Supplemental Digital Content is available in the text.
Keywords: empagliflozin, heart failure, sodium-glucose transporter 2 inhibitors
Background:
Empagliflozin reduces the risk of cardiovascular death or hospitalization for heart failure in patients with heart failure and a reduced ejection fraction, with or without diabetes, but additional data are needed about the effect of the drug on inpatient and outpatient events that reflect worsening heart failure.
Methods:
We randomly assigned 3730 patients with class II to IV heart failure with an ejection fraction of ≤40% to double-blind treatment with placebo or empagliflozin (10 mg once daily), in addition to recommended treatments for heart failure, for a median of 16 months. We prospectively collected information on inpatient and outpatient events reflecting worsening heart failure and prespecified their analysis in individual and composite end points.
Results:
Empagliflozin reduced the combined risk of death, hospitalization for heart failure or an emergent/urgent heart failure visit requiring intravenous treatment (415 versus 519 patients; empagliflozin versus placebo, respectively; hazard ratio [HR], 0.76; 95% CI, 0.67–0.87; P<0.0001). This benefit reached statistical significance at 12 days after randomization. Empagliflozin reduced the total number of heart failure hospitalizations that required intensive care (HR, 0.67; 95% CI, 0.50–0.90; P=0.008) and that required a vasopressor or positive inotropic drug or mechanical or surgical intervention (HR, 0.64; 95% CI, 0.47–0.87; P=0.005). As compared with placebo, fewer patients in the empagliflozin group reported intensification of diuretics (297 versus 414 [HR, 0.67; 95% CI, 0.56–0.78; P<0.0001]). Additionally, patients assigned to empagliflozin were 20% to 40% more likely to experience an improvement in New York Heart Association functional class and were 20% to 40% less likely to experience worsening of New York Heart Association functional class, with statistically significant effects that were apparent 28 days after randomization and maintained during long-term follow-up. The risk of any inpatient or outpatient worsening heart failure event in the placebo group was high (48.1 per 100 patient-years of follow-up), and it was reduced by empagliflozin (HR, 0.70; 95% CI, 0.63–0.78; P<0.0001).
Conclusions:
In patients with heart failure and a reduced ejection fraction, empagliflozin reduced the risk and total number of inpatient and outpatient worsening heart failure events, with benefits seen early after initiation of treatment and sustained for the duration of double-blind therapy.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977.
ClinicalPerspective.
What Is New?
Empagliflozin reduced the total number of hospitalizations for heart failure, including those that required intensive care and those that required a vasopressor or positive inotropic drug or mechanical or surgical intervention.
Patients treated with empagliflozin were less likely to require intensification of diuretics and were more likely to experience improvement (and less likely to show deterioration) in New York Heart Association functional class in the outpatient setting.
The effect of empagliflozin to reduce the combined risk of death, hospitalization for heart failure, or an emergent or urgent heart failure visit was statistically significant at 12 days after randomization.
What Are the Clinical Implications?
The effect of empagliflozin to reduce worsening heart failure events in both the outpatient and inpatient setting reinforces its previously reported benefits to reduce the combined risk of cardiovascular death and hospitalization for heart failure and to decrease the risk of serious adverse renal events.
The combined results of EMPEROR-Reduced and the DAPA-HF trials support the use of SGLT2 inhibitors as part of standard of care for patients with heart failure and a reduced ejection fraction, with or without diabetes.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce the risk of hospitalizations for heart failure in 8 large-scale trials, including those that focused on type 2 diabetes, heart failure with a reduced ejection fraction, and chronic kidney disease.1–8 Although hospitalization for heart failure has been regarded as a reliable surrogate of disease progression in heart failure,9,10 efforts to standardize the identification of events by the process of adjudication typically exclude many events that are regarded as clinically meaningful by the treating physician.11–14 Furthermore, any benefit on hospitalizations for heart failure should not be counterbalanced by an increase in other causes of hospitalizations, leading to little overall benefit when the effect on hospitalizations for any reason are analyzed.
Importantly, hospitalization for heart failure represents only a small fraction of a patient’s total life experiences.15,16 If the goal of treatment is to maintain clinical stability, an intervention should have a favorable impact on other manifestations of worsening heart failure, particularly those that occur in an outpatient setting.17 These include worsening functional capacity, worsening of symptoms that require intensification of background therapy (especially diuretics), and the need for treatment in an emergent or urgent care setting. These outpatient events carry an adverse prognostic import comparable to that of a heart failure hospitalization.18–21 Several treatments that reduce the risk of hospitalizations for heart failure in patients with heart failure and a reduced ejection fraction also favorably impact outpatient metrics of clinical instability.21–23
Little is known about the effect of SGLT2 inhibitors on the broad spectrum of both inpatient and outpatient worsening heart failure events, because these were not documented in trials with these drugs in type 2 diabetes.1–5 The EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) trial demonstrated a benefit of SGLT2 inhibition with empagliflozin to reduce both the risk and rate of heart failure hospitalizations.7 The current report expands the findings of the effects on the drug on both inpatient and outpatient worsening heart failure events.
Methods
The design and methodology used in the EMPEROR-Reduced trial have been described in detail in previous publications.7,24 The trial was a randomized, double-blind, parallel-group, placebo-controlled, and event-driven study. Ethics approval was obtained at each study site, and all patients provided informed consent to participate in the study; the registration identifier at ClinicalTrials.gov is NCT03057977. Data will be made available on request in adherence with transparency conventions in medical research and through requests to the corresponding author. The executive committee of EMPEROR has developed a comprehensive analysis plan and numerous prespecified analyses, which will be presented in future scientific meetings and publications. At a later point in time, the full database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer-ingelheim.com/transparency_policy.html).
