Abstract
Objectives
To evaluate the mechanical ventilation rates of dental treatment rooms and assess the effectiveness of aerosol removal by mechanical ventilation and a portable air cleaner (PAC) with a high-efficiency particulate air (HEPA) filter.
Methods
Volumetric airflow were measured to assess air change rate per hour by ventilation (ACHvent). Equivalent ventilation provided by the PAC (ACHpac) was calculated based on its clean air delivery rate. Concentrations of 0.3, 0.5 and 1.0 μm aerosol particles were measured in 10 dental treatment rooms with various ventilation rates at baseline, after 5-min of incense burn, and after 30-min of observation with and without the PAC or ventilation system in operation. Velocities of aerosol removal were assessed by concentration decay constants for the 0.3 μm particles with ventilation alone (Kn) and with ventilation and PAC (Kn+pac), and by times needed to reach 95 % and 100 % removal of accumulated aerosol particles.
Results
ACHvent varied from 3 to 45. Kn and Kn+pac were correlated with ACHvent (r = 0.90) and combined ACHtotal (r = 0.81), respectively. Accumulated aerosol particles could not be removed by ventilation alone within 30-min in rooms with ACHvent<15. PAC reduced aerosol accumulation and accelerated aerosol removal, and accumulated aerosols could be completely removed in 4 to 12-min by ventilation combined with PAC. Effectiveness of the PAC was especially prominent in rooms with poor ventilation. Added benefit of PAC in aerosol removal was inversely correlated with ACHvent.
Conclusions
Aerosol accumulation may occur in dental treatment rooms with poor ventilation. Addition of PAC with a HEPA filter significantly reduced aerosol accumulation and accelerated aerosol removal.
Clinical significance
Addition of PAC with a HEPA filter improves aerosol removal in rooms with low ventilation rates.
Keywords: Dental aerosols, Ventilation rate, Portable air cleaner, HEPA, COVID-19
1. Introduction
Dentistry is considered a high-risk profession during a transmissible infectious disease pandemic due to the close proximity between dental care providers (DCPs) and the patient’s respiratory tract openings during dental exams and treatments. Standard precaution in dental offices, mainly developed against contact and droplet transmissions of bloodborne diseases, may not be adequate during an outbreak of infectious respiratory diseases [1]. As COVID-19 is likely transmissible through direct contacts, droplets and aerosols [2], transmission-based precaution for aerosols need to be considered when treating patients confirmed or suspected to have the infection. In addition to basic personal protective equipment (PPE), N95 masks, protective goggles with side shields and full-length face shields are added to the armamentarium of dental professionals performing aerosol-generating procedures per the American Dental Association (ADA) and the Central for Disease Control and Prevention (CDC) guidance [3,4]. Such increased level of protections might have been effective as few clusters of COVID-19 transmission have been reported in dental offices [5,6]. Nonetheless, both the dental professionals and their patients have been adversely impacted by fear and uncertainties associated with a novel infectious respiratory disease that can be transmitted by covert patients without any symptoms [7,8]. With mounting evidence that SARS CoV-2 is airborne and COVID-19 is likely transmissible through aerosols [[9], [10], [11], [12], [13]], dental practices worldwide face challenges to develop adequate aerosol precaution measures beyond the familiar PPE, which is especially important for the protection of dental patients because they could not wear a mask during the dental exam and treatments.
Engineering controls through mechanical ventilation and air filtration are considered a higher level of precaution than PPE and are important mechanisms to reduce the risks of airborne disease transmission in an indoor environment such as the dental treatment rooms. The US CDC guidance recommends that dental offices consider improving the building mechanical ventilation systems and/or adding a portable air cleaner (PAC) to minimize potential risks associated with aerosols in dental offices [4]. Though limited evidence indicates that PAC is useful in dental settings for aerosol controls [14,15], there is no published data that allow an assessment of the role of mechanical ventilations in aerosol removal from dental offices. The dental facility construction codes in New York state stipulate that dental clinics should have a mechanical ventilation rate of at least 6 air changes per hour (ACH) [16]. Though both the US CDC and the American Society of Heating Air-conditioning and Refrigeration Engineers (ASHARE) publishes ventilation guidelines for health care facilities, their guidance on ventilation design did not include dental spaces [[17], [18], [19]]. ASHARE recommends that a minimum of 6 ACH for patients rooms, 12 ACH for airborne infection isolation room and 15 ACH for procedure rooms such as those for trauma surgery or interventional cardiology in outpatient settings [17], which is consistent with CDC guidelines for environmental infection control in healthcare facilities [19].
