Related Articles, p. 190 and p. 204
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a public health emergency. Though originally described as a respiratory virus, SARS-CoV-2 has now been shown to have multiorgan involvement.1 Chronic kidney disease (CKD) has emerged as a risk factor for adverse outcomes. In this issue of AJKD, Ng et al2 and Flythe et al3 explore the association of the spectrum of kidney disease with outcomes in patients hospitalized with COVID-19.
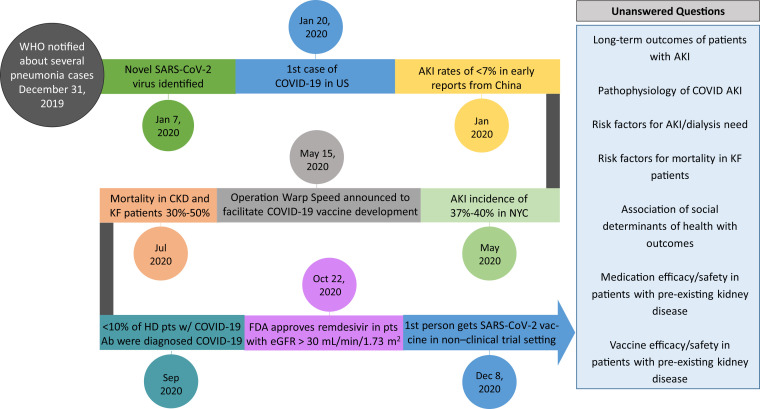
Although initial reports from China revealed a low incidence (5%-10%) of acute kidney injury (AKI), subsequent articles documented a far greater incidence of AKI in hospitalized patients with COVID-194 (Fig 1 ). In a follow-up study of hospitalized patients with COVID-19 from the Northwell Health system, Ng et al found that 40% of hospitalized patients admitted with COVID-19 developed AKI. Although not discussed in this article, urinalysis suggested causes other than acute tubular injury. In general, kidney biopsies have shown varied histopathology, including acute glomerulonephritis and acute tubular injury.
Figure 1.
Timeline of coronavirus disease 2019 (COVID-19) scientific discoveries and future directions, with special emphasis on the kidney and the unanswered questions for kidney disease and COVID-19. Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FDA, US Food and Drug Administration; HD, hemodialysis; KF, kidney failure; pt, patient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Regardless of pathophysiology, COVID-19–associated AKI is associated with increased mortality. Ng et al found these rates to be 46.4% in those not requiring kidney replacement therapy (KRT) and as high as 79.3% in those requiring KRT. At discharge, 69% of patients who required KRT and 74% of patients with AKI not requiring KRT had kidney recovery. What remains to be elucidated is the long-term consequences of COVID-19–associated AKI. Of those who did not recover kidney function or those who were discharged with continued requirement for KRT, how many of those patients ultimately recover? Conversely, for those who recovered, what proportion of those patients will have incident CKD?
Evaluation and treatment of AKI in COVID-19 patients is similar to AKI in non–COVID-19 patients, with supportive measures being the cornerstone of management. Hyperkalemia is common in patients hospitalized with COVID-19, likely due to high cell turnover similar to a hypercatabolic state and reduced kidney function. Given the possibility of limited supply of dialysis during a pandemic surge,5 the use of potassium binders in addition to other temporizing measures may help delay KRT necessity.
Approximately 19% of hospitalized patients with COVID-19–associated AKI will require KRT.4 During the pandemic and the consequent surge in patients requiring KRT, several challenges have led to reduced dialysis capacity, including nursing staff shortages, personal protective equipment shortages during the initial phases, and shortages of dialysis machines and supplies.
Intermittent hemodialysis (HD) remains the preferred option in hemodynamically stable patients, whereas continuous KRT (CKRT) is the modality of choice for hemodynamically unstable patients.6 To accommodate increased patient volume, intermittent HD treatments have often been shortened, with the use of slow continuous ultrafiltration devices and potassium binders between sessions. If CKRT is available, treatments with higher flow rates may be used to provide adequate clearance while allowing the machines to be used on multiple patients a day.7 Prolonged intermittent KRT has been used in hemodynamically unstable patients in facilities without CKRT capabilities or if demand has outstripped supply. It remains to be determined whether any of these measures had an impact on patient outcomes.
With limited personnel and dialysis resources during surges, acute peritoneal dialysis (PD) saw a resurgence because it helped bypass machine shortages and coagulopathy/vascular access issues, as well as reduce patient/nursing contact time. PD catheters placed by experienced personnel can be used within 24 to 48 hours of placement. Use of PD has questionable success rates in patients with acute respiratory distress syndrome treated with prone positioning.8 Studies of long-term outcomes of patients treated with acute PD and comparison of outcomes with HD are currently pending.
It rapidly became apparent that critically ill patients with COVID-19 were hypercoagulable, which posed yet another challenge: clotting of vascular access and dialyzers/lines.9 Many institutions implemented therapeutic anticoagulation strategies; however, there are no universally used protocols.
Given the high comorbid condition burden of patients with CKD and kidney failure, we anticipated COVID-19 to have a devastating impact on these vulnerable patients. Several studies in the United States reported mortality of up to 30%.
Flythe et al used the STOP-COVID database, a multicenter study of more than 4,000 critically ill patients admitted to an intensive care unit, to compare the clinical course of patients with and without pre-existing kidney disease and evaluate outcomes in patients with pre-existing kidney disease, both nondialysis CKD and kidney failure treated with dialysis. Although 12% of patients had pre-existing CKD, only 3% of patients had been receiving maintenance dialysis. Inflammatory marker patterns were inconsistent, with some higher in nondialysis patients with CKD and patients with kidney failure and others lower. Kidney disease remained an independent risk factor for in-hospital mortality,3 with a 28-day in-hospital mortality of 51% in those with kidney failure receiving dialysis, 49% in nondialysis CKD, and 35% in those without pre-existing kidney disease.
