Abstract
Aims
To assess low-density lipoprotein cholesterol (LDL-C) treatment target attainment among myocardial infarction (MI) patients according to the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) dyslipidaemia guidelines from 2011 (LDL-C < 1.8 mmol/L or ≥50% LDL-C reduction) and 2016 (LDL-C < 1.8 mmol/L and ≥50% LDL-C reduction).
Methods and results
Using nationwide registers, we identified 44 890 patients aged 21–74 admitted for MI, 2013–17. We included those attending follow-up visits at 6–10 weeks (n = 25 466) and 12–14 months (n = 17 117) after the event. Most patients received high-intensity statin monotherapy [84.3% (6–10 weeks) and 69.0% (12–14 months)] or statins with ezetimibe (2.7% and 10.2%). The proportion of patients attaining the 2011 LDL-C target was 63.8% (6–10 weeks) and 63.5% (12–14 months). The corresponding numbers for the 2016 LDL-C target were 31.6% (6–10 weeks) and 31.5% (12–14 months). At the 6- to 10-week follow-up, 37% of those not attaining the 2011 LDL-C target and 48% of those not attaining the 2016 target had an LDL-C level that was ≥0.5 mmol/L from the target. When comparing LDL-C measurements performed before vs. after the release of the 2016 guidelines, attainment of the 2016 LDL-C target increased from 30.2% to 35.0% (6–10 weeks) and from 27.6% to 37.6% (12–14 months).
Conclusion
In a nationwide register, one out of three patients with a recent MI had not attained the LDL-C target of the 2011 ESC/EAS guidelines and two out of three patients had not attained the LDL-C target of the 2016 guidelines.
Keywords: LDL-cholesterol, Statins, Ezetimibe, Target, Myocardial infarction
Introduction
Reduction of low-density lipoprotein cholesterol (LDL-C) with statins in patients with myocardial infarction (MI) reduces the risk of recurrent cardiovascular events.1 Compared to low- or moderate-intensity statin therapy, high-intensity statins, as well as the addition of ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, can further improve outcomes.2–5
The introduction of novel LDL-C lowering therapies and the mounting evidence regarding a causal association between LDL-C reduction and reduced cardiovascular risk have been reflected in guideline recommendations for treatment of dyslipidaemia in patients with MI. In the 2011 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) dyslipidaemia guidelines, lifestyle modifications and statin therapy were recommended to achieve an LDL-C goal of <1.8 mmol/L, or a ≥50% reduction in LDL-C level for patients who cannot achieve the LDL-C goal.6 In the 2016 update of these guidelines, the LDL-C target was changed to an LDL-C goal of <1.8 mmol/L and a ≥50% LDL-C level reduction if the baseline LDL-C level was between 1.8 and 3.5 mmol/L, with an added recommendation to consider ezetimibe and/or an PCSK9 inhibitor for patients who did not reach the target with maximally tolerated statin therapy.7 In the latest ESC/EAS dyslipidaemia guidelines, released in August 2019, the LDL-C target was updated to an LDL-C level of <1.4 mmol and a ≥50% LDL-C level reduction and the recommendations regarding use of ezetimibe and PCSK9 inhibitors were strengthened.8
While the aggressive LDL-C target and treatment recommendations of the 2019 ESC/EAS guidelines are likely to substantially affect lipid-lowering therapy among patients with MI, surprisingly little is known about to what extent LDL-C targets of the previous guidelines have been reached. Previous studies on LDL-C target attainment have used data from selected samples of patients,9–12 which limits their generalizability, included patients with a broad range of atherosclerotic cardiovascular disease (ASCVD) at various levels of risk,10,12–14 or assessed LDL-C levels a long period after the event.13 Furthermore, there is a lack of data on the attainment of the ≥50% LDL-C reduction criteria, which is an important component of the LDL-C target. Before the recommendations of the 2019 ESC/EAS guidelines are fully implemented and translated into national and local guidelines, it is crucial to assess to what extent the LDL-C targets of the previous ESC/EAS guidelines have been achieved.
We used data from nationwide registers in Sweden, 2013–17, to include patients with a recent MI and assess lipid-lowering therapy and attainment of the LDL-C targets of the 2011 and 2016 ESC/EAS guidelines at 6–10 weeks and 12–14 months after the event.
Methods
Study population
We used the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) register15 to include all patients, aged 18–74 years, admitted with an MI between 1 January 2013 and 1 October 2017, and who survived until discharge. During this period, patients aged <75 years could participate in the SWEDEHEART secondary prevention follow-up programme, including a first follow-up visit at 6–10 weeks after the MI and a second follow-up visit after 12–14 months.