The US CDC recommended using PAC equipped with high efficiency particulate air (HEPA) filters as part of the engineering control measures for dental settings during the COVID-19 pandemic [4]. Many studies showed that PAC was effective in removing ultrafine aerosol particles from indoor environments [[20], [21], [22], [23], [24]]. Addition of PAC generally improved aerosol particle removals as compared to ventilation alone [23]. Few studies have investigated the effectiveness of PAC on aerosol control in dental offices. An earlier study showed that a blower-filter apparatus with a HEPA filter could reduce the peak microbial concentrations in dental treatment rooms by 72 %–97 % after 20 min of filtering operation [14]. A more recent study using engineering simulations by computer fluidic dynamics indicated that placing a PAC close to the patient’s head in dental offices may reduce the quantity of aerosol particles reaching the breathing zone of the oral health care providers [15].
As a part of the institutional responses to the COVID-19 pandemic, we assessed the heating, ventilation and air conditioning (HVAC) systems in a multi-floor building that houses multiple dental treatment rooms and open bay clinics in an academic medical center and took several improvement measures, including upgrading the air filters to those with Minimum Efficiency Reporting Value rating of 13 (MERV 13) [25], and increasing the outside air percentage to 60 %. MERV 13 filters have a significantly higher particle removal efficiency (59 % for 0.1μm and 88 % for 1.0μm) than the MERV 7 filters (2 % for 0.1 μm and 30 % for 1.0 μm) that were replaced [26]. This building was originally built in the 1970s, though the internal clinical spaces had been successively renovated over the past 10 years. The mechanical ventilation rates of the dental treatment rooms and the open bay clinics were not known. To understand the properties of mechanical ventilation in the clinical spaces and assess the potential needs for improvements, we conducted experimental studies in collaborations with the engineers at the medical center facility to evaluate the mechanical ventilation rates of dental clinical spaces, the effectiveness of aerosol particle removal by mechanical ventilation, and the utility of air filtration by PAC in improving aerosol particle removals from dental treatment rooms.
2. Methods
2.1. Study settings
This study was conducted in a free-standing 8-floor dental facility originally constructed in 1976. The building contains 52 enclosed dental treatment rooms and 3 open bay clinics each containing 12 dental units spaced at 7–8 feet apart. A total of 3 air handling units, located on the third floor of the building, provide ventilations to the clinical areas on the concourse, first and second floors, and to the office, classroom and research lab areas on the fourth to seventh floors of the building. The present study focused on the enclosed dental treatment rooms on the 3 clinical floors on the lower levels. The dental urgent care clinic on the concourse level was constructed in 2010 and contains 7 enclosed dental operatories with ventilations provided by an air handling unit that draws 30 % outside air at the default setting. The general dentistry and specialty clinics on the first and second floors include 45 enclosed dental operatories and the 3 open bay 12-unit clinics with ventilations provided by two different air handling units that draws 60 % outside airs at the time of the study. All 3 air handling units are equipped with particulate filters that have a rating of MERV 13. The room temperatures were at 71 °F to 73 °F (22 °C–23 °C) and relative humidity at 34 %–52 % in the dental treatment rooms during the experiments.
2.2. Determination of room airflow and mechanical ventilation rates
The volumetric airflow rates of the enclosed dental treatment rooms and open bay clinics were measured in cubic feet per minute (CFM, or ft3/min. For metric unit conversion, 1 CFM = 0.0283 cubic meter per minute, or m3/min) at both the air supply inlets and air exhaust returns using an air velocity sensor integrated in a flow hood (ADM-850 L Airdata Multimeter with CFM-850 L FlowHood, Shortridge Instruments, Inc., Scottsdale, AZ). The Airdata multimeter and the flow hood were calibrated by the manufacturer following a program complies with the ANSI/NCSL Z540-1, ISO 17025, and MIL-STD 45662A standards immediately before the experiments. The volumetric sizes of the dental treatment rooms and open bay clinics were calculated in cubic feet (CF, or ft3. 1 ft3 = 0.0283 m3) based on the length, width and ceiling height of each space. The mechanical ventilation rates of each space in number of ACH was calculated as follows [27]:
| ACHs = (total air supply rate in CFM × 60 min)/volume of room in CF, and |
| ACHe = (total air exhaust rate in CFM × 60 min)/volume of room in CF. |
The supply and exhaust airflow rates for each room were measured three times and the mean values were used as the base for the final ventilation rate calculations. The larger value between ACHs and ACHe was used as the room’s mechanical ventilation rate (ACHvent) [27].