Regrettably, this study highlighted “renalism,”10 demonstrating that patients with CKD (including those with kidney failure) received experimental treatments half as often as patients without kidney disease. Given the high mortality rate, clinical trials need to enroll this at-risk group.
Patients receiving in-center HD are at a particularly higher risk for contracting SARS-CoV-2 infection due to frequent health care encounters. Patients with kidney failure are less likely to have classic COVID-19 symptoms compared with patients with and without CKD. Interestingly, they were more likely to present with altered mental status, highlighting the need for vigilant screening given the concern for “silent spread.” A recent study estimates that 10% of dialysis patients have had COVID-19, even though they represented only 3% of the critical care population in this study.
Several professional societies have issued guidelines to limit outbreaks in dialysis units. However, specific guidelines differ based on logistics, available trained personnel, and personal protective equipment.11 Immediate screening tests and isolation of patients or staff who had close contacts with cases are effective at preventing further spread of the infection.12 Although difficult to achieve in the midst of a pandemic, home modalities, which can use telemedicine platforms and reduce exposure, should be encouraged.
Since SARS-CoV-2 emerged nearly 1 year ago, the scientific community has rallied together to ensure the dissemination of knowledge regarding COVID-19. Although we have learned a great deal about COVID-19 and kidney disease, much remains to be elucidated, including long-term outcome studies and models for identification of patients at high risk for AKI and mortality. New reports have described inequalities in social determinants of health affecting COVID-19 outcomes and these need to be focused on for research. However, much work still lies ahead (Fig 1). Although COVID-19 is terrifying, it behooves us to remember words attributed to Marie Curie, “Nothing in life is to be feared, it is only to be understood. Now is the time to understand more, so that we may fear less.”13(p36)
Article Information
Authors’ Full Names and Academic Degrees
Lili Chan, MD, MSCR, Judy Hindi, MD, and Girish N. Nadkarni, MD, MPH.
Support
None.
Financial Disclosure
Dr Chan has received consulting fees from GLG consulting in the past three years and operational funding from Renal Research Institute. Dr Nadkarni receives financial compensation as a consultant and advisory board member for RenalytixAI, owns equity in RenalytixAI, is a scientific co-founder of RenalytixAI, is a consultant, advisory board member, and scientific co-founder for Pensieve Health, and has received operational funding from Goldfinch Bio and Renalytix AI and consulting fees from BioVie Inc, Variant Bio, AstraZeneca, Reata, and GLG consulting in the past 3 years.
Other Disclosures
Drs Chan and Nadkarni have contributed data to the STOP-COVID database.
Peer Review
Received October 27, 2020 in response to an invitation from the journal. Direct editorial input from an Associate Editor and a Deputy Editor. Accepted in revised form November 2, 2020.
References
- 1.Gupta A., Madhavan M.V., Sehgal K. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng J.H., Hirsch J.S., Hazzan A. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2):204–215. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flythe J.E., Assimon M.M., Tugman M.J. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77(2):190–203. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan L., Chaudhary K., Saha A. AKI in hospitalized patients with COVID-19 [published online ahead of print September 3, 2020]. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed]
- 5.Reddy Y.N.V., Walensky R.P., Mendu M.L., Green N., Reddy K.P. Estimating shortages in capacity to deliver continuous kidney replacement therapy during the COVID-19 pandemic in the United States. Am J Kidney Dis. 2020;76(5):696–709.e1. doi: 10.1053/j.ajkd.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adapa S., Aeddula N.R., Konala V.M. COVID-19 and renal failure: challenges in the delivery of renal replacement therapy. J Clin Med Res. 2020;12(5):276–285. doi: 10.14740/jocmr4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Division of Nephrology, Columbia University Vagelos College of Physicians Working Group Disaster response to the COVID-19 pandemic for patients with kidney disease in New York City. J Am Soc Nephrol. 2020;31(7):1371–1379. doi: 10.1681/ASN.2020040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivatana V., Aggarwal V., Finkelstein F.O., Naljayan M., Crabtree J.H., Perl J. Peritoneal dialysis for acute kidney injury treatment in the United States: brought to you by the COVID-19 pandemic. Kidney360. 2020;1(5):410–415. doi: 10.34067/KID.0002152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaranarayanan D., Muthukumar T., Barbar T. Anticoagulation strategies and filter life in COVID-19 patients receiving continuous renal replacement therapy: a single-center experience [published online ahead of print September 17, 2020]. Clin J Am Soc Nephrol. https://doi.org/10.2215/CJN.08430520 [DOI] [PMC free article] [PubMed]
- 10.Chertow G.M., Normand S.-L.T., McNeil B.J. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15(9):2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 11.Li S.-Y., Tang Y.-S., Chan Y.-J., Tarng D.-C. Impact of the COVID-19 pandemic on the management of patients with end-stage renal disease. J Chin Med Assoc. 2020;83(7):628–633. doi: 10.1097/JCMA.0000000000000356. 000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho J.-H., Kang S.H., Park H.C. Hemodialysis with cohort isolation to prevent secondary transmission during a COVID-19 outbreak in Korea. J Am Soc Nephrol. 2020;31(7):1398–1408. doi: 10.1681/ASN.2020040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaborg G.T. Need we fear our nuclear future? Bull Atomic Scientists. 1968;24(1):36–42. [Google Scholar]