One admission was randomly selected for patients with multiple admissions for MI during the study period. For the analyses at the 6- to 10-week follow-up visit, we then excluded patients who (i) had unknown statin or ezetimibe therapy at admission for the MI, (ii) had missing data on LDL-C at index MI, (iii) died within 10 weeks after discharge, (iv) received PCSK9 inhibitors at the index MI or follow-up visit, (v) had no registered follow-up visit, (vi) had missing data on LDL-C at the follow-up visit, or (vii) had unknown statin or ezetimibe therapy at the follow-up visit. For the analyses at the 12- to 14-month follow-up visit, we further excluded patients who (i) died before 14 months after discharge, (ii) received PCSK9 inhibitors at the 12- to 14-month follow-up, (iii) had no registered 12- to 14-month follow-up visit, (iv) had missing data on LDL-C at the 12- to 14-month follow-up, or (v) had unknown statin or ezetimibe therapy at the 12- to 14-month follow-up. We excluded patients receiving PCSK9 inhibitors as few patients received these drugs during the study period (n = 27).
Information about comorbidities was obtained from SWEDEHEART and complemented by data from the National Patient Register, which includes the inpatient care diagnoses of all hospital admissions in Sweden since 1987.16 Data concerning medications at admission, in-hospital treatments, discharge medications, and use of statins and ezetimibe at each follow-up visit were collected in SWEDEHEART. Data on filled prescriptions and the type of lipid-lowering therapy were obtained from the Prescribed Drugs Register which includes all filled prescriptions in Sweden.17 Date of death was obtained from the Swedish population registry. LDL-C levels were recorded in SWEDEHEART; the standard methods of measuring LDL-C at each hospital were used. Laboratories serving Swedish hospitals are included in a nationwide laboratory quality authority. Three times per year, the consistency of lipid measurements across the laboratories is assessed, with deviations outside pre-specified ranges of errors (total cholesterol, 5%; high-density lipoprotein-cholesterol, 10%; and LDL-C, 12%) prompting recalibration. The regional ethics committee in Stockholm approved the study (2018/1957/32).
Categorization of lipid-lowering therapies and baseline LDL-C levels
Patients who filled a statin and/or ezetimibe prescription within the previous 180 days and had a registered use of the drug in SWEDEHEART were considered as receiving statin and/or ezetimibe therapy at the index MI and at each follow-up, respectively. The patient was considered as exposed to the statin of the latest filled prescription if more than one prescription was filled within the previous 180 days. The patients’ lipid-lowering therapies were categorized into four groups: (i) not receiving statins, (ii) low- or moderate-intensity statin therapy, (iii) high-intensity statin therapy, and (iv) any statin combined with ezetimibe. High-intensity statin therapy was defined as atorvastatin ≥40 mg/day or rosuvastatin ≥20 mg/day. Statins that were not high-intensity statins were considered as low- or moderate-intensity statins.18
A baseline (pre-treatment) LDL-C level is required in order to assess the target of a ≥50% reduction in LDL-C level. Thus, for patients without ongoing lipid-lowering therapy at the index MI (80.2% of the study population), the LDL-C level as measured at the index MI was used. For those with ongoing lipid-lowering therapy (19.8%), the baseline LDL-C level was extrapolated using the LDL-C level at the index MI and the LDL-C reduction of the lipid-lowering therapy that the patient was receiving. We sampled this LDL-C reduction from β probability density functions derived from clinical trials, specific for each type and dose of lipid-lowering therapy, as presented and validated by Cannon et al.13 (Supplementary material online, Table S1).
Statistical analysis
First, we assessed lipid-lowering therapy at admission for the index MI and at each follow-up visit. For the total population and for each category of lipid-lowering therapy separately, we then assessed the proportion of the patients at each follow-up visit who (i) had neither attained an LDL-C level of <1.8 mmol/L nor an LDL-C level reduction of ≥50%, (ii) had not attained an LDL-C level of <1.8 mmol/L but attained a ≥50% LDL-C reduction, (iii) had attained an LDL-C level of <1.8 mmol/L but not an LDL-C level reduction of ≥50%, and (iv) had attained both an LDL-C level of <1.8 mmol/L and a ≥50% LDL-C level reduction. Of these categories of LDL-C target attainment, (ii)–(iv) represent attainment of the LDL-C target of the 2011 ESC/EAS guidelines and (iv) represents attainment of the LDL-C target of the 2016 ESC/EAS guidelines. To assess potential changes in lipid-lowering therapy and attainment of LDL-C targets associated with the introduction of the 2016 guidelines, we also assessed categories of lipid-lowering therapy and LDL-C target attainment at follow-up visits occurring before vs. after the release date of the 2016 guidelines (28 August 2016).
Next, we focused on the LDL-C target of the 2016 ESC/EAS guidelines. We described the patient characteristics by target attainment and assessed the distance to LDL-C target attainment by calculating the additional reduction in LDL-C level that patients who had not attained the target would need to achieve in order to reach the LDL-C target. We then assessed the association between patient characteristics, selected a priori (Supplementary material online, Table S2), and LDL-C target achievement using multivariable logistic regression.
