Supplemental Digital Content is available in the text.
Keywords: empagliflozin; glomerular filtration rate; heart failure; renal insufficiency, chronic
Background:
In EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), empagliflozin reduced cardiovascular death or heart failure (HF) hospitalization and total HF hospitalizations, and slowed the progressive decline in kidney function in patients with HF and a reduced ejection fraction, with and without diabetes. We aim to study the effect of empagliflozin on cardiovascular and kidney outcomes across the spectrum of kidney function.
Methods:
In this prespecified analysis, patients were categorized by the presence or absence of chronic kidney disease (CKD) at baseline (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73 m2 or albumin-to-creatine ratio >300 mg/g). The primary and key secondary outcomes were: (1) a composite of cardiovascular death or HF hospitalization (primary outcome); (2) total HF hospitalizations; and (3) eGFR slope. The direct impact on kidney events was investigated by a prespecified composite kidney outcome (defined as a sustained profound decline in eGFR, chronic dialysis, or transplant). The median follow-up was 16 months.
Results:
Of 3730 patients who were randomized to empagliflozin or placebo, 1978 (53%) had CKD. Empagliflozin reduced the primary outcome and total HF hospitalizations in patients with and without CKD: hazard ratio (HR)=0.78 (95% CI, 0.65–0.93) and HR=0.72 (95% CI, 0.58–0.90), respectively (interaction P=0.63). Empagliflozin slowed the slope of eGFR decline by 1.11 (0.23–1.98) ml/min/1.73 m2/yr in patients with CKD and by 2.41 (1.49–3.32) ml/min/1.73 m2/yr in patients without CKD. The risk of the composite kidney outcome was reduced similarly in patients with and without CKD: HR=0.53 (95% CI, 0.31–0.91) and HR=0.46 (95% CI, 0.22–0.99), respectively. The effect of empagliflozin on the primary composite outcome and key secondary outcomes was consistent across a broad range of baseline kidney function, measured by clinically relevant eGFR subgroups or by albuminuria, including patients with eGFR as low as 20 ml/min/1.73 m2. Empagliflozin was well tolerated in CKD patients.
Conclusions:
In EMPEROR-Reduced, empagliflozin had a beneficial effect on the key efficacy outcomes and slowed the rate of kidney function decline in patients with and without CKD, and regardless of the severity of kidney impairment at baseline.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977.
Clinical Perspective.
What Is New?
The effect of empagliflozin in patients with heart failure with reduced ejection fraction with or without chronic kidney disease has not been previously reported.
The authors studied the effect of empagliflozin on cardiovascular and kidney outcomes across the entire spectrum of kidney function.
What Are the Clinical Implications?
Empagliflozin reduced the primary outcome of death from cardiovascular causes or hospitalization for heart failure, first and repeated heart failure hospitalizations, and serious kidney outcomes in patients with and without chronic kidney disease.
This study supports the use of empagliflozin for improving the outcomes of patients with heart failure with reduced ejection fraction and chronic kidney disease, even in those with an estimated glomerular filtration rate as low as 20 mL/(min·1.73 m2).
Editorial, see p 322
The incidence and prevalence of both heart failure (HF) and kidney disease are increasing.1 Patients with HF with reduced ejection fraction have a poor quality of life and a high risk of adverse cardiovascular events and death, particularly in those with kidney impairment.2,3 Additionally, patients with chronic kidney disease (CKD) commonly develop heart failure, and the most common cause of demise is cardiovascular death.
For nearly 2 decades, both angiotensin converting enzyme inhibitors and angiotensin receptor blockers have been used for the treatment of both HF and reduced ejection fraction, as well as for the management of albuminuric CKD.4–7 In both conditions, initiation of treatment with these drugs is commonly accompanied by mild transient worsening of kidney function attributable to their hemodynamic effects on the intraglomerular pressure in the kidneys. This early azotemia frequently leads clinicians to withhold or fail to up-titrate these drugs to achieve the target doses in HF with reduced ejection fraction, and may therefore deprive patients of the clinical benefit associated with their optimal use.8,9 More recently, sacubitril/valsartan was shown to slow the decline in estimated glomerular filtration rate (eGFR) in patients with both a reduced and preserved ejection fraction.10,11
Sodium glucose contransporter-2 (SGLT2) inhibitors have been shown to reduce eGFR decline, progression of kidney disease, or kidney failure (including renal death, the need for dialysis, or kidney transplantation), as well as the risk of HF hospitalizations and cardiovascular death in patients with type 2 diabetes.12–17 In patients with type 2 diabetes and albuminuric CKD enrolled in the CREDENCE trial (Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy), canagliflozin reduced the risk of kidney failure and serious cardiovascular events compared with placebo.17 Most recently, in the DAPA-CKD trial (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in patients with Chronic Kidney Disease), which enrolled patients with albuminuric kidney disease with or without diabetes, dapagliflozin reduced the rate of serious kidney outcomes, hospitalizations for HF, and mortality.18
In EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction), empagliflozin reduced the composite of cardiovascular death or HF hospitalization and first and recurrent hospitalizations for HF, slowed the progressive decline in kidney function, and reduced the risk of serious kidney events in patients with and without diabetes.19 Importantly, in EMPEROR-Reduced, patients were eligible for enrollment with an eGFR as low as 20 ml/min/1.73 m2 and more than half of the patients had prevalent CKD at baseline. Thus, the trial afforded the opportunity of investigating the efficacy and safety of empagliflozin on cardiac and kidney outcomes according to CKD and across a wide range of kidney function; these findings were a prespecified analysis of EMPEROR-Reduced.
