As the world is grappling with the global COVID-19 pandemic, dengue epidemics continue to rage relentlessly in the tropics and subtropics.1 About 100 million dengue cases are reported every year, often overwhelming already fragile health-care systems, with the highest burden in southeast Asia followed by Latin America.2 Dengue and COVID-19 have in common that epidemic transmission is driven by population densities, and both are rapidly spread via travellers.3 The difference between the two diseases is the mode of transmission. The four dengue virus serotypes are transmitted by Aedes spp mosquitoes, which mainly proliferate in the climatic conditions of the tropics and subtropics,4 whereas severe acute respiratory syndrome coronavirus 2 is transmitted via respiratory droplets ubiquitously.
While the scientific community is racing towards developing a vaccine against COVID-19, we already have a vaccine at hand against dengue. First licensed in 2015, the tetravalent live attenuated dengue vaccine developed by Sanofi Pasteur (CYD-TDV, with the trade name of Dengvaxia) was evaluated in more than 30 000 children in ten countries in Asia and Latin America, with now more than 5 years of observation time since administration of the first dose.5 The combined phase 3 trials showed a moderate–high efficacy, with increasing efficacy with age, serotype 4, and in settings with higher seroprevalence. Further post-hoc analyses with retrospective stratification into baseline serostatus (presence or absence of previous dengue infection at the time of administration of the first dose) revealed that vaccine performance was strongly driven by serostatus: seropositive individuals benefitted from high efficacy, whereas seronegative individuals experienced no statistically significant efficacy but an increase in hospitalised dengue from year 3 onwards after administration of the first dose.5 Subsequently, WHO recommended that CYD-TDV should only be given to seropositive individuals. Hence screening for dengue serostatus before vaccination is needed.6
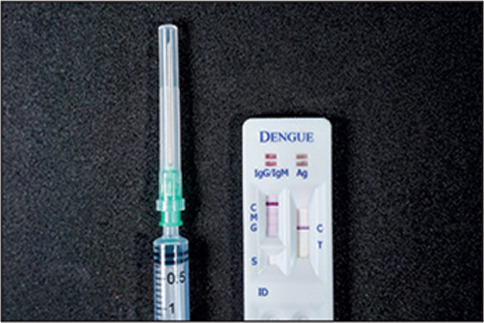
In The Lancet Infectious Diseases, Carlos DiazGranados and colleagues evaluated five commercially available immunoassays—two IgG-based ELISAs (EUROIMMUN and Panbio) and three rapid diagnostic tests (RDTs; TELL ME FAST, SD BIOLINE, and OnSite)—for their potential to classify baseline dengue serostatus, using baseline samples from more than 3000 participants in the immunogenicity subsets of the phase 3 CYD14 and CYD15 efficacy trials.7 All immunoassays exhibited high specificity (>98% for all immunoassays apart from SD BIOLINE RDT), but variable sensitivities, with higher sensitivities observed for the ELISAs (EUROIMMUN 89·2% [95% CI 87·9–90·3] and Panbio 92·5 [91·4 to 93·5]) than the RDTs (TELL ME FAST 52·5% [50·6 to 54·4], SD BIOLINE 71·1% [69·3 to 72·8], and OnSite 47·6% [45·7 to 49·5]).7 These results are encouraging, and consistent with an earlier evaluation of various ELISA against RDTs, in which sensitivities for RDTs were found to generally be lower than those of the ELISAs (≥90%).8, 9 Those studies also found that sensitivity for the assays evaluated by DiazGranados and colleagues appeared similar in samples from individuals with recent (<13 months) versus remote (3–4 years) virologically confirmed dengue.8 Additionally, cross-reactivity to other flaviviruses was low with RDTs (≤7%), but more significant with ELISAs (up to 51% for West Nile and 34% for Zika).8
DiazGranados and colleagues also re-evaluated CYD-TDV vaccine efficacy in participants identified as dengue seropositive by the five immunoassays.7 Vaccine efficacy against symptomatic virologically confirmed dengue in immunoassay-positive participants was high across all five immunoassays (from 82·8% [95% CI 66·9–91·1] by SD BIOLINE RDT to 89·7% [64·6–97·0] by OnSite RDT), as was vaccine efficacy against hospitalised virologically confirmed dengue (from 72·8% [38·9–87·9] by EUROIMMUN ELISA to 92·4% [37·8–99·1] by TELL ME FAST RDT), underpinning the public health usefulness of the first licensed dengue vaccine. Vaccine efficacy against severe virologically confirmed dengue was similarly high, but lacked precision owing to very few severe virologically confirmed dengue cases over the follow-up.