Finally, in a sensitivity analysis, we assessed lipid-lowering therapy and LDL-C target attainment among patients with available data from both the 6- to 10-week and the 12- to 14-month follow-up visit. This analysis was performed to assess whether the findings were affected by the exclusion of patients between the two follow-up visits. Analyses were performed in Stata version 15.0 (StataCorp LP, College Station, TX, USA).
Results
Study population
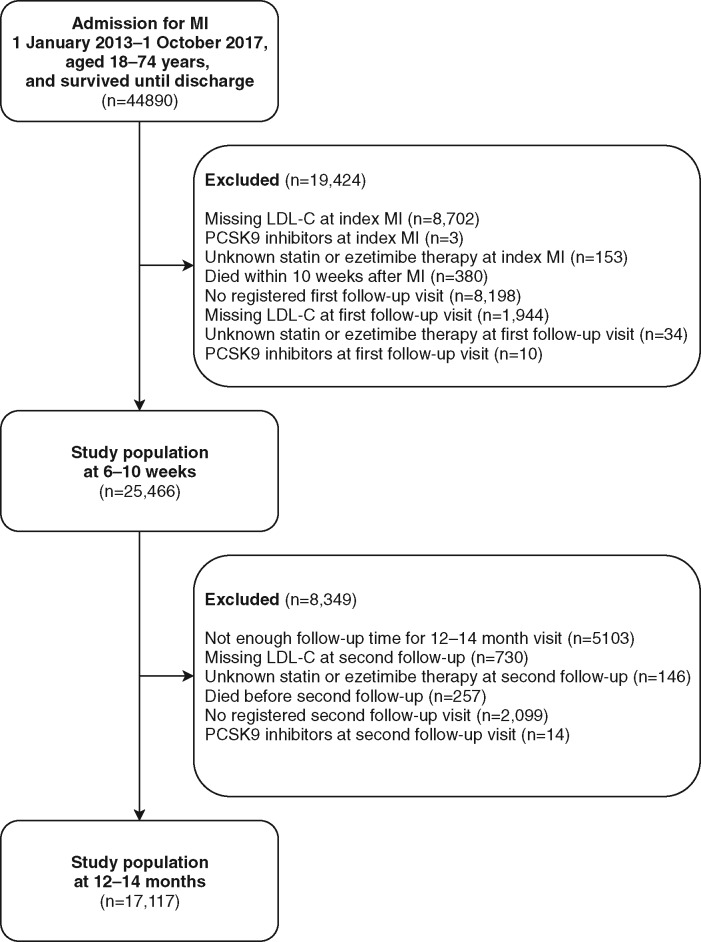
A total of 44 890 patients, aged 21–74 years, hospitalized with an MI and surviving until discharge were identified. At first follow-up and at second follow-up 25 466 and 17 117 patients, respectively, remained after exclusion criteria were applied (Figure 1). The baseline characteristics of those who were excluded and included in the analyses are shown in Supplementary material online, Table S3. Compared to the patients included in the analyses, those excluded before the 6- to 10-week follow-up were more likely to have had a non-ST-elevation MI, previous coronary intervention, more comorbidities, and to receive high-intensity statins at admission (Supplementary material online, Table S3).
Figure 1.
Flowchart for study population. LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PCSK9, proprotein convertase subtilisin/kexin type 9.
The mean [standard deviation (SD)] age of the study population at the index MI was 62 (9) years, 25% were women, 41% presented with an ST-elevation MI, 20% had diabetes mellitus, and 80% had no ongoing statin therapy. In total, 7336 (28.8%) of the 6- to 10-week follow-up visits and 6630 (38.7%) of the 12- to 14-month follow-up visits occurred after the release of the 2016 ESC/EAS guidelines. Mean (SD) LDL-C was 3.2 (1.1) mmol/L (index MI), 1.9 (0.7) mmol/L (6- to 10-week follow-up), and 1.9 (0.8) mmol/L (12- to 14-month follow-up).