2.3. Aerosol particle generation and quantification
Aerosol particles were generated by burning three sticks of incenses (Precious Lavender, HEM Corp., Mumbai, India) and size-specific quantifications for the 0.3, 0.5 and 1.0 μm-diameter aerosol particles were done using a Lasair III 310C aerosol particle counter (Particle Measuring Systems, Boulder, CO, USA). The aerosol particles produced by incense burn are mostly at or below 1.0 μm in diameter and float for an extended period of time if not removed by ventilation or filtration [22,28]. Before the experiment, we measured the aerosol concentrations near the breathing zone of the operators in dental treatment rooms during restorative and surgical procedures and found that most of the aerosol particles generated by high and low-speed dental drillings were below 1.0 μm in diameter (Supplemental Fig. 1, Supplemental Tables 1 and 2), which is consistent with the reports that aerosol particles from drilling activities in dental treatment rooms were mostly smaller than 0.5 μm in diameter and particles larger than 1 μm were rare [[29], [30], [31]]. A small oscillating fan mounted on the walls was turned on at low setting during the experiment to render the air well mixed in the dental treatment rooms. The quantitative increase in particle concentrations (#/m3) from the beginning to the end of 5-minute incense burn was defined as the aerosols accumulated in the room from the incense burn (Qe). The aerosol generation and accumulation experiments by incense burn were repeated in four rooms with different volumetric sizes with the ventilation system turned off.
2.4. Determination of effectiveness of aerosol removal by PAC
The PAC model used for this study was a Honeywell 50250 (Honeywell Inc., Charlotte, NC, USA) with a 360° circular HEPA filter purchased online in October 2020. As the particle with a diameter of 0.3 μm is considered “the most penetrating particle” [32], and the filtration efficiency rating of the HEPA filter is based on this particle size, we used the decay constants of 0.3 μm particles to assess the effectiveness of the PAC used in this study. The effectiveness of aerosol removal by PAC was determined in four treatment rooms using aerosol particles generated from incense burn [20,24]. The mechanical ventilation system was first turned off. After 15 min of baseline measurements, 3 sticks of incenses were burned for 5 min and extinguished. The aerosol particle concentrations were then continuously measured for 30 min to determine the particle decay constants (k0) of the 0.3 μm aerosol particles without mechanical ventilation. After 30 min of measurements, 3 sticks of incenses were again burned for 5 min and extinguished. The PAC, placed on the left side of the dental chair at about 2 feet away from the footrest (Supplemental Fig. 2), was turned on at high setting during the second incense burn and the aerosol particle concentrations were continuously measured again for 30 min to determine the decay constants (kpac) of the 0.3 μm aerosol particles with the PAC on. The decay constants with (kpac) and without (k0) the PAC on were calculated as described by Waring et al. [20] by fitting a linear regression line over time (h) into the negative natural log scale of time-varying concentration (Ct) normalized by the initial concentration at the time the incense was extinguished (C0).
The Honeywell 50250 PAC has a clean air delivery rate (CADR) of 250 for particles ranging from 0.1 μm to 11 μm, which signifies that the PAC delivers 250 CFM clean air [24,33]. The number of equivalent of ACH provided by the PAC (ACHpac) are dependent on the CADR and the volume of the room [34]:
| ACHpac = CADR of the PAC in CFM × 60 min/volume of the room in CF |
The experiments on aerosol removal with PAC turned on were performed in the same rooms described in the preceding section on aerosol generation.