Study Patients
We enrolled men or women with chronic heart failure (New York Heart Association [NYHA] functional class II, III, or IV) with a left ventricular ejection fraction ≤40%, who were all receiving appropriate treatments for heart failure, including diuretics, inhibitors of the renin-angiotensin system and neprilysin, β-blockers, mineralocorticoid receptor antagonists, and when indicated, cardiac devices.
We designed the trial to preferentially enroll patients with a left ventricular ejection fraction of ≤30%. To do so, we limited the number of patients with an ejection fraction >30% by requiring them to have a hospitalization for heart failure within 12 months or high levels of NT-proBNP (N-terminal prohormone B-type natriuretic peptide; ie, ≥1000 pg/ml or ≥2500 pg/ml in those with an ejection fraction of 31% to 35% or 36% to 40%, respectively) as compared with ≥600 pg/ml in those with an ejection fraction of ≤30%. These NT-proBNP thresholds were doubled in patients with atrial fibrillation.7,24
Study Assessments
Patients who fulfilled prespecified inclusion and exclusion criteria were randomized, double-blind (in a 1:1 ratio) to receive placebo or empagliflozin 10 mg daily, in addition to their usual therapy for heart failure. After randomization, all appropriate treatments for heart failure or other medical conditions could be initiated or altered at the clinical discretion of the investigator in response to changes in each patient’s clinical status. Patients were assessed at study visits for major outcomes, symptoms and functional capacity related to heart failure, changes in medications used for heart failure, vital signs, biomarkers reflecting changes in the course of heart failure or the action of SGLT2 inhibitors, and adverse events; these assessments took place every 2 to 6 months, depending on the metric and the duration of follow-up. All randomized patients were followed for the occurrence of prespecified outcomes for the entire duration of the trial, regardless of whether the study participants were taking their study medications or were compliant with study procedures.
Trial End Points
We specified 3 measures as major outcomes to be evaluated in a hierarchical manner.
The primary end point was the composite of adjudicated cardiovascular death or hospitalization for heart failure, analyzed as time-to-first-event. The first secondary end point was the occurrence of all adjudicated hospitalizations for heart failure (including first and recurrent events). The second secondary end point was the analysis of the slope of the change in estimated glomerular filtration rate during double-blind treatment, which was supported by an analysis of a composite of end-stage renal events. We planned to enroll ≈3600 patients who were expected to experience a target of 841 adjudicated primary end point events during a follow-up period of 6 to 36 months.
To ascertain these major outcomes, we prospectively collected information on all deaths, all hospitalizations for any reason, and all emergent and urgent outpatient events that may reflect worsening heart failure. Hospitalizations were classified as cardiovascular or noncardiovascular based on the judgment of the investigator. In contrast, a heart failure hospitalization was prospectively identified by a clinical-event committee in a blinded manner using prespecified criteria. To qualify as an adjudicated heart failure hospitalization, patients were required to have meaningful worsening of their clinical status and intensification of treatment for heart failure. The duration of the in-hospital stay was at least 12 hours; if the patient had not received intravenous medications for heart failure, the minimum stay of an adjudicated heart failure hospitalization was 24 hours. Investigators documented the clinical course of each hospital admission on a dedicated form, including the prescribing of medications and devices used to treat episodes of clinical decompensation. The committee also adjudicated the occurrence of myocardial infarction, stroke, and transient ischemic attack.
In addition to the adjudication and characterization of hospitalizations for heart failure, at each scheduled study visit, patients were prospectively asked about interval events and changes in the use of diuretics that reflected the occurrence of worsening heart failure since the last visit. Events that were considered to reflect clinical deterioration included: (1) worsening heart failure that required the use of an intravenous drug for heart failure in an emergency department; (2) worsening heart failure that required the use of an intravenous drug for heart failure in an outpatient urgent care setting; (3) intensification of daily doses of diuretics for worsening symptoms; and (4) worsening NYHA functional class. Events treated in an emergency department, urgent care setting, or during a hospital stay shorter than that required for an adjudicated event were grouped together. Physicians were not provided any specific guidance as to the degree or duration of diuretic intensification that would qualify as representing a clinically meaningful change. Ascertainment of these events and measures were prospectively collected in the case report form, and their inclusion in analyses of individual and composite end points was prespecified before the blind of the trial was broken.
Statistical Analysis
For time-to-first-event analyses, differences between the placebo and empagliflozin groups were assessed for statistical significance using a Cox proportional hazards model, with prespecified covariates of age, sex, geographical region, diabetes status at baseline, left ventricular ejection fraction, and estimated glomerular filtration rate at baseline. For these analyses, the assumption of proportional hazards was investigated, and no violations were observed. For the analysis of total (first and repeated) events, between-group differences were assessed using a joint frailty model, with cardiovascular death (for end points including heart failure events) or all-cause mortality (for end points including all-cause hospitalization) as competing risks, and using covariates that were used for time-to-first-event analyses. For the analysis of changes in vital signs and laboratory measurements, treatment effects were assessed based on changes from baseline using a mixed model for repeated measures that included age and baseline estimated glomerular filtration rate as linear covariates and baseline score by visit, visit by treatment, sex, region, baseline left ventricular ejection fraction, individual last projected visit based on dates of randomization and trial closure, and baseline diabetes status as fixed effects. The analysis of changes in NT-proBNP was performed on log-transformed data. All P values reported are 2-sided, and P values less than 0.05 were considered statistically significant.