Methods
Study Rationale, End Points, and Randomization Procedures
The rationale and design of EMPEROR-Reduced have been previously published.20 In short, EMPEROR-Reduced is a double-blind, placebo-controlled, parallel-group, event-driven randomized trial that randomly assigned 3730 patients with class II, III, or IV HF and an ejection fraction of ≤40% to receive either empagliflozin (10 mg once daily) or placebo, in addition to recommended heart failure therapy.
The primary outcome and the first 2 secondary outcomes were evaluated using a hierarchical testing procedure. The primary outcome was a composite of adjudicated cardiovascular death or hospitalization for heart failure, analyzed as the time to the first event. The first secondary outcome was the occurrence of all adjudicated hospitalizations for heart failure, including first and recurrent events. The second secondary outcome was the rate of the decline in eGFR during double-blind treatment. A composite kidney outcome (see Kidney Endpoint Definition section), mortality from cardiovascular causes and all-cause death were also prespecified outcomes, but were not part of the testing hierarchy.
Randomization was stratified according to geographical region (North America, Latin America, Europe, Asia, or other), diabetes status at screening, and eGFR at screening (<60 or ≥60 ml/min/1.73 m2), according to the Chronic Kidney Disease Epidemiology Collaboration equation. The median follow-up duration was 16 months.
Ethics approval was obtained at each study site, and all patients provided informed consent to participate in the study; the registration identifier at ClinicalTrials.gov is NCT03057977 (URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977).
Baseline Categorization of Kidney Impairment
For this prespecified subgroup analyses, patients were categorized by the presence or absence of CKD at baseline. We defined prevalent CKD by the presence of an eGFR <60 ml/min/1.73 m2 or urine albumin-to-creatine ratio (UACR) >300 mg/g, and no CKD was defined by an eGFR ≥60 ml/min/1.73 m2 and UACR ≤300 mg/g. We evaluated the influence in clinically relevant categories of: (1) presence or absence of CKD; (2) 5 baseline eGFR categories (<30, 30–44, 45–59, 60–89, and ≥90 ml/min/1.73 m2); and (3) 3 baseline UACR categories (<30, 30–300, and >300 mg/g) on all 3 of the hierarchically-ranked outcomes and on several cardio-kidney outcomes.
Kidney End Point Definition
The composite kidney outcome was defined as time to first occurrence of chronic dialysis, kidney transplant, sustained reduction of ≥40% eGFR, or sustained eGFR <15 ml/min/1.73 m2 if eGFR was >30 ml/min/1.73 m2 or <10 ml/min/1.73 m2 for patients with baseline eGFR ≤30 ml/min/1.73 m2.
Anemia
The analysis of anemia included all patients with available values at baseline and at least 1 on-treatment measurement. Anemia was defined by the lower limit of normal given by the central laboratory depended on sex and age.
Statistical Analyses
Patient characteristics and adverse events were analyzed descriptively. Time-to-first-event analyses were performed with a Cox proportional hazards model, and adjusted for age, sex, region, diabetes status, and ejection fraction at baseline. These analyses were performed according to the intention-to-treat principle for all randomized patients, and included data up to the end of the planned treatment period. Total (first and recurrent) hospitalizations for HF were evaluated accordingly with a joint frailty model that accounted for cardiovascular death. As prespecified, the slope of eGFR decline was analyzed based on on-treatment data (baseline and other off-treatment data were not included in the model) by a random coefficient model allowing for random intercept and random slope per patient. Continuous end points were analyzed in a mixed model with repeated measures. An analysis of covariance was used to compare eGFR baseline and eGFR 30 days after treatment discontinuation in the 2 treatment groups. The analysis of covariance, joint frailty, slope, and mixed model with repeated measures models all included the same covariates as the Cox model. To assess the consistency of effects across subgroups, subgroup-by-treatment interaction terms were added to the models. Adverse event analyses were based on patients with events occurring during the on-treatment period. SAS Version 9.4 was used for all analyses.
Data Sharing Statement
Data will be made available on request in adherence with transparency conventions in medical research and through requests to the corresponding author. The executive committee of EMPEROR-Reduced has developed a comprehensive analysis plan and numerous prespecified analyses, which will be presented in future scientific meetings and publications. At a later time point, the full database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer-ingelheim.com/transparency_policy.html).