Lipid-lowering therapy and attainment of LDL-C <1.8 mmol/L and/or ≥50% reduction
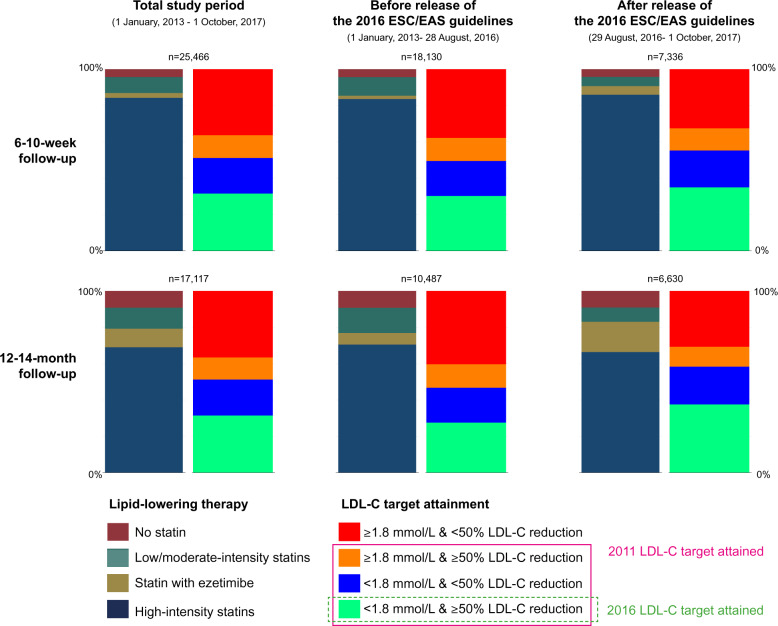
Figure 2 shows the proportion of patients by category of lipid-lowering therapy and by attainment of an LDL-C level of <1.8 mmol/L and a ≥50% reduction in LDL-C. At the 6- to 10-week follow-up, most patients had either high-intensity statin monotherapy (84.3%) or the combination of any statin with ezetimibe (2.7%). The corresponding numbers at the 12- to 14-month follow-up were 69.0% and 10.2%. In total, 63.8% (6–10 weeks) and 63.5% (12–14 months) of the patients had attained the LDL-C target of the 2011 guidelines as they had achieved an LDL-C level of <1.8 mmol/L or an LDL-C reduction of ≥50%. Roughly half of the patients [51.2% (6–10 weeks) and 51.3% (12–14 months)] reached an LDL-C of <1.8 mmol/L, while the proportion attaining an LDL-C reduction of ≥50% was 44.2% (6–10 weeks) and 43.7% (12–14 months). The proportion of patients attaining both an LDL-C level of <1.8 mmol/L and an LDL-C reduction of ≥50% (the 2016 ESC/EAS target) was 31.6% at the 6- to 10-week follow-up and 31.5% at the 12- to 14-month follow-up.
Figure 2.
Achievement of an LDL-C level of <1.8 mmol/L and a ≥50% reduction in LDL-C level and lipid-lowering therapies at 6–10 weeks and 12–14 months after myocardial infarction for the total study period and for follow-up visits occurring before and after the release of the 2016 ESC/EAS guidelines. ESC, European Society of Cardiology; EAS, European Atherosclerosis Society; LDL-C, low-density lipoprotein cholesterol.
When follow-up visits occurring before and after the introduction of the 2016 guidelines were analysed separately, we observed an increase in the use of combination therapy with statins and ezetimibe, especially at the 12- to 14-month follow-up. Concurrently, a larger proportion of patients at the 6- to 10-week follow-up had attained an LDL-C level of <1.8 mmol/L (49.5% before the release of the 2016 guidelines vs. 55.3% after the release) and an LDL-C reduction of ≥50% (43.0% vs. 47.3%), with similar findings at the 12- to 14-month follow-up. Accordingly, a larger proportion of patients attained the 2016 LDL-C target after the introduction of the 2016 guidelines (35.0% at 6–10 weeks and 37.6% at 12–14 months) compared to before these guidelines were released (30.2% and 27.6%) (Figure 2 and Supplementary material online, Table S4).
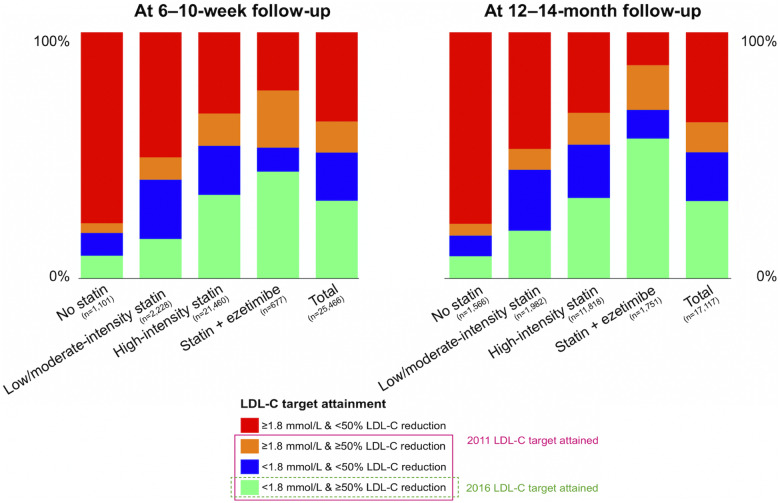
Among patients receiving statins with ezetimibe, 53.2% attained an LDL-C level of <1.8 mmol/L at the 6- to 10-week follow-up and 43.4% attained the 2016 ESC/EAS target of both this LDL-C goal and an LDL-C reduction of ≥50% (Figure 3). The corresponding numbers were 53.9% and 34.0% for those receiving monotherapy with high-intensity statins and 40.2% and 16.1% for those with low- or moderate-intensity statins. LDL-C target attainment by category of lipid-lowering therapy at follow-up visits occurring before vs. after the release of the 2016 ESC guidelines are shown in Supplementary material online, Figure S1.