Results
A total of 3730 patients at 520 centers in 20 countries were randomly assigned to placebo (n=1867) or to empagliflozin (n=1863). As previously reported,7 the 2 groups comprised patients with mild, moderate, and severe heart failure, as reflected by left ventricular ejection fraction and circulating levels of NT-proBNP, and were well-balanced with respect to baseline characteristics.
Effect on Combined Risk of Death or Hospitalization
There were 512 patients who died for any reason or were hospitalized for heart failure in the placebo group and 407 such patients in the empagliflozin group, corresponding to annualized rates of 23.3% and 17.8%, respectively. These differences reflected a 24% lower risk as a result of treatment with empagliflozin as compared with placebo (hazard ratio [HR], 0.76; 95% CI, 0.67–0.87; P<0.0001; Table). There were 674 patients who died for any reason or were hospitalized for a cardiovascular reason in the placebo group and 556 such patients in the empagliflozin group, corresponding to annualized rates of 33.4% and 26.1%, respectively. These differences reflected a 22% lower risk as a result of treatment with empagliflozin as compared with placebo (HR, 0.78; 95% CI, 0.70–0.87; P<0.0001). There were 860 patients who died or were hospitalized for any reason in the placebo group and 743 such patients in the empagliflozin group, corresponding to annualized rates of 47.3% and 38.4%, respectively. These differences reflected a 19% lower risk as a result of treatment with empagliflozin as compared with placebo (HR, 0.81; 95% CI, 0.74–0.90; P<0.0001; Table; Figure I in the Data Supplement).
Table.
Major Outcomes and Inpatient and Outpatient Worsening Heart Failure Events
Effect of Empagliflozin on Hospitalizations
As compared with the placebo group, patients in the empagliflozin group had fewer total (first and recurrent) hospitalizations for heart failure (553 versus 388 [HR, 0.70; 95% CI, 0.58–0.85; P=0.0003]), fewer total (first and recurrent) hospitalizations for a cardiovascular reason (999 versus 819 [HR, 0.78; 95% CI, 0.67–0.91; P<0.0001]), and fewer total (first and recurrent) hospitalizations for any reason (1570 versus 1364 [HR, 0.85; 95% CI, 0.75–0.95; P=0.007]; Table). Adjudicated cardiovascular events not related to heart failure were uncommon (occurring in 3%) and similar in the 2 treatment groups (Table I in the Data Supplement).
The effect of empagliflozin on total hospitalizations for heart failure is further described in Table I in the Data Supplement. As compared with placebo, fewer patients in the empagliflozin group were hospitalized for heart failure once (160 versus 231), hospitalized for heart failure twice (50 versus 61), and hospitalized for heart failure ≥3 times (36 versus 50). The effect of empagliflozin on first and recurrent hospitalizations for heart failure was consistent in 12 prespecified subgroups, except for a nominally significant treatment-by-race interaction (Figure II in the Data Supplement).
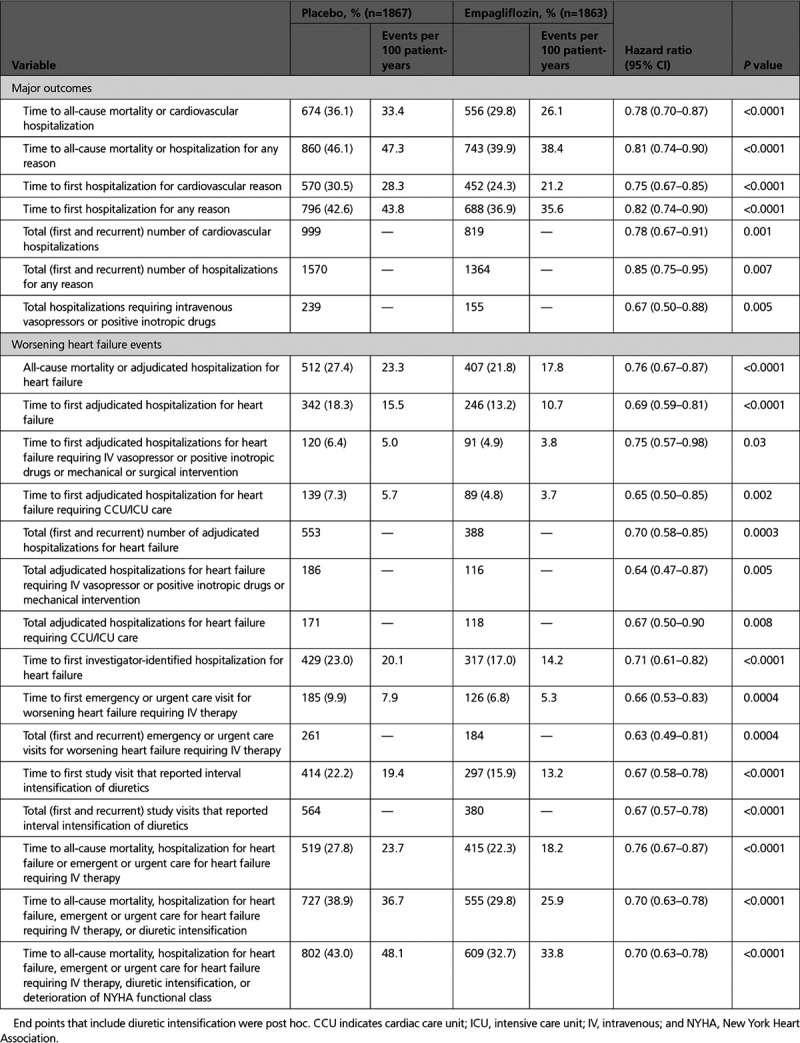
Empagliflozin also reduced the severity of heart failure admissions and the frequency of utilization of a broad range of interventions used for the management of decompensated heart failure. Empagliflozin decreased the time to the first heart failure hospitalization that required intensive care (HR, 0.65; 95% CI, 0.50–0.85; P=0.002) and also decreased the total number of such heart failure admissions (HR, 0.67; 95% CI, 0.50–0.90; P=0.008; Table; Figure 1). When compared with the placebo group, the empagliflozin group experienced fewer total hospitalizations for heart failure that required only oral or intravenous diuretics (219 versus 293), fewer hospitalizations for heart failure that required intravenous vasodilators but no vasopressor or positive inotropic agents or mechanical support (36 versus 54), fewer admissions for heart failure that required a vasopressor or positive inotropic agent but no mechanical support (92 versus 152), and fewer hospitalizations for heart failure that required mechanical or surgical intervention (24 versus 34; Table I in the Data Supplement).