Results
Patient Characteristics
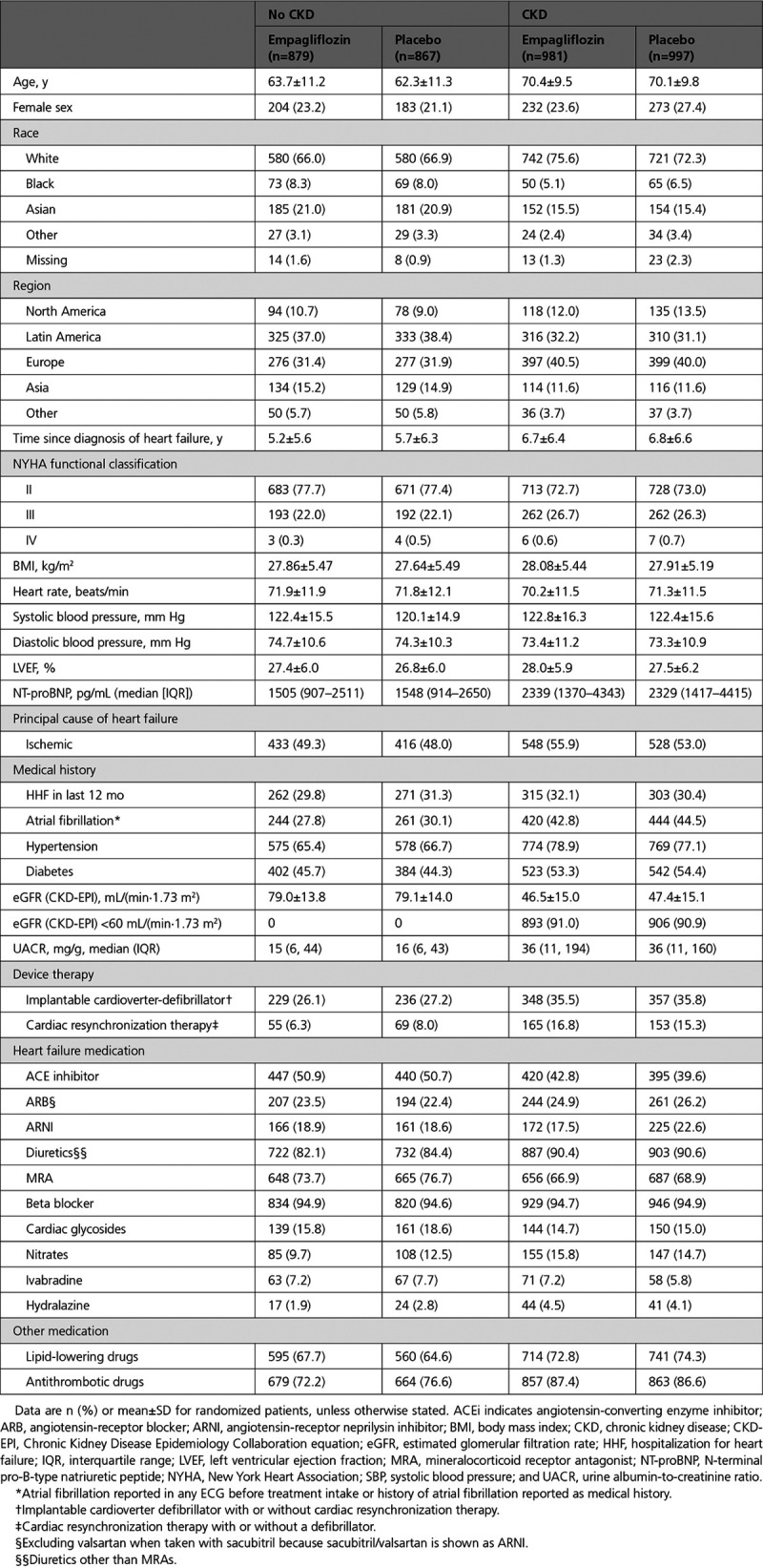
Among the 3730 patients included in the trial, 1978 (53%) had prevalent CKD at baseline. Compared with patients without CKD, those with CKD were older (70 versus 63 years), had a longer duration of HF (mean time since diagnosis, 6.8 versus 5.5 years), were more likely to have diabetes (54% versus 45%), and were less likely to be treated with angiotensin converting enzyme inhibitors or angiotensin receptor blockers (67% versus 73%). Reflective of the subgroup definition for prevalent CKD, eGFR was much lower for patients with CKD (mean, 47 versus 79 ml/min/1.73 m2), and the UACR was much higher (median, 36 versus 15 mg/g). The baseline characteristics in the placebo and empagliflozin groups were well balanced in patients with and without CKD (Table 1). The patient characteristics across the 5 eGFR categories follow the same pattern as previously described and are presented in Table I of the Data Supplement. The use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers and mineralocorticoid receptor antagonists less frequent in patients with lower eGFR at baseline. Patient characteristics across the 3 UACR categories are presented in Table II of the Data Supplement.
Table 1.
Baseline Characteristics of Patients by CKD Status at Baseline
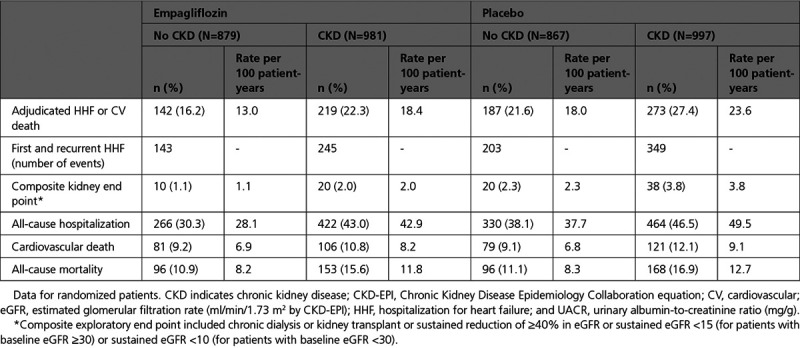
Event Rates of Patients With and Without Kidney Impairment
Patients with prevalent CKD had a higher rate of the primary composite outcome, first and recurrent hospitalizations for HF, composite kidney outcome, all-cause hospitalizations, and cardiovascular and all-cause death (Table 2).
Table 2.
Event rates by CKD Status
Primary and Key Secondary End Points
Empagliflozin reduced the primary outcome of time-to-first–cardiovascular death or HF hospitalization in patients with and without prevalent CKD (HR, 0.78; 95% CI, 0.65–0.93 [with]; HR, 0.72; 95% CI, 0.58–0.90 [without]; interaction P=0.63). Empagliflozin also reduced the key secondary endpoint of first and recurrent HF hospitalizations in patients with and without prevalent CKD (interaction P=0.78) (Figure 1).
Figure 1.
