.x. [DOI] [PubMed] [Google Scholar]
- 120.Vijay B. S., Bedi V., Mitra S., Das B. Role of tranexamic acid in reducing postoperative blood loss and transfusion requirement in patients undergoing hip and femoral surgeries. Saudi Journal of Anaesthesia. 2013;7(1):29–32. doi: 10.4103/1658-354X.109803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Volquind D., Zardo R. A., Winkler B. C., Londero B. B., Zanelatto N., Leichtweis G. P. Use of tranexamic acid in primary total knee replacement: effects on perioperative blood loss. Brazilian Journal of Anesthesiology. 2016;66(3):254–258. doi: 10.1016/j.bjan.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Wang C., Kang P., Ma J., Yue C., Xie J., Pei F. Single-dose tranexamic acid for reducing bleeding and transfusions in total hip arthroplasty: a double-blind, randomized controlled trial of different doses. Thrombosis Research. 2016;141:119–123. doi: 10.1016/j.thromres.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 123.Wang C. G., Sun Z. H., Liu J., Cao J. G., Li Z. J. Safety and efficacy of intra-articular tranexamic acid injection without drainage on blood loss in total knee arthroplasty: a randomized clinical trial. International Journal of Surgery. 2015;20:1–7. doi: 10.1016/j.ijsu.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 124.Wang D., Luo Z. Y., Yu Z. P., et al. The antifibrinolytic and anti-inflammatory effects of multiple doses of oral tranexamic acid in total knee arthroplasty patients: a randomized controlled trial. Journal of Thrombosis and Haemostasis. 2018;16(12):2442–2453. doi: 10.1111/jth.14316. [DOI] [PubMed] [Google Scholar]
- 125.Wang D., Yang Y., He C., et al. Effect of multiple doses of oral tranexamic acid on haemostasis and inflammatory reaction in total hip arthroplasty: a randomized controlled trial. Thrombosis and Haemostasis. 2019;119(1):92–103. doi: 10.1055/s-0038-1676625. [DOI] [PubMed] [Google Scholar]
- 126.Wang G., Wang D., Wang B., Lin Y., Sun S. Efficacy and safety evaluation of intra-articular injection of tranexamic acid in total knee arthroplasty operation with temporarily drainage close. International Journal of Clinical and Experimental Medicine. 2015;8(8):14328–14334. [PMC free article] [PubMed] [Google Scholar]
- 127.Wang J., Wang Q., Zhang X., Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. The Journal of Arthroplasty. 2017;32(11):3385–3389. doi: 10.1016/j.arth.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 128.Wang J. W., Chen B., Lin P. C., Yen S. H., Huang C. C., Kuo F. C. The efficacy of combined use of rivaroxaban and tranexamic acid on blood conservation in minimally invasive total knee arthroplasty a double-blind randomized, controlled trial. The Journal of arthroplasty. 2017;32(3):801–806. doi: 10.1016/j.arth.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 129.Wei W., Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. The Journal of Arthroplasty. 2014;29(11):2113–2116. doi: 10.1016/j.arth.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 130.Wong J., Abrishami A., el Beheiry H., et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. The Journal of Bone and Joint Surgery American Volume. 2010;92(15):2503–2513. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 131.Xu X., Li X., Liu W., Wang Z. Longtime soaking of high concentration tranexamic acid in total hip arthroplasty: a prospective randomized controlled trial in 224 patients. Pakistan Journal of Medical Sciences. 2015;31(6):1306–1311. doi: 10.12669/pjms.316.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu X., Jiang J., Liu W., Li X., Lu H. Application of thromboelastography to evaluate the effect of different routes administration of tranexamic acid on coagulation function in total hip arthroplasty. Journal of Orthopaedic Surgery and Research. 2019;14(1):p. 430. doi: 10.1186/s13018-019-1497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamasaki S., Masuhara K., Fuji T. Tranexamic acid reduces blood loss after cementless total hip arthroplasty-prospective randomized study in 40 cases. International Orthopaedics. 2004;28(2):69–73. doi: 10.1007/s00264-003-0511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Y., Lv Y. M., Ding P. J., Li J., Ying-Ze Z. The reduction in blood loss with intra-articular injection of tranexamic acid in unilateral total knee arthroplasty without operative drains: a randomized controlled trial. European Journal of Orthopaedic Surgery & Traumatology. 2015;25(1):135–139. doi: 10.1007/s00590-014-1461-9. [DOI] [PubMed] [Google Scholar]