Figure 1.
Total (first and recurrent) adjudicated heart failure hospitalizations requiring admission to cardiac care unit or intensive care unit in in the placebo and empagliflozin groups. Shown are mean cumulative function curves for placebo (shown in red) and for empagliflozin (shown in blue). HR indicates hazard ratio.
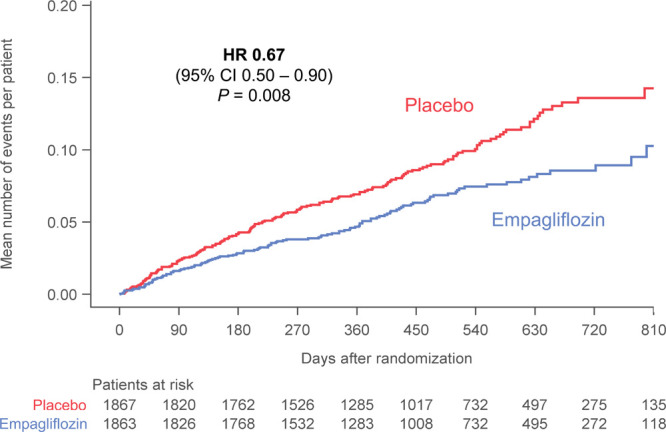
For hospitalizations that required a vasopressor or positive inotropic support or mechanical or surgical interventions, empagliflozin not only reduced the time-to-first-event (HR, 0.75; 95% CI, 0.57–0.98; P=0.03), but also decreased the risk of a first or recurrent event (HR, 0.64; 95% CI, 0.47–0.87; P=0.005; Table; Figure 2). When considering all hospitalizations for heart failure, the 2 groups were similar with respect to the median duration of each admission for heart failure (9.0 [interquartile range, 6–15] for placebo versus 8.0 days [interquartile range, 5–15] for empagliflozin).
Figure 2.
Total (first and recurrent) adjudicated hospitalization for heart failure requiring intravenous vasopressor or positive inotropic drug or mechanical or surgical intervention in the placebo and empagliflozin groups. Shown are mean cumulative function curves for placebo (shown in red) and for empagliflozin (shown in blue). HR indicates hazard ratio.
Effect of Empagliflozin on Worsening Heart Failure Events Other Than Hospitalizations
Patients in the empagliflozin group experienced fewer emergency department or urgent care visits for worsening heart failure (Table I in the Data Supplement). Empagliflozin reduced the time to a first emergent/urgent care for heart failure (HR, 0.66; 95% CI, 0.53–0.83; P=0.0004) and decreased the risk of total (first or recurrent) emergent/urgent care visits for heart failure (HR, 0.63; 95% CI, 0.49–0.831; P=0.0004); Table). Many of the emergent and urgent heart failure events were soon followed by admission to a hospital on the same day, but among those that represented stand-alone events, there were fewer events in the empagliflozin group than in the placebo group (43 and 56 events, respectively).
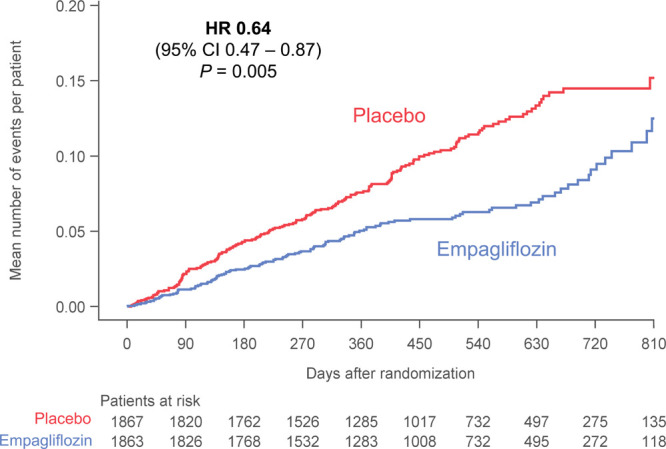
When a worsening heart failure event is defined as death, hospitalization for heart failure or an emergent or urgent heart failure visit requiring intravenous treatment, empagliflozin reduced the risk of a worsening heart failure event (HR, 0.76. 95% CI, 0.67–0.87; P<0.0001; Figure 3). The benefit of empagliflozin on this end point first reached statistical significance at 12 days after randomization, and statistical significance was sustained from day 34 onwards (Figure III in the Data Supplement).
Figure 3.
Time-to-first-event analysis of all-cause mortality, heart failure hospitalization, or emergent/urgent care visit for worsening heart failure in the placebo and empagliflozin groups. Shown are Kaplan-Meier curves for placebo (shown in red) and for empagliflozin (shown in blue). Only emergent and urgent care visits requiring intravenous therapy are included. HR indicates hazard ratio.