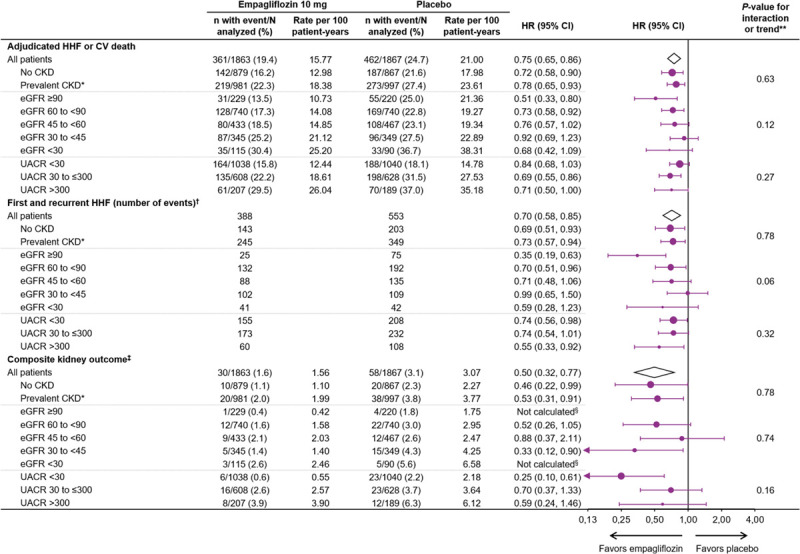
Clinical outcomes in patients by CKD status, eGFR and UACR categories at baseline. Data for randomized patients; HR and 95% CI from Cox proportional hazards model unless otherwise noted. CKD-EPI indicates Chronic Kidney Disease Epidemiology Collaboration equation; CV, cardiovascular; eGFR, estimated glomerular filtration rate (CKD-EPI; ml/min/1.73 m2); HHF, hospitalization for heart failure; HR, hazard ratio; and UACR, urinary albumin-to-creatinine ratio (mg/g). *Prevalent CKD defined as eGFR (CKD-EPI) <60 ml/min/1.73 m2 or UACR >300 mg/g. †Evaluated using a joint frailty model together with CV death. ‡Composite exploratory endpoint included chronic dialysis or kidney transplant or sustained reduction of ≥40% in eGFR or sustained eGFR (CKD-EPI) <15 ml/min/1.73 m2 (for patients with baseline eGFR ≥30) or sustained eGFR <10 (for patients with baseline eGFR <30). §Not calculated as less than 14 events in this subgroup. **P value for interaction shown for CKD subgroups; P value for trend test shown for eGFR and UACR subgroups.
Empagliflozin reduced the slope of decline in eGFR by 1.11 (0.23–1.98) ml/min/1.73 m2 per year in patients with CKD and by 2.41 (1.49–3.32) ml/min/1.73 m2 per year in patients without CKD (interaction P=0.045) (Table 3). The change of eGFR over time for patients with and without CKD is shown in Figure 2. An early eGFR decrease (“dip”) was observed in the empagliflozin-treated group. At week 4, the placebo-corrected eGFR dip between the treatment groups was similar (2.4 ml/min/1.73 m2 for patients with CKD and 2.7 ml/min/1.73 m2 without CKD; interaction P=0.59). Thereafter, stabilization and recovery toward baseline was observed in the empagliflozin group, whereas eGFR declined progressively in the placebo group.
Table 3.
eGFR Slope Analyses by CKD Status, eGFR, and UACR Categories at Baseline
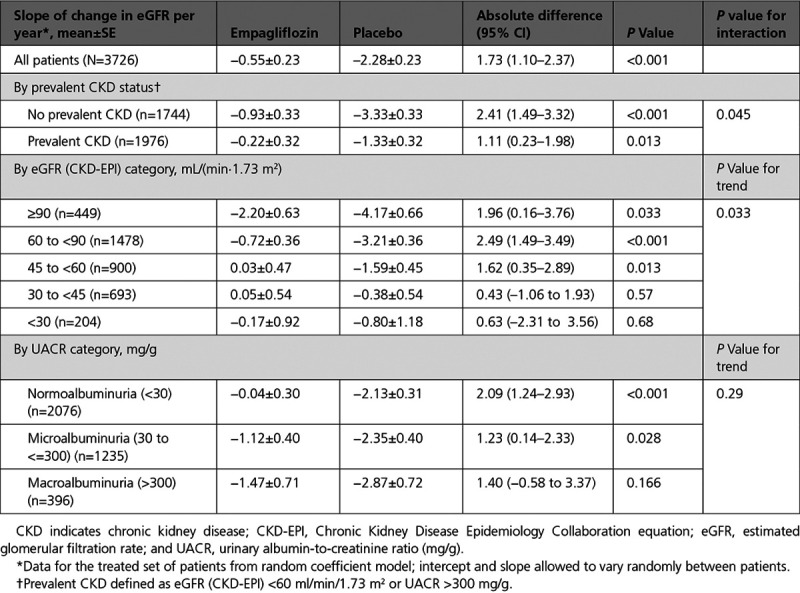
Figure 2.