2.5. Effects of mechanical ventilation and PAC on aerosol particle removal
With the building mechanical ventilation system turned on, aerosol particle concentrations in 10 selected dental treatment rooms were first measured for 15 min at baseline, followed by 5 min during incense burning, then continuously for 30 min to observe the removal of the particles by mechanical ventilation alone after the incenses were extinguished. After 30 min of mechanical ventilation, 3 sticks of incenses were again burned for 5 min with the PAC turned on at this time and continued for an additional 30 min after incense burn to observe the effectiveness of aerosol particle removal by the PAC combined with mechanical ventilation. The size specific quantification was done for particles 0.3, 0.5, 1.0 μm in diameters using the Lasair III 310C aerosol particle counter and a Lighthouse 3016 airborne particle counter (Lighthouse Worldwide Solutions, Fremont, CA, USA). The decay constants for the 0.3 μm aerosol particles were calculated for mechanical ventilation alone (kn) and for PAC combined with mechanical ventilation (kn+pac) as described above. The experiments were performed in dental treatment rooms with varying ventilation rates to determine the association between mechanical ventilation rates and effectiveness of aerosol particle removal with and without the PAC turned on. Percentage of 0.3 μm aerosol particles removed was calculated for each room at 5, 10, 15, 20, 25 and 30 min after the incenses were extinguished, with and without the PAC being turned on, to assess the effectiveness of PAC in rooms with different mechanical ventilation rates. Calculations of aerosol removal percentage were based on the decrease of those 0.3 μm aerosol particles accumulated from incense burn only (Qe, or the numbers of particles per cubic meter added to the baseline level after 5 min of incense burn).
When both the mechanical ventilation system and the PAC are turned on, total ACH for the room (ACHtotal) is calculated as the sum of ACHvent and ACHpac.
2.6. Noise level in dental operatories
Sound levels in the selected dental treatment rooms were recorded continuously at 0.5-second interval during the experiments using the Decibel X Pro (SkyPaw Co., Hanoi, Vietnam) sound meter application [35] to assess the noise level added by the PAC. The average A-weighted sound levels (dBA) were compared between the periods with and without the PAC in operation. The sound meter was placed by the headrest of the dental chair and was approximately 6 feet away from the PAC near the footrest of the dental chair.
2.7. Statistical analysis
To understand the effects of mechanical ventilation and air filtration on the speed of aerosol particle removal from the dental treatment rooms, a simple regression model was used to assess the correlation coefficients (Pearson’s r) of particle decay constants and those of speeds of aerosol removals between different experimental conditions using StatView (SAS Institute Inc., Cary, NC, USA). Aerosol particle removal rates of the PAC were analyzed in dental treatment rooms with varying mechanical ventilation rates to explore potential interactions between ventilation rates and aerosol particle removal efficiency of the PAC. Average sound levels were compared between periods with and without the PAC turned on to investigate the additional noises introduced by the PAC. Quantitative comparisons between two experimental conditions (with and without the PAC) were done with paired-t tests. All statistical tests were two-tailed at the significance level of 0.05.
3. Results
The volumes and mechanical ventilation rates of dental treatment rooms and open bay clinics are provided in Supplemental Table 3. The 52 treatment rooms are on average 878 CF (24.9 m3) in volume (range 396–1646 CF, or 11.2 to 46.6 m3). ACHvent varied from 3 to 45 with a mean of 14 (±10). Thirteen of the treatment rooms have approximately equal supply and exhaust airflow rates with the differentials between ACHs and ACHe not greater than one. Twenty-seven rooms have greater supply than exhaust air flow rates with positive differentials between ACHs and ACHe at two or greater; and 12 rooms have greater exhaust than supply airflow rates with negative differentials between ACHs and ACHe at two or greater. Most of the treatment rooms (92 %) have ACHvent at or greater than 6, and 60 % of the rooms had ACHvent at or greater than 10. The rooms with poor ventilation (ACHvent<6) are invariably located at the far ends of corridors where the supply ducts end. Two of the rooms had unfunctional air exhaust vents and had an exhaust airflow rate of zero.
Table 3.