As compared with placebo, fewer patients in the empagliflozin group reported intensification of diuretics since the previous study visit (297 versus 414), and there were fewer total study visits that reported interval diuretic intensification in the empagliflozin group (380 versus 564) (Table; Table I in the Data Supplement). Conversely, the empagliflozin group had somewhat more patients (281 versus 246) and more study visits (334 versus 291) where the dose of diuretics was reported to have been reduced. Empagliflozin decreased the time to the first study visit that reported diuretic intensification (HR, 0.67; 95% CI, 0.58–0.78; P<0.0001) as well as the total number of study visits that reported intensification of diuretics since the previous visit (HR, 0.67; 95% CI, 0.57–0.78; P<0.0001; Table; Figure IV in the Data Supplement).
Additionally, at prespecified study visits, patients in the empagliflozin group had a 20% to 40% higher odds of experiencing an improvement in NYHA functional class and had a 20% to 40% lower odds of experience worsening of NYHA functional class. The effect of empagliflozin for shifts in NYHA class were apparent at every scheduled assessment throughout the first year of follow-up; the pattern of benefit was similar whether or not worst score imputations were used for patients who died or had missing data attributable to being lost to follow-up or withdrawal of consent (Figure 4). Statistical significance for both improvement and deterioration in NYHA class was apparent as early as 28 days after randomization and was sustained for 52 weeks.
Figure 4.
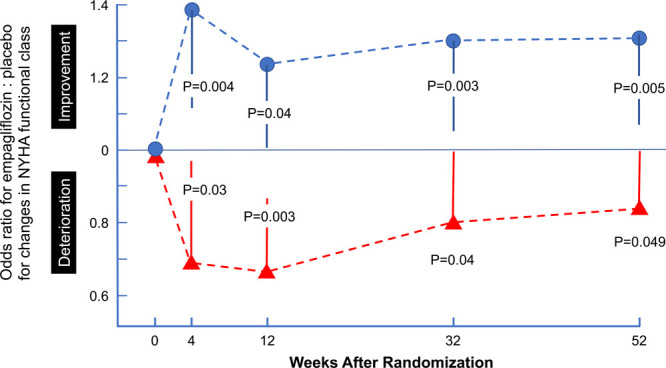
Odds of improvement or deterioration in NYHA functional class during first 52 weeks as a result of treatment with empagliflozin. Shown are the odds ratios for empagliflozin vs placebo for improvement in NYHA functional class (top, shown in blue) and for deterioration in NYHA functional class (bottom, shown in red). An odds ratio of 1.3 indicates 30% higher odds of improvement, whereas an odds ratio of 0.7 indicates 30% lower odds of deterioration. Patients who died, were lost to follow-up, or declined consent were assigned worst rank, but very similar results were seen when the analysis was repeated without these worst rank assignments. P values represent the significance of the differences between the 2 treatment groups. NYHA indicates New York Heart Association.
When a worsening heart failure event is defined as death, hospitalization for heart failure, an emergent or urgent heart failure visit requiring intravenous treatment, diuretic intensification or deterioration in NYHA functional class, a worsening heart failure event occurred in 802 patients in the placebo group, but in only 609 patients in the empagliflozin group, corresponding to annualized rates of 48.1% and 33.8%, respectively (HR, 0.70; 95% CI, 0.63–0.78; P<0.0001) (Table). The effect of empagliflozin on this composite of inpatient and outpatient worsening heart failure events first reached statistical significance at 12 days after randomization, and statistical significance was sustained from day 12 onwards.
Serial changes in laboratory tests and vital signs are shown in Figures V to IX in the Data Supplement. Empagliflozin produced significant increases in hematocrit and decreases in uric acid, which were apparent as early as 4 weeks after randomization and were maintained for the first 100 weeks. Patients treated with empagliflozin experienced an early and sustained decrease in body weight, which averaged <1.0 kg. Treatment with empagliflozin was accompanied by small decreases in NT-proBNP after 4 weeks, and the effect increased in magnitude over time (Figure VIII in the Data Supplement). In contrast, the effects of empagliflozin on blood pressure seen after 4 weeks (of approximately 1–2 mm Hg) waned during prolonged treatment (Figure IX in the Data Supplement).
Discussion
In the EMPEROR-Reduced trial, empagliflozin reduced the risk of cardiovascular death or hospitalization for heart failure, a benefit that was driven primarily by an effect of the drug to reduce first and recurrent admissions for worsening heart failure.7 Herein we show that this benefit on disease progression is not dependent on the definition of this end point or on the identification and adjudication of events. The effect of empagliflozin was similar whether the analysis focused on cardiovascular death or all-cause mortality, and the benefit of the drug was not offset by an increase in events unrelated to heart failure. Even when the analysis includes all deaths and all hospitalizations regardless of cause, empagliflozin’s effect on morbidity and mortality remained clinically meaningful and highly significant. As expected, the magnitude of the benefit on hospitalization declined when the focus of the analysis broadened beyond heart failure events, decreasing from 30% for total heart failure hospitalizations to 22% for total cardiovascular hospitalizations to 15% for total hospitalizations for any reason. This decline is expected whenever hospitalizations that are not favorably influenced by a treatment are progressively added to the analysis of events in a clinical setting where only half of the hospital admissions in patients with a meaningfully reduced ejection fraction are related to worsening heart failure.