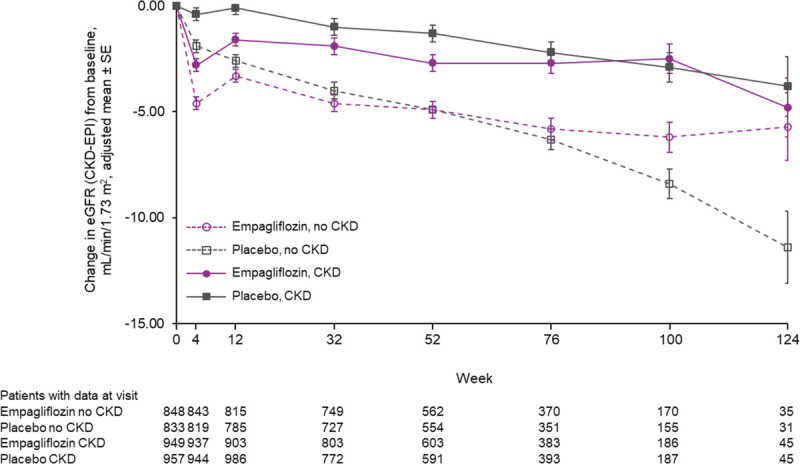
eGFR over time by CKD status at baseline. Data for treated patients from a mixed model for repeated measures based on on-treatment data. Prevalent CKD defined as eGFR (CKD-EPI) <60 ml/min/1.73 m2 or UACR >300 mg/g. CKD indicates chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; eGFR, estimated glomerular filtration rate; and UACR, urinary albumin-to-creatinine ratio.
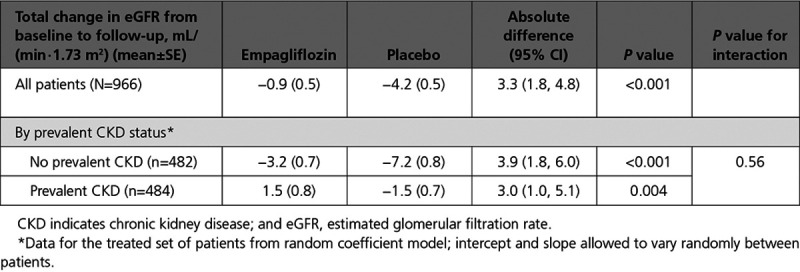
In a subset of 966 patients (484 with and 482 without CKD) that had measurements of eGFR at baseline and at 23 to 45 days after the discontinuation of treatment, empagliflozin reduced the decline in eGFR during the course of the trial by 3.0 (95% CI, 1.0–5.1) ml/min/1.73 m2 in patients with CKD and by 3.9 (95% CI, 1.8–6.0) ml/min/1.73 m2 in patients without CKD (interaction P=0.56) (Table 4).
Table 4.
Change of eGFR from Baseline to 30 Days After Treatment Discontinuation by CKD Status at Baseline
The effect of empagliflozin on the primary composite outcome and the key secondary outcomes across the 5 eGFR categories, including patients with an eGFR between 20 and 30 ml/min/1.73 m2, and 3 UACR categories is shown in Figure 1 and Table 3. Baseline kidney function did not influence the benefits of empagliflozin on the primary endpoint or on total hospitalizations for HF, but baseline eGFR had a modest effect on the magnitude of the absolute treatment difference on the eGFR slope (interaction P=0.033).
Other Prespecified Outcomes
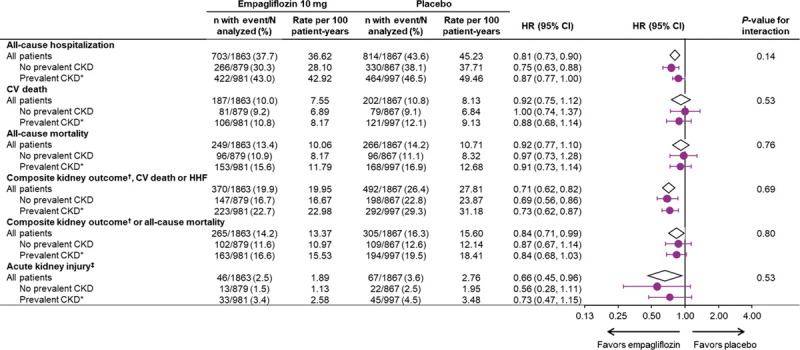
The exploratory composite kidney outcome of sustained profound decline in eGFR, chronic dialysis, or kidney transplant was reduced similarly in patients with and without CKD (HR, 0.53; 95% CI, 0.31–0.91 in patients with CKD; HR, 0.46; 95% CI, 0.22–0.99 in patients without CKD; interaction P=0.78). The absolute differences of event rates between placebo and empagliflozin were 1.17 per 100 patient-years for patients without CKD and 1.78 per 100 patient-years for patients with CKD (Figure 1).
When baseline eGFR was analyzed by 5 subgroups, kidney function did not influence the magnitude of the effect of the drug on the risk of the composite kidney outcome (interaction P=0.74). Investigator reported acute kidney injury was less frequent in the empagliflozin group, both in patients with CKD and without CKD (HR, 0.73; 95% CI, 0.47–1.15 [with]; HR, 0.56; 95% CI, 0.28–1.11 [without]; interaction P=0.53). Furthermore, baseline kidney function did not influence the effect of empagliflozin on all-cause hospitalizations or cardiovascular or all-cause mortality (Figure 3).
Figure 3.
Additional clinical outcomes by CKD status at baseline. Data for randomized patients; HR and 95% CI from Cox proportional hazards model. CKD indicates chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HHF, hospitalization for heart failure; HR, hazard ratio; MedDRA, Medical Dictionary for Regulatory Activities; and UACR, urinary albumin-to-creatinine ratio. *Prevalent CKD defined as eGFR (CKD-EPI) <60 ml/min/1.73 m2 or UACR >300 mg/g. †Composite exploratory endpoint included chronic dialysis or kidney transplant or sustained reduction of ≥40% in eGFR or sustained eGFR (CKD-EPI) <15 ml/min/1.73 m2 (for patients with baseline eGFR ≥30) or sustained eGFR <10 (for patients with baseline eGFR <30). ‡Acute kidney injury based on MedDRA preferred term based on investigator-reported adverse events.