Decay constants for 0.3 μm particles and time needed to remove 95 % and 100 % aerosol particles accumulated from incense burn without room ventilation and with the portable air cleaner off and on.
| RM # | Qe (#/m3) |
ACHpac | Ko | Kpac | Time to 95 % removal |
Time to 100 % removal |
|||
|---|---|---|---|---|---|---|---|---|---|
| pac off | pac on | pac off | pac on | pac off | pac on | ||||
| 022 | 7.8 × 107 | 6.3 × 107 | 18 | 0.4 | 20.2 | >30 | 8 | >30 | 9 |
| 031 | 7.3 × 107 | 6.0 × 107 | 16 | 1.1 | 16.8 | >30 | 9 | >30 | 11 |
| 032 | 6.6 × 107 | 3.4 × 107 | 22 | −0.4 | 23.2 | >30 | 5 | >30 | 5 |
| 033 | 5.9 × 107 | 5.4 × 107 | 15 | −0.6 | 14.7 | >30 | 9 | >30 | 9 |
| Mean | 6.9 × 107 | 5.2 × 107 | 17.5 | 0.1 | 18.7 | – | 8 | – | 9 |
| SD | 8.3 × 106 | 1.3 × 107 | 3.2 | 0.8 | 3.7 | – | 2 | – | 3 |
Qe– number of 0.3 μm particles per cubic meter (#/m3) accumulated from 5 min of incense burn with portable air cleaner (pac) off or on. ACHpac – equivalent air change per hour provided by the portable air cleaner. Ko – decay constants with portable air cleaner off. Kpac – decay constants with portable air cleaner on.
3.1. Aerosol particle generation and characterization
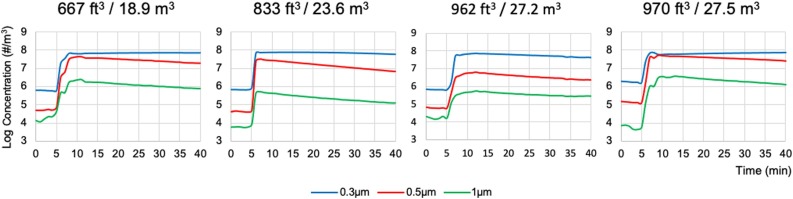
Aerosol particles 0.3, 0.5 and 1.0 μm in diameters increased to a great extent (on average 7.0 × 107, 3.1 × 107 and 1.6 × 106/m3, respectively) after 5 min of incense burn and their concentrations stayed high throughout the 30 min observation period after the incenses were extinguished (Fig. 1 , Table 1 ). This was especially true for the 0.3 μm aerosol particles, which decreased only 6.5 % on average in concentration after 30 min, compared to a 59.5 % decrease for the 0.5 μm and a 61.0 % decrease for the 1.0 μm particles (Table 1).
Fig. 1.
Size-specific aerosol particle concentrations in logarithmic scale at base line, after 5 min of incense burn and after 30 min of observation in four dental treatment rooms with various volumetric sizes in cubic feet (ft3) or cubic meters (m3) with ventilation turned off. Incense burn started at the 5th minutes and stopped at the 10th minutes. Particle concentrations were measured at 1-minute interval for the 30 min period from the 10th and 40th minutes to observe the natural particle concentration decays without the effect of ventilation or air filtration.
Table 1.
Aerosol particle concentrations (#/m3, Mean ± SD) before and after 5 min of incense burn and after 30 min of observation with room ventilation off.
| 0.3 μm | 0.5 μm | 1.0 μm | Total | |
|---|---|---|---|---|
| Baseline | 9.6 × 105 ± 4.8 × 105 | 7.9 × 104 ± 3.7 × 104 | 1.8 × 104 ± 1.7 × 104 | 1.1 × 106 ± 5.0 × 105 |
| After incense burn | 7.0 × 107 ± 8.0 × 106 | 3.1 × 107 ± 2.0 × 107 | 1.6 × 106 ± 1.4 × 106 | 1.0 × 108 ± 1.6 × 107 |
| Aerosol accumulated (Qe) | 6.9 × 107 ± 8.4 × 106 | 3.1 × 107 ± 2.0 × 107 | 1.6 × 106 ± 1.3 × 106 | 1.0 × 108 ± 1.6 × 107 |
| After 30 min | 6.4 × 107 ± 1.5 × 107 | 1.4 × 107 ± 1.1 × 107 | 6.1 × 105 ± 5.2 × 105 | 7.8 × 107 ± 2.6 × 107 |
| % Aerosol removed | 6.5 ± 30 | 59.5 ± 11.2 | 61 ± 13 | 25.7 ± 15.2 |
The concentration decay constants K0 for the 0.3 μm aerosol particles ranged from -0.62 to 1.09 (Mean 0.11, SD 0.78) when no active ventilation or air filtration was present. The slight changes in concentrations over the 30-minute measurement time were likely due to deposition of the particles on interior surfaces of the room and air leakage around the room doors (Fig. 1, Table 3).