The effect of empagliflozin to reduce hospitalizations for heart failure was apparent across a broad spectrum of event severity. The drug reduced admissions that were treated only with oral or intravenous diuretics as well as hospitalizations that required therapy with intravenous vasopressors or positive inotropic agents, and treatment also decreased the risk of hospitalizations for heart failure that required intensive care. The risk of the latter types of admission was reduced by 35% by treatment with the SGLT2 inhibitor. At the same time, empagliflozin also led to a 34% decrease in the risk of patients seeking emergent or urgent treatment for worsening heart failure that were treated with intravenous diuretics. A similar risk reduction in urgent outpatient worsening heart failure events was also seen with dapagliflozin in a large-scale trial in patients with heart failure and a reduced ejection fraction (DAPA-HF [Dapagliflozin on Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure]).23 Importantly, the lower risk of hospital admissions was not counterbalanced by a longer length of stay when patients in the empagliflozin group were hospitalized for heart failure.
In addition to these events requiring an acute intervention, treatment with empagliflozin had a meaningful effect on the risk of worsening heart failure events in the nonurgent outpatient setting. Patients in the empagliflozin group had a 20% to 40% higher odds of experiencing an improvement in NYHA functional class and had a 20% to 40% lower odds of suffering worsening of NYHA functional class. Importantly, these benefits were statistically significant at the first visit at which NYHA functional class was assessed (ie, after only 28 days after randomization). When we identified a worsening heart failure event in a manner similar to the primary end point of the DAPA-HF trial (eg, death, hospitalization for heart failure, or an emergent or urgent heart failure visit requiring intravenous treatment), a benefit of empagliflozin on this end point first reached statistical significance at 12 days after randomization.
The effect of empagliflozin to prevent a meaningful deterioration in functional capacity was paralleled by an effect of the drug to reduce the need for intensification of diuretic therapy, a benefit that was particularly apparent during prolonged treatment. Interestingly, the effect of empagliflozin to prevent intensification of diuretics was twice as great as the effect of the drug to promote decreases in the dose of diuretics. These observations are similar to those reported with dapagliflozin in heart failure and a reduced ejection fraction, where the SGLT2 inhibitor also reduced the need for intensification of diuretic therapy, but had minimal effects on the average dose of prescribed diuretics over the course of double-blind treatment.25 Importantly, when all outpatient and inpatient worsening events are considered together, the risk of a worsening heart failure event per 100 patient-years of follow-up was nearly 50, indicating that only half of our patients with chronic heart failure maintained clinical stability when followed for 1 year, even though our cohort comprised patients with primarily class II symptoms at the start of follow-up (Table).
We observed consistent effects of empagliflozin on a comprehensive series of measures of clinical stability in 12 predefined subgroups. Importantly, concomitant treatment with mineralocorticoid receptor antagonists and neprilysin inhibitors did not attenuate the response to SGLT2 inhibition. In our original publication and in a meta-analysis of the DAPA-HF and EMPEROR-Reduced trials, we noted a somewhat lesser benefit in patients with ejection fraction of 31% to 40% and in patients with class III symptoms.7,26 However, it should be noted that our patients with an ejection fraction of 31% to 40% were not representative of a clinical practice cohort, in that they could be enrolled in our trial only if they had markedly increased levels of natriuretic peptides, and moreover, patients with class III symptoms represented only a quarter of the patients in the trial. Therefore, it is reassuring that when we repeated our subgroup analyses in the current paper, with a focus on total heart failure hospitalizations, the treatment-by-subgroup interaction effects for both ejection fraction and NYHA functional class were no longer apparent.
The mechanisms by which empagliflozin exerts these broad-based effects to maintain clinical stability remain to be defined. Although the early benefits of empagliflozin to prevent clinical deterioration may suggest the possibility of a hemodynamic or natriuretic effect, the magnitude of such an action appears to be modest and is typically short-lived.27–29 Conventional diuretics typically produce immediate declines in natriuretic peptides as well as meaningful and durable changes in systolic blood pressure, generally without a change in hematocrit. In contrast, the early effect of empagliflozin on natriuretic peptides was very small in our trial, although the magnitude increased after months of therapy; in contrast, the initial decline in blood pressure waned considerably during the course of double-blind therapy. This time-dependent pattern suggests that changes in natriuretic peptides may have been the result of (rather than the determinant) of favorable long-term effects of SGLT2 inhibitors on cardiac structure and function. Similar modest changes in natriuretic peptides and systolic blood pressure were reported with dapagliflozin in patients with heart failure and a reduced ejection fraction in 2 trials.30,31 Finally, empagliflozin produced meaningful and sustained increases in hematocrit and decreases in uric acid in our study; both have been identified as important determinants of the heart failure benefits of these drugs in trials of patients with type 2 diabetes.32,33 Both erythrocytosis and uric acid lowering may represent biomarkers for the effect of SGLT2 inhibitors to promote energy deprivation signaling and reduce oxidative stress.34,35
Our findings should be considered in light of certain limitations. The median duration of follow-up was 16 months, which was shorter than many trials of neurohormonal antagonists in heart failure. Although the effects of SGLT2 inhibitors to reduce heart failure events beyond the trial duration has not been established, there was no indication that the effects of empagliflozin on worsening heart failure events waned during the course of the current trial. We prospectively assessed changes in the Kansas City Cardiomyopathy Questionnaire in our trial, but we did not include changes in this measure as a worsening heart failure event, because these are not typically recognized as such in clinical practice; furthermore, the effect of empagliflozin on this metric are being reported in a separate manuscript. Finally, outpatient events were defined by the investigator and were not reviewed or adjudicated. However, it is noteworthy that, in the current study, the process of adjudication in the current trial did not modify the size of the benefit of empagliflozin on heart failure hospitalizations (Table).