Figure 3.
Achievement of an LDL-C level of <1.8 mmol/L and a ≥50% reduction in LDL-C level by category of lipid-lowering therapy at 6–10 weeks and 12–14 months after myocardial infarction. LDL-C, low-density lipoprotein cholesterol.
Factors associated with attainment of the 2016 ESC LDL-C target
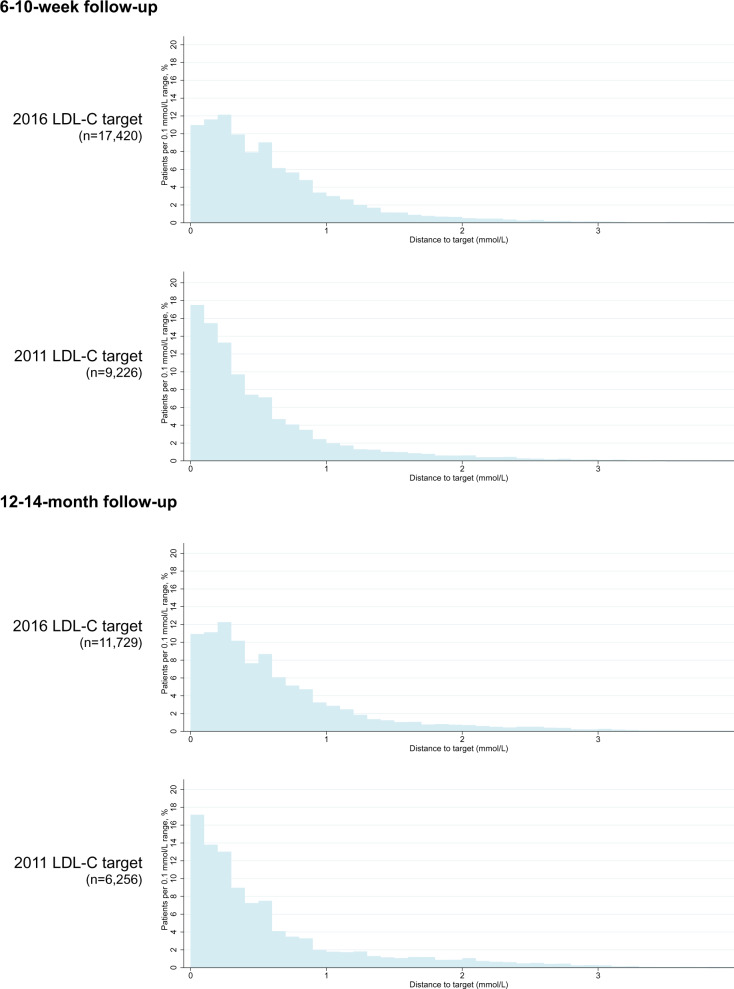
Characteristics of patients included in the analysis at each follow-up by achievement of 2016 LDL-C target are shown in Table 1. In the multivariable logistic regression analyses, several factors were associated with LDL-C target achievement; for example, at both follow-ups, current smoking, previous MI, and previous stroke were associated with a lower likelihood of achieving the LDL-C target, while more intensive lipid-lowering therapy and diabetes mellitus were associated with a higher likelihood of achieving the LDL-C target (Supplementary material online, Table S5). The median (interquartile range) distance to the 2011 ESC/EAS LDL-C target among those who had not attained the target was 0.3 (0.1–0.7) mmol/L at the 6- to 10-week follow-up and 0.4 (0.2–0.8) mmol/L at the 12- to 14-month follow-up with 37% and 40% of the patients at each of the follow-up visits having a distance to the target that was ≥0.5 mmol/L. The corresponding numbers for the 2016 ESC/EAS LDL-C target were 0.5 (0.2–0.8) mmol/L at the 6- to 10-week follow-up and 0.5 (0.2–0.9) mmol/L at the 12- to 14-month follow-up. At both follow-up visits, 48% of these patients had a distance to the 2016 target that was ≥0.5 mmol/L (Figure 4, Supplementary material online, Table S6). In the sensitivity analyses of patients who had LDL-C data available at both the 6- to 10-week and 12- to 14-month follow-up, findings regarding lipid-lowering therapy and LDL-C target attainment were largely similar to those in the main analyses (Supplementary material online, Table S7).
Table 1.