DiazGranados and colleagues' findings suggest that current commercially available immunoassays and RDTs could be used for pre-vaccination screening for CYD-TDV. Although a more sensitive or convenient test would improve the performance and efficiency of pre-vaccination screening programmes, countries can start to choose from existing screening tests for their vaccination programmes. The key considerations for selection are accuracy, ease of use, and affordability. Ideally, a screening test should be both highly sensitive and specific to minimise false positives and negatives to yield maximal population level benefit and minimise harm by correctly screening for seropositive individuals only.6 It should also be affordable, simple to use, and provide a rapid result so that vaccination can be given immediately after serostatus is confirmed. RDTs fulfil these ease-of-use requirements as they can use finger-pricked blood samples and the test can be done outside of laboratory settings with results available in 15–20 min.
While ELISAs are highly sensitive, they can only be done with serum or plasma and the blood samples have to be sent to a laboratory for testing. This requires phlebotomy and delays for obtaining the results. However, phlebotomies during school programmes for the purpose of school-based implementation combined with screening for other diseases could enhance the uptake and acceptance by schools and communities. In private clinics and travel medicine settings,10 blood is often taken before hepatitis B vaccination to check for hepatitis B serostatus, and thus there is precedence for pre-vaccination screening. Similar approaches can be taken for CYD-TDV.
As vaccine safety is a top priority, perhaps the most important consideration for test selection is test specificity. A test with high specificity would lead to few false-positive results, thus reducing possible harm from vaccinating a person who is dengue naive. As the number of false-positive results is affected by both test specificity and seroprevalence of dengue in the population, countries need to estimate the positive and negative predictive values of a screening test for each use setting, in addition to considerations of affordability. An additional consideration might be the use of a two-test algorithm: for instance, people are screened first with a RDT and, for those who are negative, a venous blood sample is collected for ELISA, or in areas where other flaviviruses are co-circulating, ELISA-positive results are confirmed with a more specific RDT.
During the COVID-19 pandemic, we cannot neglect the rapidly increasing dengue burden. The study by DiazGranados and colleagues has shown that we have the tools needed to maximise the public health impact of a dengue vaccine.
© 2021 iStock - Avid Photographer
Acknowledgments
We declare no competing interests. AW-S is consultant to WHO. None of the views expressed here necessarily present the views of WHO.
References
- 1.Wilder-Smith A, Tissera H, Ooi EE, Coloma J, Scott TW, Gubler DJ. Preventing dengue epidemics during the COVID-19 pandemic. Am J Trop Med Hyg. 2020;103:570–571. doi: 10.4269/ajtmh.20-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanaway JD, Shepard DS, Undurraga EA. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26:350–363. doi: 10.1093/jtm/taz062. [DOI] [PubMed] [Google Scholar]
- 5.Sridhar S, Luedtke A, Langevin E. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Hombach J, Ferguson N. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis. 2019;19:e31–e38. doi: 10.1016/S1473-3099(18)30494-8. [DOI] [PubMed] [Google Scholar]
- 7.DiazGranados CA, Bonaparte M, Wang H. Accuracy and efficacy of pre-dengue vaccination screening for previous dengue infection with five commercially available immunoassays: a retrospective analysis of phase 3 efficacy trials. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30695-2. published online Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaparte M, Zheng L, Garg S. Evaluation of rapid diagnostic tests and conventional enzyme-linked immunosorbent assays to determine prior dengue infection. J Travel Med. 2019;26 doi: 10.1093/jtm/taz078. [DOI] [PubMed] [Google Scholar]
- 9.Hunsperger E, Peeling R, Gubler DJ, Ooi EE. Dengue pre-vaccination serology screening for the use of Dengvaxia. J Travel Med. 2019;26 doi: 10.1093/jtm/taz092. [DOI] [PubMed] [Google Scholar]
- 10.Turner DP, McGuinness SL, Cohen J, Waring LJ, Leder K. Use of pre-travel vaccine-preventable disease serology as a screening tool to identify patients in need of pre-travel vaccination: a retrospective audit. J Travel Med. 2017;24 doi: 10.1093/jtm/tax011. [DOI] [PubMed] [Google Scholar]


