Abstract
Background
The outbreak of coronavirus disease 2019 (COVID-19) infection has led to the reorganization of hospital care in several countries. The objective was to report the postoperative mortality after elective digestive resections in a nationwide cohort during the lockdown period.
Methods
This analytic study was performed using a national billing database (the Programme de Médicalisation des Systèmes d’Informations). Patients who underwent elective digestive resections were divided in 2 groups: the lockdown group defined by hospital admissions between March 17 and May 11, 2020; and the control group, defined by hospital admissions during the corresponding period in 2019. Groups were matched on propensity score, geographical region, and surgical procedure. The primary outcome was the postoperative mortality.
Results
The overall population included 15,217 patients: 9,325 patients in the control group and 5,892 in the lockdown group. The overall surgical activity was decreased by 37% during the lockdown period. The overall in-hospital mortality during the hospital stay was 2.7%. After matching and adjustment, no difference in mortality between groups was reported (OR = 1.05; 95% CI: 0.83–1.34; P = .669). An asymptomatic COVID-19 infection was a risk factor for a 2-fold increased mortality, whereas a symptomatic COVID-19 infection was associated with a 10-fold increased mortality.
Conclusion
Despite a considerable reduction in the surgical activity for elective digestive resections during the lockdown period, mortality remained stable on a nationwide scale in COVID-free patients. These findings support that systematic COVID-19 screening should be advocated before elective gastrointestinal surgery and that all efforts should be made to maintain elective surgical resection for cancer during the second wave in COVID-free patients.
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) infection has led to the reorganization of hospital care in several countries. In France, on March 12, 2020, the government imposed national restrictions to elective surgery. Several guidelines were rapidly published to help surgeons to adapt their practice to the pandemic context.1, 2, 3, 4, 5, 6, 7
The first reason for postponing elective surgery was optimizing manpower and healthcare resources to favor COVID-19 patients’ treatment, impacting the overall activity. The COVIDsurg collaborative study estimated a worldwide decrease in elective surgery of about 70%, representing more than 28 million people during 3 months of disruption with a major effect on benign disease surgery.8 The same trend has been estimated in France, in 14 academic hospitals, with a reduction of 50% of elective colorectal surgeries during the lockdown period.9 Nevertheless, it has been suggested that these estimations could be biased because of a possible transfer of oncological patients from hospitals massively impacted by COVID-19 (especially public and academic hospitals) to institutions less involved in COVID-19 management (in particular, private institutions).9
The second reason for this strategy was the prevention of hospital-acquired COVID-19 infection for surgical candidates and the prevention of postoperative complications in COVID-19-positive patients.5 , 10 , 11 In fact, a study by Aminian et al reported an uncommonly high mortality rate after 4 frequent procedures during the COVID-19 pandemic: cholecystectomy, hernia repair, gastric bypass, and hysterectomy.12 These findings were confirmed by the work of the COVIDsurg collaborative, which reported a 30-day mortality rate of 24% in more than 1,100 COVID-19 patients after elective surgery.13 Mortality was strongly associated with postoperative pulmonary complications, especially in older men (age >70 years).
The bias of data mostly reported from high-volume hospitals (not representative of the entire healthcare system) could be amended by information on a larger scale. Nationwide solid data on the decrease of elective surgery during the lockdown period are lacking.14, 15, 16 The objective of the present study was to assess the impact of lockdown on postoperative mortality and on surgical activity in elective digestive surgery on a national scale using administrative data covering the entire healthcare system in France.
Methods
Study design
This is an analytical observational study comparing surgical activity during the COVID-19 pandemic lockdown with the surgical activity of a historical control group from the corresponding period of 2019.
Data were collected from a National Health Database (the Programme de Médicalisation des Systèmes d’Informations; PMSI), which is a mandatory billing tool for all hospital stays in France, either for public or private institutions.17 The purpose of this database is to establish the financial budget of hospitals based on the number of patients admitted and the type of procedures performed. Information in the database includes patient and hospital identifier; length of stay; date of admission and discharge; any diagnosis reported according to the International Classification of Diseases, 10th version (ICD-10); and any surgical procedure coded according to specific procedural classification (the Classification Commune des Actes Médicaux), which is used in France to classify any surgical, endoscopic, or radiological act.18 The validity of this database has been tested by cross-referencing with other cohort databases.19, 20, 21 Access to the database was requested from and granted by the National Commission on Informatics and Liberty. As the identifier is anonymous, patient consent was not required.