In conclusion, in patients with heart failure and a reduced ejection fraction, empagliflozin reduced the risk of inpatient and outpatient worsening heart failure events, benefits that were seen within 12 to 28 days of initiation of treatment and were sustained for the duration of double-blind therapy. The effect of SGLT2 inhibitors to maintain clinical stability is akin to that which has been reported with β-blockers, mineralocorticoid receptor antagonists, and angiotensin receptor neprilysin inhibitors, but are seen in patients already receiving treatment with these drugs.
Sources of Funding
This work was supported in part by Boehringer Ingelheim and Eli Lilly and Company.
Disclosures
Dr Packer reports consulting fees from Boehringer Ingelheim and Akcea received during the conduct of the study; and consulting fees from Abbvie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, all outside of the submitted work. Dr Anker reports grants and/or personal fees from Abbott Vascular, AstraZeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Respircardia, Servier, and Vifor Pharma; and personal fees from Boehringer Ingelheim received during the conduct of the study. Dr Butler reports research support from the National Institutes of Health, Patient Centered Outcomes Research and the European Union, and consulting fees from Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, LinaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, V-Wave Limited, and Vifor. Dr Fillipatos reports consulting fees from Boehringer Ingelheim during the study and consulting/lecture fees from Novartis, Vifor, Servier, Medtronic and Merck. Dr Ferreira is a consultant for Boehringer Ingelheim. Dr Pocock is a consultant for Boehringer Ingelheim, and reports personal fees received from Boehringer Ingelheim during the conduct of the study. Dr Carson reports consulting fees from Boehringer Ingelheim and IQVIA related to work on the Clinical Events Committee during the conduct of the study. Dr Anand reports personal/consulting fees from ARCA, Amgen, Boston Scientific Corporation, Novartis, LivaNova, and Zensun. Dr Doehner reports personal fees from Aimediq, Bayer, Boehringer Ingelheim, Medtronic, Pfizer, Sanofi-Aventis, Sphingotec, and Vifor Pharma; and research support from the European Union (Horizon2020), German Ministry of Education and Research, German Center for Cardiovascular Research, Vifor Pharma, and ZS Pharma. Dr Haass reports consulting fees from Boehringer Ingelheim related to work on the Clinical Events Committee during the conduct of the study. Dr Komajda reports consulting fees from Boehringer Ingelheim related to work on the Clinical Events Committee during the conduct of the study; and personal fees from Novartis, Servier, Amgen, Sanofi, Bayer, AstraZeneca, Lilly, and Torrent. Dr Miller reports consulting fees from Abbott, Boehringer Ingelheim, Respicardia, CVRx, Pfizer, and Abbvie. Dr Pehrson reports consulting fees and/or lecture fees from Boehringer Ingelheim, Glaxo Smith Kline, Celgene, Bristol Myers Squibb, Bayer, Johnson & Johnson. Dr Teerlink reports grants and/or consulting fees from Abbott, Amgen, Astra-Zeneca, Bayer, Boehringer-Ingelheim, Cytokinetics, Daxor, EBR Systems, LivaNova, Medtronic, Merck, Novartis, Relypsa, Servier, Windtree Therapeutics, and ZS Pharma. Drs Brueckmann and Jamal, C. Zeller, and S. Schnaidt are employees of Boehringer Ingelheim. Dr Zannad has received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius; and personal fees from Boehringer Ingelheim during the conduct of the study.
Supplemental Materials
Data Supplement Figures I–IX
Data Supplement Table I
Supplementary Material
Footnotes
Sources of Funding, see page 335
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.051783.
Contributor Information
Stefan D. Anker, Email: s.anker@cachexia.de.
Javed Butler, Email: jbutler4@umc.edu.
Gerasimos Filippatos, Email: gfilippatos@gmail.com.
João Pedro Ferreira, Email: jp7ferreira@hotmail.com.
Stuart J. Pocock, Email: stuart.pocock@lshtm.ac.uk.
Peter Carson, Email: peter.carson@va.gov.
Inder Anand, Email: inder.anand001@gmail.com.
Wolfram Doehner, Email: wolfram.doehner@charite.de.
Markus Haass, Email: m.haass@theresienkrankenhaus.de.
Michel Komajda, Email: komajda.michel@orange.fr.
Alan Miller, Email: alan.miller@jax.ufl.edu.
Steen Pehrson, Email: steen.pehrson@REGIONH.DK.
John R. Teerlink, Email: john.teerlink@ucsf.edu.
Martina Brueckmann, Email: martina.brueckmann@boehringer-ingelheim.com.
Waheed Jamal, Email: waheed.jamal@boehringer-ingelheim.com.
Cordula Zeller, Email: cordula.zeller@boehringer-ingelheim.com.
Sven Schnaidt, Email: sven_yannik.schnaidt@boehringer-ingelheim.com.
Faiez Zannad, Email: f.zannad@chru-nancy.fr.