Empagliflozin reduced the risk of the composite outcome of the composite kidney endpoint or all-cause mortality in patients with CKD (HR, 0.84; 95% CI, 0.68–1.03) and without CKD (HR, 0.87; 95% CI, 0.67–1.14) (interaction P=0.80) (Figure 3).
Empagliflozin increased hemoglobin levels versus placebo, both in patients with and without prevalent CKD. Adjusted mean increases from baseline at week 52 were 0.67 g/dL (95% CI, 0.55–0.80) in patients with CKD and 0.75 g/dL (95% CI, 0.62–0.88) in patients without CKD (interaction P=0.42). Of the 1905 patients with prevalent CKD, 535 (28%) had anemia at baseline. Empagliflozin reduced the proportion of patients with anemia; anemia persisted to last value on treatment in 71% (184 of 259) patients in the placebo group and in 50% (138 of 276) of patients in the empagliflozin group.
Adverse Events
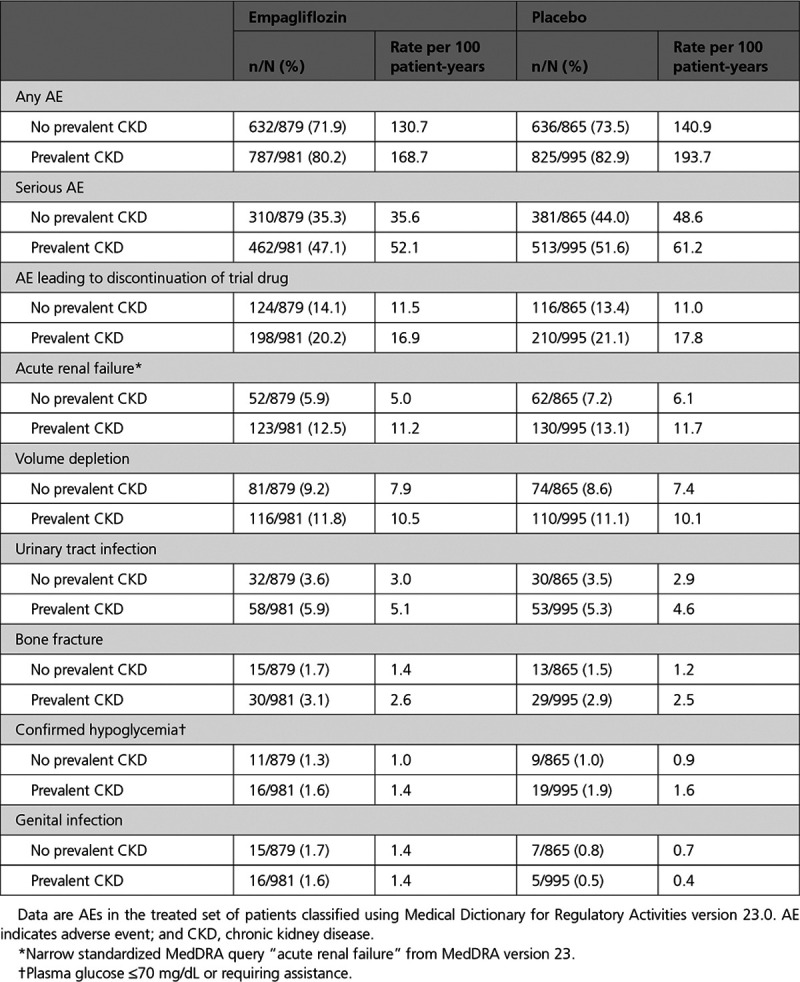
Adverse events (AEs) by baseline CKD status and across the 5 eGFR categories were generally consistent with the AE profile in the overall population. As expected, the incidence of AE rates (including serious AEs and AEs leading to discontinuation of trial drug) was higher in patients with prevalent CKD at baseline and in patients with lower eGFR ranges. Generally, empagliflozin and placebo groups showed similar frequencies of the listed AEs across these subgroups (Table 5).
Table 5.
Adverse Events by Presence and Absence of Prevalent CKD at Baseline
Discussion
EMPEROR-Reduced enrolled a substantial proportion of patients with prevalent CKD and allowed patients to be included with an eGFR as low as 20 ml/min/1.73 m2. The current study demonstrates the favorable effects of empagliflozin on the primary efficacy outcome of time-to-first–cardiovascular death or HF hospitalization and the key secondary end points of total HF hospitalizations and eGFR slope, as well as a reduction in serious kidney outcomes in patients with and without CKD and across the spectrum of kidney function, irrespective of degree of kidney injury measured by eGFR or albuminuria. Empagliflozin was well-tolerated in patients with severely impaired kidney function.
In patients with diabetes and increased cardiovascular risk, SGLT2 inhibitors have reduced the risk of cardiovascular death and hospitalizations for HF, including in patients with baseline CKD.21,22 The findings of EMPEROR-Reduced along with those of DAPA-HF (Dapagliflozin and Adverse Events in Chronic Heart Failure and a Reduced Ejection Fraction) extend these findings to patients with HF and a reduced ejection fraction, with or without diabetes.16,19,23 Patients in DAPA-HF had a higher baseline eGFR than those in EMPEROR-Reduced (66 versus 62 ml/min/1.73 m2); the proportion of patients with CKD at baseline was higher in EMPEROR-Reduced. In DAPA-HF, dapagliflozin reduced the primary outcome of cardiovascular death or worsening HF leading to hospitalization or an urgent care visit similarly in patients with or without CKD. These findings are highly concordant with the findings of EMPEROR-Reduced reported herein.