Population
All adult patients operated on for elective digestive resections, as defined by Supplementary Appendix 1, were included. These comprised esophageal, gastric, colic, rectal, pancreatic, and hepatic resections. Patients with multiple resections (more than 2 procedures) and patients undergoing emergency surgery (defined by a hospital admission after an emergency department visit) were excluded.
All data from January 1, 2019, to June 30, 2020, were extracted from the database. Two groups were created: the lockdown group defined by hospital admissions between March 17, 2020, and May 11, 2020, corresponding to the lockdown period in France, and the control group selected as hospital admissions occurring between March 19, 2019, and May 13, 2019 (ie, corresponding to the same calendar days, from the second day of the 12th week of the year to the first day of the 20th week of the year).
Covariates
Patient characteristics and demographics extracted from the database included age, sex, hospital, and region. The ICD-10 codes of previous hospital stays in the past 3 years were used to calculate the comorbidity index according to the Charlson Comorbidity Index (CCI) defined in Appendix 2, and neoadjuvant chemotherapy or radiotherapy (codes Z511 and Z510).22 , 23 Nutritional status (E43, E44, E46) and obesity (E66) were also recorded.
Data on the type of surgical resection were collected. Different groups of surgery were created to match patients: esophagectomy; total and partial gastrectomy; right, transversal, left, and total colectomy; low anterior rectal resection, rectal resection associated with colo-anal anastomosis, total colo-protectomy with ileo-anal anastomosis, abdomino-perineal resection, and prolapsus surgery; minor or major (≥3 segments) hepatectomy; and pancreatic head or other pancreatic resections.
The volume of resection per week per center was categorized using the quartiles of surgical activity. The COVID-19 infection status was defined according to the ICD-10 codes and classified as symptomatic (U0710, U0711, U0714, and U0715) or asymptomatic (U0712 and U0713) as defined in Supplementary Appendix 3. A high viral spreading zone was defined as a rate over 0.1 of outpatients for COVID-19 at emergency departments in a region or as a rate over 0.8 of intensive care unit hospitalization for COVID-19 in a region on May 4, 2020 (governmental announcement defining the type of zone).
Outcomes
The primary outcome was the in-hospital postoperative mortality, which is defined as death occurring during the hospital stay for surgery, irrespective of the time between the surgery and the event.
The secondary outcomes were the change in the number of hospital admissions for digestive surgery and in the postoperative morbidity rate. The decrease in surgical activity was calculated by reporting the difference between 2020 and 2019. In the assessment of postoperative morbidity, we included the following complications: surgical site infection, bleeding, thromboembolic disease, pulmonary complication, and renal failure. The ICD-10 codes used to identify any selected complication are reported in Appendix 3.
Statistical analysis
Description of data was performed with absolute values and percentage for categorical variables and mean and standard deviation for continuous variables. Univariate analysis was realized with a univariate general linear model. A propensity score was calculated on the probability of being operated on in 2020 using the age, sex, CCI,22 , 23 nutritional status, cancer status, neoadjuvant chemotherapy or radiotherapy, and hospital volume. A matching between the lockdown and control groups was performed using the nearest neighbor for propensity score and the exact method for surgical procedure and region to perfectly match on the same procedure in the same region where the surgery was performed. A multivariate analysis for in-hospital mortality was conducted on this matched population including the group (lockdown and control) and type of COVID-19 infection (none, asymptomatic, symptomatic) in a general linear model. The same type of analysis was performed using morbidity as dependent variable. Odds ratio (OR) and 95% confidence intervals (CI) were estimated. Statistical analyses were performed with R software. The R-package Matchit was used to perform the group matching. 24 , 25
Results
Characteristics of the study population
The overall population included 15,217 patients, 5,892 in the lockdown group and 9,325 in the control group. Patients’ characteristics are summarized in Table I . The mean age was 65 ± 15 years, with 54% being male patients. In the lockdown group, there were more severe patients as the CCI was higher (control = 2.2 ± 2.7 vs lockdown = 2.3 ± 2.5, P < .0001), with a higher prevalence of resection for cancer (control = 5,791, 62% vs lockdown = 4,445, 75%; P < .0001). Moreover, neoadjuvant chemotherapy or radiotherapy were more common among patients in the lockdown group (control = 1,504, 16% vs lockdown = 1,064, 18%; P = .002). In total, 574 of 5,892 (8.7%) surgical patients were diagnosed COVID-19 positive in 2020, and 61 (1.0%) were classified as symptomatic. The characteristics according type of COVID-19 infection is reported in Appendix 4. Patients with infection were more severe patients as the CCI was higher, with a higher rate of malnutrition. The most common symptom of COVID-19 infection was respiratory symptoms (n = 56 of 61, 92%).