References
- 1.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016; 37:1526–1534. doi: 10.1093/eurheartj/ehv728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018; 138:458–468. doi: 10.1161/CIRCULATIONAHA.118.034222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019; 139:2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130 [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. ; VERTIS CV Investigators. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383:1425–1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 7.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1414–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 8.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 9.Rosano GMC. Clinical trial design, endpoints, and regulatory requirements. Handb Exp Pharmacol. 2017; 243:67–78. doi: 10.1007/164_2016_75 [DOI] [PubMed] [Google Scholar]
- 10.Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014; 7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI). 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018; 137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 12.Tyl B, Lopez Sendon J, Borer JS, Lopez De Sa E, Lerebours G, Varin C, De Montigny A, Pannaux M, Komajda M. Comparison of outcome adjudication by investigators and by a central end point committee in heart failure trials: experience of the SHIFT heart failure study. Circ Heart Fail. 2020; 13:e006720 doi: 10.1161/CIRCHEARTFAILURE.119.006720 [DOI] [PubMed] [Google Scholar]
- 13.Carson P, Fiuzat M, O’Connor C, Anand I, Plehn J, Lindenfeld JA, Silver M, White M, Miller A, Davis G, et al. Determination of hospitalization type by investigator case report form or adjudication committee in a large heart failure clinical trial (β-Blocker Evaluation of Survival Trial [BEST]). Am Heart J. 2010; 160:649–654. doi: 10.1016/j.ahj.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001; 345:1667–1675. doi: 10.1056/NEJMoa010713 [DOI] [PubMed] [Google Scholar]
- 15.Cleland JG. How to assess new treatments for the management of heart failure: composite scoring systems to assess the patients’ clinical journey. Eur J Heart Fail. 2002; 4:243–247. doi: 10.1016/s1388-9842(02)00039-9 [DOI] [PubMed] [Google Scholar]
- 16.Cleland JG, Charlesworth A, Lubsen J, Swedberg K, Remme WJ, Erhardt L, Di Lenarda A, Komajda M, Metra M, Torp-Pedersen C, Poole-Wilson PA; COMET Investigators. A comparison of the effects of carvedilol and metoprolol on well-being, morbidity, and mortality (the “patient journey”) in patients with heart failure: a report from the Carvedilol Or Metoprolol European Trial (COMET). J Am Coll Cardiol. 2006; 47:1603–1611. doi: 10.1016/j.jacc.2005.11.069 [DOI] [PubMed] [Google Scholar]
- 17.Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018; 3:252–259. doi: 10.1001/jamacardio.2017.5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallick A, Gandhi PU, Gaggin HK, Ibrahim N, Januzzi JL. The importance of worsening heart failure in ambulatory patients: definition, characteristics, and effects of amino-terminal pro-B-type natriuretic peptide guided therapy. J Am Coll Cardiol. Heart Fail. 2016; 4:749–755. doi: 10.1016/j.jchf.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, et al. ; PARADIGM-HF Investigators and Committees*. Importance of clinical worsening of heart failure treated in the cutpatient setting: evidence from the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF). Circulation. 2016; 133:2254–2262. doi: 10.1161/CIRCULATIONAHA.115.020729 [DOI] [PubMed] [Google Scholar]
- 20.Skali H, Dwyer EM, Goldstein R, Haigney M, Krone R, Kukin M, Lichstein E, McNitt S, Moss AJ, Pfeffer MA, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur J Heart Fail. 2014; 16:560–565. doi: 10.1002/ejhf.71 [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, et al. ; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002; 106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf [DOI] [PubMed] [Google Scholar]
- 22.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. ; PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015; 131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748 [DOI] [PubMed] [Google Scholar]
- 23.Docherty KF, Jhund PS, Anand I, Bengtsson O, Böhm M, de Boer RA, DeMets DL, Desai AS, Drozdz J, Howlett J, et al. Effect of Dapagliflozin on Outpatient Worsening of Patients With Heart Failure and Reduced Ejection Fraction: a prespecified analysis of DAPA-HF. Circulation. 2020; 142:1623–1632. doi: 10.1161/CIRCULATIONAHA.120.047480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, et al. ; EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019; 21:1270–1278. doi: 10.1002/ejhf.1536 [DOI] [PubMed] [Google Scholar]
- 25.Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA, Böhm M, Boulton DW, Chopra VK, DeMets DL, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation. 2020; 142:1040–1054. doi: 10.1161/CIRCULATIONAHA.120.047077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020; 396:819–829. doi: 10.1016/S0140-6736(20)31824-9 [DOI] [PubMed] [Google Scholar]
- 27.Yasui A, Lee G, Hirase T, Kaneko T, Kaspers S, von Eynatten M, Okamura T. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018; 9:863–871. doi: 10.1007/s13300-018-0385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Mörschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014; 16:1087–1095. doi: 10.1111/dom.12322 [DOI] [PubMed] [Google Scholar]
- 29.Ohara K, Masuda T, Murakami T, Imai T, Yoshizawa H, Nakagawa S, Okada M, Miki A, Myoga A, Sugase T, Sekiguchi C, Miyazawa Y, Maeshima A, Akimoto T, Saito O, Muto S, Nagata D. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: A comparison study with furosemide and tolvaptan. Nephrology (Carlton). 2019; 24:904–911. doi: 10.1111/nep.13552 [DOI] [PubMed] [Google Scholar]
- 30.Inzucchi SE, McGuire D, Pitt B, Scirica BM, Austin B, Drazner M, Fong M, Givertz MM, Gordon R, Jermyn R, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019; ;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929 [DOI] [PubMed] [Google Scholar]
- 31.Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, DeMets DL, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J. 2020; 41:3402–3418. doi: 10.1093/eurheartj/ehaa496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018; 41:356–363. doi: 10.2337/dc17-1096 [DOI] [PubMed] [Google Scholar]
- 33.Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. J Am Coll Cardiol. Heart Fail. 2020; 8:57–66. doi: 10.1016/j.jchf.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 34.Packer M. Critical examination of mechanisms underlying the reduction in heart failure events with SGLT2 inhibitors: identification of a molecular link between their actions to stimulate erythrocytosis and to alleviate cellular stress. Cardiovasc Res. 2020cvaa064 doi: 10.1093/cvr/cvaa064 [DOI] [PubMed] [Google Scholar]
- 35.Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J Card Fail. 2020; 26:977–984. doi: 10.1016/j.cardfail.2020.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