- 135.Yen S. H., Lin P. C., Chen B., Huang C. C., Wang J. W. Topical tranexamic acid reduces blood loss in minimally invasive total knee arthroplasty receiving rivaroxaban. BioMed Research International. 2017;2017 doi: 10.1155/2017/9105645.9105645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan X., Li B., Wang Q., Zhang X. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. The Journal of Arthroplasty. 2017;32(9):2738–2743. doi: 10.1016/j.arth.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 137.Zekcer A., Del Priori R., Tieppo C., da Silva R. S., Severino N. R. Topical vs. intravenous administration of tranexamic acid in knee arthroplasty and prevalence of deep venous thrombosis: a randomized clinical trial. Jornal Vascular Brasileiro. 2016;15(2):120–125. doi: 10.1590/1677-5449.007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yi Z., Bin S., Jing Y., Zongke Z., Pengde K., Fuxing P. Tranexamic acid administration in primary total hip Arthroplasty. The Journal of Bone and Joint Surgery American Volume. 2016;98(12):983–991. doi: 10.2106/JBJS.15.00638. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y., Zhang L., Ma X., et al. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Der Orthopade. 2016;45(7):616–621. doi: 10.1007/s00132-016-3252-y. [DOI] [PubMed] [Google Scholar]
- 140.Zhang S., Wang C., Shi L., Xue Q. Multi-route applications of tranexamic acid to reduce blood loss after total knee arthroplasty: a randomized controlled trial. Medicine. 2019;98(30, article e16570) doi: 10.1097/MD.0000000000016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang S., Xu H., Xie J., Cao G., Lei Y., Pei F. Tranexamic acid attenuates inflammatory effect and modulates immune response in primary total knee arthroplasty: a randomized, placebo-controlled, pilot trial. Inflammopharmacology. 2020;6:p. 1. doi: 10.1007/s10787-020-00695-6. [DOI] [PubMed] [Google Scholar]
- 142.Zhang S., Xie J., Cao G., Lei Y., Huang Q., Pei F. Six-dose intravenous tranexamic acid regimen further inhibits postoperative fibrinolysis and reduces hidden blood loss following total knee arthroplasty. The journal of knee surgery. 2019;21 doi: 10.1055/s-0039-1694768. [DOI] [PubMed] [Google Scholar]
- 143.Zhao H., Xiang M., Xia Y., Shi X., Pei F. X., Kang P. Efficacy of oral tranexamic acid on blood loss in primary total hip arthroplasty using a direct anterior approach: a prospective randomized controlled trial. International Orthopaedics. 2018;42(11):2535–2542. doi: 10.1007/s00264-018-3846-6. [DOI] [PubMed] [Google Scholar]
- 144.Zhou K. D., Wang H. Y., Wang Y., Liu Z. H., He C., Feng J. M. Is topical or intravenous tranexamic acid preferred in total hip arthroplasty? A randomized, controlled, noninferiority clinical trial. PLoS One. 2018;13(10, article e0204551) doi: 10.1371/journal.pone.0204551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou X.‐. D., Zhang Y., Jiang L.‐. F., et al. Efficacy and safety of tranexamic acid in intertrochanteric fractures: a single-blind randomized controlled trial. Orthopaedic Surgery. 2019;11(4):635–642. doi: 10.1111/os.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zohar E., Fredman B., Ellis M., Luban I., Stern A., Jedeikin R. A comparative study of the postoperative allogeneic blood-sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesthesia and Analgesia. 1999;89(6):1382–1387. doi: 10.1097/00000539-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 147.Zohar E., Ellis M., Ifrach N., Stern A., Sapir O., Fredman B. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesthesia and Analgesia. 2004;99(6):1679–83, table of contents. doi: 10.1213/01.ANE.0000136770.75805.19. [DOI] [PubMed] [Google Scholar]
- 148.Zufferey P. J., Lanoiselée J., Chapelle C., et al. Intravenous tranexamic acid bolus plus infusion is not more effective than a single bolus in primary hip arthroplasty: a randomized controlled trial. Anesthesiology. 2017;127(3):413–422. doi: 10.1097/ALN.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 149.Zufferey P. J., Miquet M., Quenet S., et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial. British Journal of Anaesthesia. 2010;104(1):23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 150.Ahmed A. A., Abdelrhman H., Zaky M., Abdallah M. Allogeneic- blood-sparing effect of tranexamic acid versus acute normovolemic hemodilution for total hip replacement. Egyptian Journal of Anaesthesia. 2010;26(1):23–30. [Google Scholar]
