Dear Editor
While fever, cough, and dyspnea are the main symptoms of coronavirus disease-2019 (COVID-19), nonrespiratory presentations have been increasingly recognized, including neurological manifestations (1, 2, 3). Herein, we describe the first report of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced facial nerve palsy assessed by 18fluoro-2-deoxy-d-glucose (18FDG) positron emission tomography-computed tomography (PET/CT).
A 60-year-old healthy man with COVID-19, confirmed by polymerase chain reaction assay performed due to close contact with a COVID-19 case, developed sudden-onset right-sided facial nerve palsy. The patient had no systemic, respiratory, or auditory symptoms, nor facial pain. He was subsequently admitted with fever, cough, and dyspnea, and received remdesivir, dexamethasone, and oxygen. There was peripheral right-sided facial nerve palsy, involving mouth, eye, and forehead, consistent with Bell's palsy. Electromyography revealed decreased compound muscle action potential (∼60% axonal degeneration) in the right facial nerve.
Given the likelihood of neuropathic impact of SARS-CoV-2(3), we performed 18FDG-PET/CT to assess the metabolic activity of the facial nerve and the related processing neurons. The study was approved by the Institutional Review Board of our institution and the patient provided written consent. 18FDG-PET/CT was performed after 6 hours of fasting, in a semidarkened, noiseless, and odorless room with the patient's eyes covered for 20 minutes, subsequent to which 18FDG was administered intravenously (4.6 MBq/kg, Masih Daneshvari hospital, Tehran, Iran). Whole body PET/CT was performed after 60 minutes by sequential TOF-PET/CT (Discovery 690 PET/CT, GE Healthcare, USA). The imaging data were acquired in the 3D mode with scan duration of 10 minutes for brain and 2 minutes per bed position for whole-body scan. Low-dose CT with shallow breathing was used for attenuation correction and diagnostic purposes. Scan parameters were as follows: tube voltage 120-140 kV, tube current with automated dose modulation 60-440 mA/slice, field of view 50 cm. Images with a transverse pixel size = 0.625 and a slice thickness = 3.75 mm were reconstructed in axial, coronal, and sagittal planes. The images were reviewed by a nuclear medicine physician and a radiologist with substantial experience in PET/CT imaging.
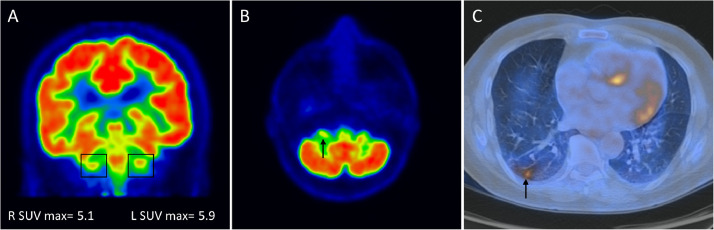
Brain PET/CT in axial and coronal planes identified decreased 18FDG uptake in the right facial nerve compared with the contralateral side, spanning from the cerebellopontine angle to the internal auditory canal (Fig 1 ). Maximal standardized uptake value in the right facial nerve was 5.1, which was significantly (ie, >10%) lower than the standardized uptake value in the left facial nerve (5.9). Additionally, hypermetabolic areas colocalizing with the ground-glass opacities were identified in the lung field (Fig 1).
Figure 1.
18FDG-PET/CT scan of a 60-year-old man with sudden-onset right sided facial nerve palsy and positive PCR for SARS-CoV-2. Coronal and axial planes of brain (a, b) and axial image of lung (c) are shown. (a) Maximal standardized uptake value (SUVmax) in the right facial nerve was 5.1, which was >10% lower than the SUV in the left facial nerve (5.9). (b) There was decreased 18FDG uptake in the right facial nerve (arrow) compared with the contralateral side, spanning from the cerebellopontine angle to the internal auditory canal. (c) Hypermetabolic areas colocalizing with ground-glass opacities were identified in the lung (arrow). (Color version of figure is available online.)
Bell's palsy is the most common etiology of facial nerve palsy. The condition is idiopathic in most cases, and less commonly is secondary to trauma, or viral or bacterial infection (3). It is typically self-limiting, with recovery occurring within 2-8 weeks in the majority of cases (4). If clinically indicated, neuro-imaging is performed to exclude other causes of facial nerve palsy, for example, space occupying lesions. A reduction in the metabolic activity in the facial nerve in the affected side compared with the contra-lateral side in the present case may suggest a reduction in the blood flow to the nerve secondary to microthrombosis in the perineural arteriovenous plexus—a phenomenon that occurs due to SARS-CoV-2-induced endothelial injury in the pulmonary and several other microvascular beds (5). In addition to vascular injury, an alternative or concurrent mechanism may be the neurotropathic effects of SARS-CoV-2 by infecting the neurons or supportive non-neural cells such as glia, as suggested by expression of the cellular entry proteins for SARS-CoV-2 in these cell types (3).
In conclusion, to the best of our knowledge, this is the first description of the hypometabolism of the facial nerve on 18FDG-PET/CT in COVID-19-induced Bell's palsy. Further research is warranted to elucidate the putative neuro-vascular underpinnings of the SARS-CoV-2-induced peripheral facial nerve palsy.
Acknowledgments
Prior Presentations
No
Author Contribution
M.K, M.B, N.R and A.Y have provided the case and images and M.K and S.H. have written the article.
Funding Sources/Disclosures
M.K, M.B, N.R, A.Y and S.H report no conflict of interest or funding sources.
References
- 1.Galougahi M.K., Ghorbani J., Bakhshayeshkaram M. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi-Galougahi M., Yousefi-Koma A., Bakhshayeshkaram M. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper K.W., Brann D.H., Farruggia M.C. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020 doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleicher J.N., Hamiel S., Gengler J.S. A survey of facial paralysis: etiology and incidence. Ear Nose Throat J. 1996;75:355–358. [PubMed] [Google Scholar]
- 5.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]