In addition to their benefits on heart failure hospitalizations, SGLT2 inhibitors also slow the progression of chronic kidney disease, as reflected by a decrease in the rate of decline in eGFR over time and a reduction in the risk of end-stage kidney disease. In EMPEROR-Reduced, empagliflozin reduced the risk of the composite kidney endpoint by 50%, and this risk reduction was statistically significant, both in patients with and without CKD. Of note, the effect of dapagliflozin on a composite of serious kidney events was not statistically significant (HR 0.71; 95% CI, 0.44–1.16), but this estimate was based on comparatively few events. It seems highly unlikely that dapagliflozin does not have a kidney benefit similar to empagliflozin, since dapagliflozin reduced the risk of end-stage kidney disease in the DAPA-CKD trial, which enrolled patients with albuminuric CKD who did not have HF (DAPA-CKD enrolled patients with a baseline eGFR as low as 25 ml/min/1.73 m2, whereas EMPEROR-Reduced allowed the participation of patients with a baseline eGFR as low as 20 ml/min/1.73 m2). Consequently, the patients with CKD in EMPEROR-Reduced had a baseline eGFR similar to those enrolled in DAPA-CKD (47 and 43 ml/min/1.73 m2, respectively), and lower than the mean eGFR of patients with type 2 diabetes and albuminuric CKD enrolled in the CREDENCE trial (56 ml/min/1.73 m2).17,18 Accordingly, when the results of DAPA-HF and EMPEROR-Reduced are combined in a meta-analysis, SGLT2 inhibition with these drugs reduced the risk of major kidney outcomes by 38% (HR, 0.62; 95% CI, 0.43–0.90) without evidence for heterogeneity.20
Kidney function declines progressively during the course of chronic heart failure.24,25 We showed a favorable effect of empagliflozin on kidney function using 3 metrics, ie, slowing of the slope of eGFR over time, attenuation of the decline in eGFR from baseline to the off-treatment assessment at the end of the trial, and a composite of serious kidney events. We noted a nominally significant treatment-by–baseline eGFR interaction for the effect of empagliflozin on the eGFR slope, which was apparent irrespective of whether the analysis categorized patients into 2 or 5 eGFR subgroups. However, these analyses are model-dependent and are based on absolute differences. Given the lower baseline values for eGFR in patients with CKD, the magnitude of benefit on eGFR slope with empagliflozin was proportionally similar in patients with and without CKD; furthermore, absolute declines in eGFR are likely to be more clinically meaningful in patients with CKD, since they accelerate the loss of eGFR to levels that are indicative of end-stage kidney disease. The importance of the effect of empagliflozin on the course of kidney disease in patients with CKD is highlighted by the fact that, when compared with patients without CKD, patients with compromised eGFR experienced similar benefits of empagliflozin to prevent the decline in eGFR. The analyses that compared effects between off-treatment values at baseline and after treatment discontinuation were unconfounded by the presence of the study medication and show that empagliflozin protected the kidney regardless of CKD status. Additionally, both subgroups experienced similar reductions in the risk of the composite kidney outcome.
Potential mechanisms for the kidney benefits of SGLT2 inhibition include a reduction in single nephron hyperfiltration, decreased tubulointerstitial damage, suppression of hyperglycemia-induced production of reactive oxygen species and angiotensinogen, reduction of mitochondrial damage, and enhanced nutrient deprivation signaling.26–29 The latter mechanism may explain why empagliflozin increased hemoglobin and reduced the proportion of patients with anemia, irrespective of presence or absence of CKD at baseline.30
Despite an expected higher rate of adverse events in patients with CKD, there were no excess adverse events in patients receiving empagliflozin, as compared to patients receiving placebo, across all categories of kidney function. The initial dip in eGFR seen more commonly in empagliflozin-treated patients was not associated with an excess risk of investigator-reported acute kidney injury, regardless of the presence or absence of CKD. The rapid increase in eGFR seen when empagliflozin was withdrawn at the end of the trial indicates that the initial dip in eGFR represents a reversible functional change in intrarenal hemodynamics, which is seen in patients with and without CKD. This initial decline in eGFR did not prevent empagliflozin from exerting its long-term beneficial effects on heart failure events or on kidney function.
Limitations
Several limitations should be highlighted in this study. Although this was a prespecified secondary analysis, EMPEROR-Reduced was not powered to assess the effect of empagliflozin in subgroups such as CKD, much less to assess the effect in smaller subgroups such as the 5 categories of eGFR and 3 UACR categories. Although the benefits on the composite kidney outcome were based on relatively few events, as reflected in the confidence intervals, the point estimate of the magnitude of our findings of a benefit on kidney outcomes is very similar to that seen in large-scale trials of patients with diabetes or with chronic kidney disease.
Conclusions
In EMPEROR-Reduced, empagliflozin reduced the key efficacy outcomes of time-to-first–HF hospitalization or cardiovascular death and first and recurrent HF hospitalizations, and slowed the rate of kidney function decline, in patients with and without CKD and across a broad kidney function spectrum. Moreover, in patients with and without CKD, empagliflozin reduced a kidney composite outcome that incorporated clinically meaningful and sustained deterioration in kidney function, chronic dialysis, and kidney transplant; treatment with the drug reduced the proportion of CKD patients with anemia. These efficacy benefits and the consistent safety profile with the overall trial population support the clinical use of empagliflozin in patients with HF with reduced ejection fraction across the broad spectrum of kidney function, including those with severe kidney function impairment down to an eGFR of 20 ml/min/1.73 m2 and irrespective of the degree of albuminuria.
Acknowledgments
Graphical assistance, supported financially by Boehringer Ingelheim, was provided by Matthew Smith and Giles Brooke of Elevate Scientific Solutions. C. Zeller (an employee of Boehringer Ingelheim) did the statistical analysis. Drs Zannad and Ferreira (not employees of the sponsor) drafted the first version of the manuscript and subsequent revisions. All the other authors read and edited the manuscript. All the authors approved the final version and the decision to submit the manuscript.