Characteristics of the study population 6–10 weeks (n = 25 466) and 12–14 months (n = 17 117) after MI, by attainment of the LDL-C target of the 2016 ESC/EAS dyslipidaemia guidelines
| 6- to 10-week follow-up |
12- to 14-month follow-up |
|||
|---|---|---|---|---|
| LDL-C target not reached (n = 17 420) | LDL-C target reached (n = 8046) | LDL-C target not reached (n = 11 729) | LDL-C target reached (n = 5388) | |
| At index event | ||||
| Age (years) | 63 ± 8 | 62 ± 9 | 63 ± 8 | 62 ± 8 |
| Age category (years), n (%) | ||||
| 18–44 | 542 (3) | 323 (4) | 351 (3) | 165 (3) |
| 45–54 | 2579 (15) | 1363 (17) | 1767 (15) | 852 (16) |
| 55–64 | 5737 (33) | 2619 (33) | 3854 (33) | 1768 (33) |
| 65–74 | 8562 (49) | 3741 (46) | 5757 (49) | 2603 (48) |
| Women, n (%) | 4402 (25) | 1852 (23) | 2933 (25) | 1180 (22) |
| BMI category (kg/m2) | ||||
| <25 | 4798 (28) | 2006 (26) | 3256 (28) | 1339 (26) |
| 25 to <30 | 7686 (45) | 3695 (47) | 5272 (46) | 2456 (47) |
| ≥30 | 4475 (26) | 2149 (27) | 2897 (25) | 1453 (28) |
| Total cholesterol (mmol/L) | 5.0 ± 1.3 | 5.1 ± 1.0 | 5.0 ± 1.3 | 5.2 ± 1.1 |
| LDL-cholesterol (mmol/L) | 3.1 ± 1.2 | 3.2 ± 0.9 | 3.1 ± 1.2 | 3.3 ± 1.0 |
| HDL-cholesterol (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 |
| Triglycerides (mmol/L) | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 |
| Type of MI, n (%) | ||||
| STEMI | 7341 (42) | 3227 (40) | 4840 (41) | 2239 (42) |
| NSTEMI | 10 079 (58) | 4819 (60) | 6889 (59) | 3149 (58) |
| Received PCI | 9908 (85) | 12 003 (87) | 10 033 (86) | 4702 (87) |
| Lipid-lowering therapy at admission, n (%) | ||||
| No statins | 13 782 (79) | 6762 (84) | 9376 (80) | 4415 (82) |
| Low- or moderate-intensity statin | 2613 (15) | 862 (11) | 1746 (15) | 688 (13) |
| High-intensity statin | 915 (5) | 363 (5) | 539 (5) | 246 (5) |
| Statin with ezetimibe | 110 (1) | 59 (1) | 68 (1) | 39 (1) |
| Smoking status, n (%) | ||||
| Never smoker | 5810 (34) | 2988 (38) | 4055 (35) | 1984 (37) |
| Former smoker | 6183 (36) | 2801 (35) | 4198 (36) | 1911 (36) |
| Current smoker | 5088 (30) | 2115 (27) | 3285 (28) | 1414 (27) |
| Comorbidities, n (%) | ||||
| Hypertension | 8939 (51) | 4394 (55) | 5538 (48) | 2430 (45) |
| Diabetes mellitus | 3396 (19) | 1685 (21) | 2256 (19) | 1066 (20) |
| Previous MI | 2904 (17) | 849 (11) | 1801 (15) | 623 (12) |
| Previous PCI | 2505 (14) | 749 (9) | 1574 (13) | 557 (10) |
| Previous CABG | 694 (4) | 206 (3) | 420 (4) | 158 (3) |
| Congestive heart failure | 514 (3) | 127 (2) | 277 (2) | 99 (2) |
| Previous stroke | 844 (5) | 278 (3) | 536 (5) | 163 (3) |
| COPD | 896 (5) | 280 (3) | 554 (5) | 183 (3) |
| Renal insufficiencya | 1806 (11) | 676 (9) | 1169 (10) | 445 (8) |
| Peripheral artery disease | 512 (3) | 154 (2) | 323 (3) | 86 (2) |
| Cancer | 259 (1) | 106 (1) | 160 (1) | 59 (1) |
| At 6- to 10-week follow-up | ||||
| LDL-cholesterol (mmol/L) | 2.1 ± 0.7 | 1.3 ± 0.3 | 2.0 ± 0.7 | 1.6 ± 0.6 |
| Lipid-lowering therapy, n (%) | ||||
| No statin | 999 (6) | 102 (1) | 554 (5) | 100 (2) |
| Low- or moderate-intensity statin | 1870 (11) | 358 (4) | 1348 (11) | 286 (5) |
| High-intensity statin | 14 168 (81) | 7292 (91) | 9627 (82) | 4854 (90) |
| Statin with ezetimibe | 383 (2) | 294 (4) | 200 (2) | 148 (3) |
| At 12- to 14-month follow-up | ||||
| LDL-cholesterol (mmol/L) | — | — | 2.2 ± 0.8 | 1.3 ± 0.3 |
| Lipid-lowering therapy, n (%) | ||||
| No statin | — | — | 1424 (12) | 142 (3) |
| Low- or moderate-intensity statin | — | — | 1597 (14) | 385 (7) |
| High-intensity statin | — | — | 7952 (68) | 3866 (72) |
| Statin with ezetimibe | — | — | 756 (6) | 995 (18) |
Values are represented as means ± standard deviation or n (%).