Table I.
Characteristics of patients
| Variable | Control (N = 9,325) | Lockdown (N = 5,892) | P value |
|---|---|---|---|
| Age, mean (SD) | 65 ± 15 | 66 ± 14 | <0.001 |
| Male | 4,951 (53) | 3,225 (55) | 0.05 |
| Obesity | 1,251 (13) | 764 (13) | 0.44 |
| Malnutrition | 2,129 (23) | 1,339 (23) | 0.90 |
| Charlson score, mean (SD) | 2.18 ± 2.65 | 2.34 ± 2.53 | <0.001 |
| Charlson score categorization | |||
| 0 | 3,458 (37) | 1,719 (29) | |
| 1–2 | 3,509 (38) | 2,608 (44) | |
| 3–4 | 1,046 (11) | 755 (13) | |
| >4 | 1,312 (14) | 810 (14) | <0.001 |
| Number of resections per week per center | |||
| 0–1 | 1,931 (21) | 1,154 (20) | |
| >1–2 | 2,135 (23) | 1,416 (24) | |
| >2–4 | 2,999 (32) | 1,915 (33) | |
| >4–17 | 2,260 (24) | 1,407 (24) | 0.02 |
| Resection for cancer | 5,791 (62) | 4,445 (75) | <0.001 |
| Neoadjuvant chemotherapy or radiotherapy | 1,504 (16) | 1,064 (18) | 0.002 |
| Type of COVID infection | |||
| No infection | 9,325 (100) | 5,318 (90) | |
| Asymptomatic | 0 (0) | 513 (9) | |
| Symptomatic | 0 (0) | 61 (1) | <0.001 |
Data are reported as number (%) unless otherwise specified.
COVID-19, coronavirus disease 2019; SD, standard deviation.
Decrease in surgical activity
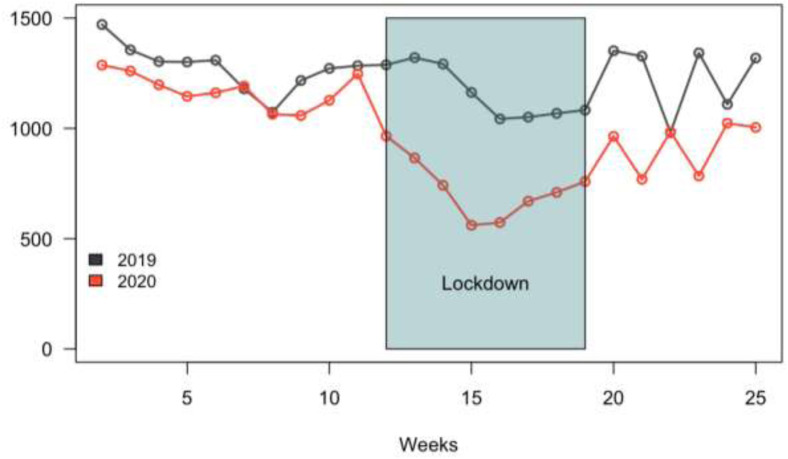
Figure 1 compares the overall surgical activity during 2019 and 2020. The overall activity decreased by 37% during the lockdown period. The lowest activity per week occurred during the 15th week of 2020 (Apr 6–12, 2020). A resumption of activity was observed the week after the lockdown without reaching the activity of 2019. The drop of activity in 2019 between the 15th and 24th weeks occurred during the Easter holidays and national holidays.
Fig 1.
Overall digestive elective resections according years per weeks.
Table II reports the decrease in activity by type of surgery. Hepatic resections were the most impacted by the lockdown with a significant decrease of 44% (P = .02). The decrease was more important in a high viral propagation zone (high viral spreading zone: 44%, low viral spreading zone: 32%, P < .001).
Table II.