- 151.Bradshaw A., Monoghan J., Campbell D. Oral tranexamic acid reduces blood loss in total knee replacement arthroplasty. Current Orthopaedic Practice. 2012;23(3):209–212. doi: 10.1097/BCO.0b013e318247f1d5. [DOI] [Google Scholar]
- 152.Malhotra R., Kumar V., Garg B. The use of tranexamic acid to reduce blood loss in primary cementless total hip arthroplasty. European Journal of Orthopaedic Surgery & Traumatology. 2011;21(2):101–104. doi: 10.1007/s00590-010-0671-z. [DOI] [Google Scholar]
- 153.Prabhu T., Deepak M., Harish R., Narasimhan V. Efficacy of tranexamic acid in conservation of blood loss in total knee arthroplasty patients. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2015;6(2):987–992. [Google Scholar]
- 154.Raviraj A., Anand A., Chakravarthy M., Kumarswamy S., Prabhu A., Pai S. Tranexamic acid reduces blood loss in simultaneous bilateral total knee arthroplasty: a randomized control trial. European Journal of Orthopaedic Surgery & Traumatology. 2012;22(5):381–386. doi: 10.1007/s00590-011-0845-3. [DOI] [Google Scholar]
- 155.Sadeghi M., Mehr-Aein A. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. Acta Medica Iranica. 2007;45(6) [Google Scholar]
- 156.Hiippala S., Strid L., Wennerstrand M., et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. British journal of anaesthesia. 1995;74(5):534–537. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 157.Baruah R. K., Borah P. J., Haque R. Use of tranexamic acid in dynamic hip screw plate fixation for trochanteric fractures. Journal of Orthopaedic Surgery. 2016;24(3):379–382. doi: 10.1177/1602400322. [DOI] [PubMed] [Google Scholar]
- 158.Carson J. L., Duff A., Poses R. M., et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. The Lancet. 1996;348(9034):1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 159.Wu Q., Zhang H. A., Liu S. L., Meng T., Zhou X., Wang P. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. European journal of orthopaedic surgery & traumatology. 2015;25(3):525–541. doi: 10.1007/s00590-014-1568-z. [DOI] [PubMed] [Google Scholar]
- 160.Wei Z., Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfusion Medicine. 2015;25(3):151–162. doi: 10.1111/tme.12212. [DOI] [PubMed] [Google Scholar]
- 161.Tyagi V., Tomaszewski P., Lukasiewicz A., Theriault S., Pelker R. The role of intraoperative intermittent pneumatic compression devices in venous thromboembolism prophylaxis in total hip and total knee arthroplasty. Orthopedics. 2018;41(1):e98–e103. doi: 10.3928/01477447-20171114-06. [DOI] [PubMed] [Google Scholar]
- 162.Fisher W. D. Impact of venous thromboembolism on clinical management and therapy after hip and knee arthroplasty. Canadian journal of surgery Journal canadien de chirurgie. 2011;54(5):344–351. doi: 10.1503/cjs.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Z. H., Shen B., Yang J., Zhou Z. K., Kang P. D., Pei F. X. Risk factors for venous thromboembolism of total hip arthroplasty and total knee arthroplasty: a systematic review of evidences in ten years. BMC Musculoskeletal Disorders. 2015;16(1):p. 24. doi: 10.1186/s12891-015-0470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McCormack P. L. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585–617. doi: 10.2165/11209070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 165.Fillingham Y. A., Ramkumar D. B., Jevsevar D. S., et al. The Safety of Tranexamic Acid in Total Joint Arthroplasty: A Direct Meta- Analysis. The Journal of arthroplasty. 2018;33(10):3070–3082.e1. doi: 10.1016/j.arth.2018.03.031. e1. [DOI] [PubMed] [Google Scholar]
- 166.Franchini M., Mengoli C., Marietta M., et al. Safety of intravenous tranexamic acid in patients undergoing majororthopaedic surgery: a meta-analysis of randomised controlled trials. Blood transfusion=Trasfusione del sangue. 2018;16(1):36–43. doi: 10.2450//2017.0219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Everhart J. S., Sojka J. H., Mayerson J. L., Glassman A. H., Scharschmidt T. J. Perioperative allogeneic red blood-cell transfusion associated with surgical site infection after total hip and knee arthroplasty. The Journal of bone and joint surgery American volume. 2018;100(4):288–294. doi: 10.2106/JBJS.17.00237. [DOI] [PubMed] [Google Scholar]
- 168.Friedman R., Homering M., Holberg G., Berkowitz S. D. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. The Journal of bone and joint surgery American volume. 2014;96(4):272–278. doi: 10.2106/JBJS.L.01268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during the current study are available from the corresponding author upon reasonable request.