Missing values (first follow-up; second follow-up): total cholesterol (n = 165; 80), triglycerides (n = 1989; 1141), HDL-cholesterol (n = 217; 91), BMI (n = 657; 444), smoking status (n = 481; 270), and renal insufficiency (n = 346; 271).
BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; ESC, European Society of Cardiology; EAS, European Atherosclerosis Society; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, based on the Chronic Kidney Disease Epidemiology Collaboration equation.
Figure 4.
Distance to the LDL-C target for patients not attaining the LDL-C target according to the 2011 and 2016 ESC/EAS guidelines, respectively, at each of the 6- to 10-week and 12- to 14-month follow-up visits. EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; LDL-C, low-density lipoprotein cholesterol.
Discussion
In this study including patients with a recent MI from a nationwide register (2013–17), we found that although most of the patients received high-intensity statins at 12–14 months after the event, around one in three patients had not attained an LDL-C level of <1.8 mmol/L or an LDL-C reduction of ≥50%, as was recommended in the 2011 ESC/EAS guidelines for the management of dyslipidaemias. Half of the patients had not attained the LDL-C goal of <1.8 mmol/L and two out of three patients had not attained this LDL-C goal and reached a ≥50% reduction in LDL-C levels, as was recommended in the 2016 ESC/EAS guidelines. Around 4 out of 10 of the patients who did not attain the 2011 LDL-C target and half of those not attaining the 2016 LDL-C target had an LDL-C level that was ≥0.5 mmol/L from the target.
Reflecting the mounting data of benefits associated with LDL-C lowering and the introduction of new LDL-C lowering therapies, each update of the ESC/EAS dyslipidaemia guidelines has included a more aggressive LDL-C target for patients with MI. The recently released ESC/EAS guidelines now recommend an LDL-C goal of <1.4 mmol/L and a ≥50% LDL-C reduction for this group of patients. Meanwhile, uncertainty has remained regarding attainment of the LDL-C targets recommended in previous guidelines for patients with MI. In a study based on a convenience sample of patients from 18 countries, 37% of the 1071 patients with acute coronary syndrome had reached an LDL-C of <1.8 mmol/L at 120 days after the event.9 In another study including 7824 patients from 27 countries, interviewed 0.5–2 years after an elective coronary artery bypass grafting, elective percutaneous coronary intervention, or an acute coronary syndrome, only 29.0% of the patients had attained an LDL-C level of <1.8 mmol/L.12 However, these studies as well as other previous analyses of LDL-C target attainment have had limitations, including the use of data from selected samples of patients with limited generalizability,9–12 or broad samples of patients with a wide range of ASCVD at various levels of risk,10,12–14 as well as LDL-C level assessments performed a long period after the event.13 Our study expands the knowledge of LDL-C target attainment by including a large population representative of patients seen in routine clinical practice and by using LDL-C data from the time of the MI, as well as from follow-up visits at 6–10 weeks and 12–14 months after the event. Importantly, while previous studies have reported the attainment of an LDL-C level of <1.8 mmol/L, none has assessed the criteria of achieving a ≥50% reduction in LDL-C. Our analyses showed that around two out of five patients who had attained an LDL-C level of <1.8 mmol/L did not reach the LDL-C target of the 2016 ESC/EAS guidelines as they had not achieved a ≥50% reduction in LDL-C levels.