Description of surgical activity according group
| Type of Surgery | Control |
(%) | Lockdown |
(%) | (%) of Decrease |
P value |
|---|---|---|---|---|---|---|
| (n = 9,325) | (n = 5,892) | 37 | ||||
| Esophagectomy | 209 | (2) | 109 | (2) | 48 | 0.11 |
| Gastrectomy | 390 | (4) | 263 | (4) | 33 | 0.42 |
| Total | 148 | (2) | 146 | (2) | 1 | |
| Other | 242 | (3) | 117 | (2) | 52 | |
| Colectomy | 5,022 | (54) | 3,200 | (54) | 36 | 0.59 |
| Right | 2,292 | (25) | 1,687 | (29) | 26 | |
| Transversal | 157 | (2) | 95 | (2) | 39 | |
| Left | 2,336 | (25) | 1,265 | (21) | 46 | |
| Total | 237 | (3) | 153 | (3) | 35 | |
| Rectal resection | 2143 | (23) | 1361 | (23) | 36 | 0.88 |
| Low anterior resection | 1,563 | (17) | 1,011 | (17) | 35 | |
| Colo-anal or ileo-anal anastomosis | 362 | (4) | 258 | (4) | 29 | |
| Abdomino-perineal resection | 99 | (1) | 62 | (1) | 37 | |
| Prolapsus resection | 119 | (1) | 30 | (1) | 75 | |
| Hepatic resection | 988 | (11) | 555 | (9) | 44 | 0.02 |
| Minor | 747 | (8) | 416 | (7) | 44 | |
| Major | 241 | (3) | 139 | (2) | 42 | |
| Pancreatic resection | 573 | (6) | 404 | (7) | 29 | 0.08 |
| Head | 377 | (4) | 270 | (5) | 28 | |
| Others | 196 | (2) | 134 | (2) | 32 | |
| Volume of center in 2019 | ||||||
| Low ≤2 per week | 3,223 | (36) | 2,212 | (38) | 31 | |
| Medium 2–4 per week | 2,690 | (29) | 1,624 | (28) | 39 | 0.001 |
| High >4 per week | 3,412 | (35) | 1,997 | (34) | 41 |
Data are reported as nb (%) unless otherwise specified.
The decrease of “high” volume center (>4 resections by week) was more important than the decrease of activity of lower volume center (P = .001).
Mortality during the lockdown period
The in-hospital mortality was 2.5% (n = 233 of 9,325) in the historical control group and 3.0% (n = 174 of 5,892) in the lockdown group (P = .1). After matching, 5,823 patients were included in each group. The characteristics of patients after matching are presented in Appendix 4. There was no significant difference between groups in the variables included in the propensity score, region, and type of surgery.
The multivariate analysis on in-hospital mortality is reported in Table III . There was no difference in mortality before and after adjustment on COVID-19 infection between the 2 groups (OR: 1.05 [95% CI: 0.83–1.34], P = .669). An asymptomatic COVID-19 infection was a risk factor for a 2-fold increased mortality (OR = 2.00, 1.27–3.05, P = .002), whereas a symptomatic COVID-19 infection was associated with a 10-fold increased mortality (OR = 10.51, 5.35–19.31, P < .001).
Table III.
Impact of the lockdown on mortality and morbidity compared to control group on the matched population and adjusted on the COVID-19 infection
| No death N = 1,1337 | Death N = 309 | OR (Univariable) | OR (Multivariable) | |
|---|---|---|---|---|
| Group | ||||
| Control | 5,684 (50) | 139 (45) | ||
| Lockdown | 5,653 (50) | 170 (55) | 1.23 (0.98–1.54, P = .074) | 1.05 (0.83–1.34, P = .669) |
| COVID-19 infection | ||||
| No infection | 10,805 (95) | 271 (88) | ||
| Asymptomatic | 484 (4) | 25 (8) | 2.06 (1.32–3.07, P = .001) | 2.00 (1.27–3.05, P = .002) |
| Symptomatic | 48 (1) | 13 (4) | 10.80 (5.56–19.58, P < .001) | 10.51 (5.35–19.31, P < .001) |
Data are reported as nb (%) unless otherwise specified.
COVID-19, coronavirus disease 2019; OR, odds ratio.