Sources of Funding
EMPEROR-Reduced was funded by Boehringer Ingelheim.
Disclosures
Dr Zannad reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, Merck, Bayer, and Cellprothera outside of the submitted work; and other support from cardiovascular clinical trialists and Cardiorenal, outside of the submitted work. Dr Ferreira reports consulting fees from Boehringer Ingelheim during the conduct of the study. Dr Pocock reports personal fees from Boehringer Ingelheim during the conduct of the study. Dr Anker reports grants from Vifor; personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Impulse Dynamics, Cardiac Dimensions, and Thermo Fisher Scientific; and grants and personal fees from Abbott Vascular, outside of the submitted work. Dr Butler reports consultancy fees from Boehringer Ingelheim during the conduct of the study; and consultancy fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave, and Vifor, outside of the submitted work. Dr Filippatos reports receiving payment from Boehringer Ingelheim for being a trial committee member during the conduct of the study and from Medtronic, Vifor, Servier, and Novartis for being a trial committee member outside of the submitted work. Dr Wanner reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Akebia, AstraZeneca, Bayer, Eli Lilly, GlaxoSmithKline, Gilead, Merck Sharp & Dohme, Mundipharma, Sanofi-Genzyme, and Vifor Fresenius outside of the submitted work. Dr Packer reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from AbbVie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk outside of the submitted work. Drs Hauske, Brueckmann, and Schnee, C. Zeller, and E. Pfarr are employees of Boehringer Ingelheim.
Supplementary Material
Footnotes
Sources of Funding, see page 320
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.051685.
This work was presented as an abstract at the American Society of Nephrology Annual Meeting, October 22 to October 25, 2020.
Contributor Information
João Pedro Ferreira, Email: jp7ferreira@hotmail.com.
Stuart J. Pocock, Email: stuart.pocock@lshtm.ac.uk.
Cordula Zeller, Email: cordula.zeller@boehringer-ingelheim.com.
Stefan D. Anker, Email: s.anker@cachexia.de.
Javed Butler, Email: butlzih@gmail.com.
Gerasimos Filippatos, Email: gfilippatos@gmail.com.
Sibylle Jenny Hauske, Email: sibylle.hauske@boehringer-ingelheim.com.
Martina Brueckmann, Email: martina.brueckmann@boehringer-ingelheim.com.
Egon Pfarr, Email: egon.pfarr@boehringer-ingelheim.com.
Janet Schnee, Email: janet.schnee@boehringer-ingelheim.com.
Christoph Wanner, Email: wanner_c@medizin.uni-wuerzburg.de.
Milton Packer, Email: milton.packer@baylorhealth.edu.
References
- 1.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD; Resource Utilization Among Congestive Heart Failure (REACH) Study. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002; 39:60–69. doi: 10.1016/s0735-1097(01)01700-4 [DOI] [PubMed] [Google Scholar]
- 2.Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, Hsu G, Sung SH, Magid DJ, Go AS. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013; 6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD Chronic Kidney Disease Collaboration.. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 395:709–733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999; 354:359–364. doi: 10.1016/S0140-6736(98)10363-X [DOI] [PubMed] [Google Scholar]
- 5.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006; 354:131–140. doi: 10.1056/NEJMoa053107 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 8.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019; 73:2365–2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017; 38:1883–1890. doi: 10.1093/eurheartj/ehx026 [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018; 6:547–554. doi: 10.1016/S2213-8587(18)30100-1 [DOI] [PubMed] [Google Scholar]
- 11.McCausland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020; 142:1236–1245. doi: 10.1161/CIRCULATIONAHA.120.047643 [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015; 373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018; 137:119–129. doi: 10.1161/CIRCULATIONAHA.117.028268 [DOI] [PubMed] [Google Scholar]
- 14.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 Diabetes. N Engl J Med. 2016; 375:323–334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 15.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 16.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 17.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 18.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. ; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney Disease. N Engl J Med. 2020; 383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 19.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 20.Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, et al. ; EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019; 21:1270–1278. doi: 10.1002/ejhf.1536 [DOI] [PubMed] [Google Scholar]
- 21.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. doi: 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 22.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019; 7:845–854. doi: 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 23.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020; 396:P819–829. doi: 10.1016/S0140-6736(20)31824-9 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, Greene T, Tighiouart H, Matsushita K, Ballew SH, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020; 75:84–104. doi: 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, Simon AL, Ying J, Beck GJ, Wanner C, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled rrials. J Am Soc Nephrol. 2019; 30:1735–1745. doi: 10.1681/ASN.2019010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR. Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. 2019; 68:248–257. doi: 10.2337/dbi18-0007 [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi Y, Matsui T, Yamagishi S. Tofogliflozin, a highly selective inhibitor of SGLT2 blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res. 2016; 48:191–195. doi: 10.1055/s-0035-1555791 [DOI] [PubMed] [Google Scholar]
- 28.Satou R, Cypress MW, Woods TC, Katsurada A, Dugas CM, Fonseca VA, Navar LG. Blockade of sodium-glucose cotransporter 2 suppresses high glucose-induced angiotensinogen augmentation in renal proximal tubular cells. Am J Physiol Renal Physiol. 2020; 318:F67–F75. doi: 10.1152/ajprenal.00402.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Packer M. Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J Am Soc Nephrol. 2020; 31:907–919. doi: 10.1681/ASN.2020010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019; 139:1985–1987. doi: 10.1161/CIRCULATIONAHA.118.038881 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