While estimation of the effect of expanded lipid-lowering therapy was outside the scope of this paper, we note that 84.3% of the patients received high-intensity statins and 2.7% received statins with ezetimibe at the 6- to 10-week follow-up, with the corresponding numbers being 69.0% and 10.2% at the 12- to 14-month follow-up. As such, the potential for further LDL-C reduction with improved uptake of statin therapy seems limited, while a larger proportion of patients were not receiving ezetimibe. However, we also note that only 4 out of 10 of the patients who received combination therapy with statins and ezetimibe attained the 2016 LDL-C target at 6–10 weeks after the event, indicating a need for use of PCSK9 inhibitors if lower LDL-C levels are to be achieved in these patients. In line with these observations, simulation studies in broad populations of patients with ASCVD indicate that although improvements in LDL-C target attainment can be achieved by increasing statin coverage and intensity and add-on treatment with ezetimibe, a non-negligible proportion would also need PCSK9 inhibitors.13,19 For example, in a simulation model study of patients with ASCVD in the USA, only 25.2% had an LDL-C of <1.8 mmol/L; when intensification of lipid-lowering therapy was modelled, 99.3% of the patients could achieve an LDL-C level of <1.8 mmol/L, including 67.3% with statin monotherapy, 18.7% with statins plus ezetimibe, and 14% with add-on PCSK9 inhibitor.19
In our analyses of LDL-C measurements performed before vs. after the introduction of the 2016 guidelines, we observed that the proportion of patients attaining the 2016 ESC/EAS LDL-C target had increased from 30.2% to 35.0% at 6–10 weeks after the event and from 27.6% to 37.6% at 12–14 months after the event. Concurrently, use of ezetimibe had increased, although the proportion receiving this drug was still small. The increase in treatment intensity may reflect uptake of the LDL-C target of the 2016 guidelines, as well as the publication of the IMPROVE-IT trial of ezetimibe in 2015,3 and the subsequent inclusion of the drug in the 2016 ESC/EAS dyslipidaemia guidelines, as well as the 2015 ESC guidelines for management of acute coronary syndromes.7,20
Our study has limitations. For the analyses including patients attending the 6- to 10-week follow-up visit, we excluded 19 424 (43.3%) of the patients meeting the inclusion criteria, the majority due to missing data on LDL-C levels or no registered follow-up visit. Compared to those included in the analyses, the excluded patients were slightly older, less likely to have ST-elevation MI and more likely to have had a previous MI, although their LDL-C levels were comparable to those of the study population. As patients who do not attend follow-up visits may have decreased adherence to lifestyle recommendations and drug treatment, it is possible that we have overestimated the proportion attaining the LDL-C target. Moreover, exposure to drugs was defined based on filled prescriptions; we had no data on adherence. Finally, the methodology for measurement of LDL-C levels varied across hospitals in Sweden. This reflects the situation in real-world clinical practice.
In this study using nationwide data from patients with a recent MI who were followed up to 12–14 months after the event, one out of three patients had not attained an LDL-C level of <1.8 mmol/L or a ≥50% reduction in LDL-C levels, as recommended in the 2011 ESC/EAS guidelines and two out of three had not attained both an LDL-C level of <1.8 mmol/L and a ≥50% reduction in LDL-C levels, as recommended in the 2016 ESC/EAS guidelines. Attainment of the LDL-C targets recommended in the previous ESC/EAS guidelines for the management of dyslipidaemia has been limited.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Funding
This work was supported by funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of interest: A.A. reports institutional grants from MSD. T.J. reports grant from MSD, during the conduct of the study; other from Astra-Zeneca, MSD, Bayer, Novartis, and Sanofi, outside the submitted work. D.L., S.S., and R.B. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may hold shares and stock options in Merck & Co., Inc., Kenilworth, NJ, USA. E.H. has received payment for lecturing and consulting from Bayer, Sanofi, Amgen, and research grants from Sanofi and Amgen, outside the submitted work. P.L. reports minor consulting and lecture fees from Sanofi, Boehringer Ingelheim, and Amgen. J.S. reports grants from Amgen outside the submitted work. P.U. has no conflict of interest to disclose.
Supplementary Material
References
- 1. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C. et al. ; Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 4. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R. et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 6.European Association for Cardiovascular Prevention & Rehabilitation Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen M-R, Wiklund O. et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 7. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H. et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 8. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 9. Gitt AK, Lautsch D, Ferrieres J, De Ferrari GM, Vyas A, Baxter CA. et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: results from the Dyslipidemia International Study II. Atherosclerosis 2017;266:158–166. [DOI] [PubMed] [Google Scholar]
- 10. Waters DD, Brotons C, Chiang C-W, Ferrières J, Foody J, Jukema JW. et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation 2009;120:28–34. [DOI] [PubMed] [Google Scholar]
- 11. Ferrieres J, De Ferrari GM, Hermans MP, Elisaf M, Toth PP, Horack M. et al. Predictors of LDL-cholesterol target value attainment differ in acute and chronic coronary heart disease patients: results from DYSIS II Europe. Eur J Prev Cardiol 2018;25:1966–1976. [DOI] [PubMed] [Google Scholar]
- 12. De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Z, Ryden L. et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019;285:135–146. [DOI] [PubMed] [Google Scholar]
- 13. Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ.. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox KM, Tai MH, Kostev K, Hatz M, Qian Y, Laufs U.. Treatment patterns and low-density lipoprotein cholesterol (LDL-C) goal attainment among patients receiving high- or moderate-intensity statins. Clin Res Cardiol 2018;107:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A. et al. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 16. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C. et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U. et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–735. [DOI] [PubMed] [Google Scholar]
- 18. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 19. Virani SS, Akeroyd JM, Nambi V, Heidenreich PA, Morris PB, Nasir K. et al. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk): insights from the Department of Veterans Affairs. Circulation 2017;135:2572–2574. [DOI] [PubMed] [Google Scholar]
- 20. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F. et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.