Postoperative complications
Table IV reports postoperative complications. In the univariate analysis on the matched groups, overall morbidity and other postoperative complications were not different between the control and lockdown groups. In the multivariate analysis, the lockdown group was not associated with higher overall morbidity (OR: 0.95 [95% CI: 0.87–1.03], P = .227).
Table IV.
Univariate analysis of mortality and morbidity for 2020 patients according to COVID-19 infection status
| Outcomes | No Infection (N = 5,318) | Asymptomatic (N = 513) | Symptomatic (N = 61) | P value (no infection versus asymptomatic) | P value (no infection versus symptomatic) |
|---|---|---|---|---|---|
| Mortality | 136 (2.6) | 25 (4.9) | 13 (21.3) | <.001 | <.001 |
| Overall morbidity | 1,459 (27.4) | 240 (46.8) | 49 (80.3) | <.001 | <.001 |
| Bleeding | 343 (6.4) | 44 (8.6) | 6 (9.8) | .08 | .42 |
| Surgical site infection | 744 (14.0) | 127 (24.8) | 19 (31.1) | <.001 | <.001 |
| Thromboembolic disease | 172 (3.2) | 28 (5.5) | 7 (11.5) | .01 | .001 |
| Pulmonary infection | 155 (2.9) | 62 (12.1) | 38 (62.3) | <.001 | <.001 |
| Pulmonary insufficiency | 305 (5.7) | 88 (17.2) | 24 (39.3) | <.001 | <.001 |
| Overall pulmonary complications | 395 (7.4) | 116 (22.6) | 40 (65.6) | <.001 | <.001 |
| Renal insufficiency | 327 (6.1) | 60 (11.7) | 13 (21.3) | <.001 | <.001 |
Data are reported as nb (%) unless otherwise specified.
COVID-19, coronavirus disease 2019.
The overall morbidity was higher in the asymptomatic group compared with the COVID-19–free group (no infection= 27% vs asymptomatic = 47%, P < .001) and even higher in the symptomatic group (no infection= 27% vs symptomatic = 80%, P < .001). The rates of pulmonary complications, surgical site infection, and thromboembolic disease were higher for COVID-19–infected patients compared to COVID-19–free patients.
Discussion
In this nationwide data analysis, the overall postoperative mortality after elective digestive surgery during the COVID-19 pandemic lockdown was comparable to the mortality rate observed during the same period in 2019. However, COVID-19 infection, either symptomatic or asymptomatic, was a strong independent risk factor of postoperative mortality and morbidity. We observed a reduction of more than 3,400 procedures (37%) in gastrointestinal surgical resections during the lockdown.
During the most acute phase of the pandemic, several national and international guidelines indicated that nonurgent surgery had to be postponed to allocate more resources to care for patients with COVID-19 patients.1 , 2 , 10 , 26 Elective surgery decreased more markedly in regions where the viral surge was higher, probably because of restrictions to favor treatment for patients with COVID-19. As reported in other countries, reduction of activity was motivated by reallocation of anesthesiologists, nursing staff, and mechanical ventilators in medical COVID-19 units or ICU COVID-19 units.27 , 28 Soreide et al furthermore described operating theaters and surgical intensive care units converted to rescue additional ICU units for patients with severe COVID requiring mechanical ventilation.29 Those facts contributed to a higher decrease in surgical activity, especially in high viral spreading zones.
In our study, despite the important reduction in elective surgery during the lockdown period, the percentage of patients operated on for cancer increased. In fact, all guidelines published in that context recommended to postpone nonurgent elective surgery, primarily delaying surgery for benign conditions. This could explain why in 2020 patients were older and presented with more severe comorbidities.8 , 10 In the present study, the comparison between groups showed a greater proportion of patients who had a higher Charlson Comorbidity Index and received neoadjuvant treatment during the lockdown period.
For the overall population, we did not observe any significant difference in postoperative mortality between the lockdown period and the corresponding period in 2019, before and after matching. The only increase was observed in the COVID-19–infected population, as already reported by several authors. Effectively, we reported a 2-fold increased mortality for asymptomatic COVID-19 infection, whereas a symptomatic COVID-19 infection was associated with a 10-fold increased mortality. Doglietto et al analyzed 41 surgical patients with positive results at the COVID-19 screening and showed results similar to ours in symptomatic COVID-19 patients.30 The COVIDSurg collaborative study also reported a mortality rate of 24% for patients undergoing surgery with an active COVID-19 infection.13 The observed increase in mortality rate in the COVID-19-positive patients supports the need for systematic screening before elective surgery, and we think that, whenever possible, elective surgery for cancer in COVID-19–free patients should not be delayed in order to limit a potential increased risk of overstaging. This fact is supported by a recent study showing a low risk of postoperative mortality for patients operated on for oncologic elective surgery with a COVID-19–free surgical pathway.31 In light of these results, we think that surgery for benign disease or for early cancer should be postponed after a positive screening. On the other hand, for locally advanced cancer, in case of symptomatic COVID-19 infection the surgery should be delayed because of a high risk of postoperative death. In case of asymptomatic COVID-19 infection, the decision should be discussed with multidisciplinary staff, including anesthesiologists, oncologists, and surgeons.
The analysis of postoperative morbidity showed increased risks of several complications after COVID-19 infection in patients operated on in 2020, such as thromboembolic disease, surgical site infection, and pulmonary complications. This finding is consistent with other studies.8 , 30 Doglietto et al also reported 10% of thrombotic complications and 60% of pulmonary complications for the COVID-19 patients group (n = 41).30
This study has some limitations. First, the real impact of COVID-19 infection on postoperative mortality and morbidity should be interpreted with caution. Data on systematic screening in each institution and delay of reverse transcription polymerase chain reaction before surgery were not available. Hence, undiagnosed asymptomatic patients could have been assigned to the COVID-free group. This ascertainment bias has already been suggested for the aforementioned study of the COVIDSurg collaborative and could also be argued in our analysis.32 Moreover, the mortality of patients with COVID-19 could be associated with a more severe patient status. We tried to limit this bias by excluding patients operated on in an emergency setting and by adjusting for neoadjuvant treatment and surgery for cancer in the statistical analysis. We think that the strong association between mortality and COVID-19 status could not be only attributed to a selection bias. Second, the PMSI database does not allow accurate screening of the cause of death because of unavailable information.
The main strength of this study is the data source. Because the PMSI database is mandatory for all hospitals and clinics, it gives a pragmatic and comprehensive picture of the surgical activity on a national scale, allowing a true assessment of in-hospital mortality.
In conclusion, despite a considerable reduction in the surgical activity for elective digestive resections during the lockdown period, mortality remained stable on a nationwide scale in COVID-free patients. In contrast, an asymptomatic COVID-19 infection was a risk factor for a 2-fold increased mortality, whereas a symptomatic COVID-19 infection was associated with a 10-fold increased mortality.
These findings support that systematic COVID-19 screening should be advocated before elective GI surgery and that all efforts should be made to maintain elective surgical resection for cancer during the second wave of the pandemic viral infection in COVID-free patients.
Funding/Support
No funding for research or publication.
Conflict of interest/Disclosure
No conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.12.036.
Supplementary materials
References
- 1.Tuech J.J., Gangloff A., Schwarz L. Our challenge is to adapt the organization of our system to the six stages of the epidemic to go beyond the COVID-19 crisis: surgical organization beyond the COVID-19 crisis. Br J Surg. 2020;107 doi: 10.1002/bjs.11639. e189–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuech J.-J., Gangloff A., Fiore F.D., et al. Strategy for the practice of digestive and oncological surgery during the COVID-19 epidemic. J Visc Surg. 2020 doi: 10.1016/j.jviscsurg.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besnier E., Tuech J.-J., Schwarz L. We asked the experts: COVID-19 outbreak: is there still a place for scheduled surgery? Reflection from pathophysiological data. World J Surg. 2020;44:1695–1698. doi: 10.1007/s00268-020-05501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glehen O., Kepenekian V., Bouché O., et al. [Treatment of primary and metastatic peritoneal tumors in the Covid-19 pandemic proposals for prioritization from the RENAPE and BIG-RENAPE groups] J Chirurgie Viscerale. 2020 doi: 10.1016/j.jchirv.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiore F.D., Bouché O., Lepage C., et al. COVID-19 epidemic: proposed alternatives in the management of digestive cancers: a French intergroup clinical point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver Dis. 2020 doi: 10.1016/j.dld.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baud G., Brunaud L., Lifante J.C., et al. Endocrine surgery during and after the COVID-19 epidemic: expert guidelines from AFCE. J Visc Surg. 2020 doi: 10.1016/j.jviscsurg.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coccolini F., Perrone G., Chiarugi M., et al. Surgery in COVID-19 patients: operational directives. World J Emerg Surg. 2020;15:25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborative C., Nepogodiev D., Bhangu A. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard M.K., Lefèvre J.H., Batteux F., et al. COVID-19 heath crisis: less colorectal resections and yet no more peritonitis or bowel obstruction as a collateral effect? Colorectal Dis. 2020 doi: 10.1111/codi.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaborative C. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020 doi: 10.1002/bjs.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wexner S.D., Cortés-Guiral D., Gilshtein H., Kent I., Reymond M. COVID-19: impact on colorectal surgery. Colorectal Dis. 2020;22:635–640. doi: 10.1111/codi.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aminian A., Safari S., Razeghian-Jahromi A., Ghorbani M., Delaney C.P. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020:1. doi: 10.1097/sla.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nepogodiev D., Glasbey J.C., Li E., et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maspero M., Mazzola M., Bertoglio C.L., et al. Major cancer surgery during the coronavirus pandemic: experience from a tertiary referral center and COVID-19 hub in Northern Italy. Br J Surg. 2020;107(10):e440–e441. doi: 10.1002/bjs.11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farid Y., Schettino M., Kapila A.K., et al. Decrease in surgical activity in the COVID-19 pandemic: an economic crisis. Br J Surg. 2020;107 doi: 10.1002/bjs.11738. e300–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirracchio R., Mavrothalassitis O., Mathis M., Kheterpal S., Legrand M. The response of US hospitals to elective surgical cases in the COVID-19 pandemic. Brit J Anaesth. 2020 doi: 10.1016/j.bja.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domin J.-P. Le Programme de médicalisation des systèmes d’information (PMSI): de l’indicateur de comptabilité hospitalière au mode de tarification (1982–2012) Histoire Médecine Santé. 2013:69–87. [Google Scholar]
- 18.Bousquet C., Trombert B., Souvignet J., Sadou E., Rodrigues J.-M. Evaluation of the CCAM hierarchy and semi structured code for retrieving relevant procedures in a hospital case mix database. AMIA Annu Symposium Proc. 2010:61–65. [PMC free article] [PubMed] [Google Scholar]
- 19.Quantin C., Cottenet J., Vuagnat A., et al. Qualité des données périnatales issues du PMSI: comparaison avec l’état civil et l’enquête nationale périnatale 2010. Journal de Gynécologie Obstétrique et Biologie de la Reproduction. 2014;43:680–690. doi: 10.1016/j.jgyn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Quantin C., Benzenine E., Hägi M., et al. Estimation of national colorectal-cancer incidence using claims databases. J Cancer Epidemiol. 2012:1–7. doi: 10.1155/2012/298369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierron A., Revert M., Goueslard K., et al. Évaluation de la qualité métrologique des données du programme de médicalisation du système d’information (PMSI) en périnatalité: étude pilote réalisée dans 3 CHU. Revue d’Épidémiologie et de Santé Publique. 2015;63:237–246. doi: 10.1016/j.respe.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Charlson M., Wells M.T., Ullman R., King F., Shmukler C. The Charlson Comorbidity Index can be used prospectively to identify patients who will incur high future costs. PLoS One. 2014;9:e112479. doi: 10.1371/journal.pone.0112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- 25.Ho D.E., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42 doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 26.Diaz A., Sarac B.A., Schoenbrunner A.R., Janis J.E., Pawlik T.M. Elective surgery in the time of COVID-19. Amer J Surg. 2020 doi: 10.1016/j.amjsurg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinelli A., Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020;107:785–787. doi: 10.1002/bjs.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ives J., Huxtable R. Surgical ethics during a pandemic: moving into the unknown? Br J Surg. 2020 doi: 10.1002/bjs.11638. [DOI] [PubMed] [Google Scholar]
- 29.Soreide K., Hallet J., Matthews J.B., et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg. 2020 doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doglietto F., Vezzoli M., Gheza F., et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155 doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glasbey J.C., Bhangu A., Collaborative C.O. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2020:JCO2001933. doi: 10.1200/JCO.20.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myles P.S., Maswime S. Mitigating the risks of surgery during the COVID-19 pandemic. Lancet. 2020 doi: 10.1016/s0140-6736(20)31256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.