3.2. Effectiveness of aerosol particle removal by the PAC
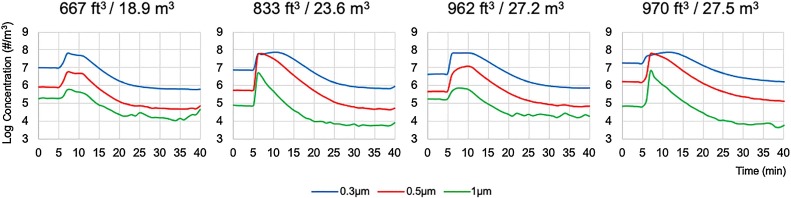
As shown in Fig. 2 and Table 2 , the concentrations for the 0.3, 0.5 and 1.0 μm particles showed rapid decrease after the incenses were extinguished with the ventilation system remained off but the PAC was turned on at high setting. The concentration decay constants kpac for the 0.3 μm particles ranged from 14.7–23.2 (Mean 18.7, SD 3.7) with the PAC on (Table 3 ). Average concentrations for the 0.3 μm aerosol particle (9.6 × 105/m3) was 10-fold lower than the baseline concentration (1.0 × 107/m3) after 30 min of observation, indicating that the PAC removed not only the aerosol particles from incense burn but also some particles already existed in the room at baseline (Fig. 2, Table 2). Times needed to reach 95 % removal of aerosol particles accumulated from incense burn were 8 (±2) minutes which were associated with the room sizes and peak particle concentrations (r = 0.99, p < 0.05) (Table 3). Larger rooms with higher concentrations of particles needed longer time to achieve 100 % removal by the PAC.
Fig. 2.
Effectiveness of aerosol particle removal by the portable air cleaner in four dental treatment rooms with various volumetric sizes in cubic feet (ft3) or cubic meters (m3) with ventilation turned off. Baseline particle concentrations were measured for 5 min. Incense burns started at the 5th min and stopped at the 10th minutes with the portable air cleaner turned on.
Table 2.
Aerosol particle concentrations (#/m3, Mean ± SD) before and after 5 min of incense burn and after 30 min of observation with ventilation off but portable air cleaner on.
| 0.3 μm | 0.5 μm | 1.0 μm | Total | |
|---|---|---|---|---|
| Baseline | 1.0 × 107 ± 6.4 × 106 | 8.8 × 105 ± 5.9 × 105 | 1.3 × 105 ± 6.6 × 104 | 1.1 × 107 ± 7.0 × 106 |
| After incense burn | 6.3 × 107 ± 1.3 × 107 | 1.7 × 107 ± 1.2 × 107 | 4.6 × 105 ± 2.2 × 105 | 8.0 × 107 ± 2.4 × 107 |
| Aerosol accumulated (Qe) | 5.2 × 107 ± 1.3 × 107 | 1.6 × 107 ± 1.1 × 107 | 3.2 × 105 ±2.5 × 105 | 6.9 × 107 ± 2.2 × 107 |
| After 30 min | 9.6 × 105 ± 4.8 × 105 | 7.9 × 104 ± 3.6 × 104 | 1.9 × 104 ± 1.7 × 104 | 1.0 × 106 ± 5.0 × 105 |
| % Aerosol removed* | 119.0 ± 13.2 | 108.3 ± 9.2 | 150.5 ± 34.7 | 116 ± 11 |
Aerosol concentrations were lower than the baseline after 30-min, indicating that the portable air cleaner removed all the aerosol particles accumulated from incense burn and some particles already existed in the room at baseline.
3.3. Aerosol particle removal through mechanical ventilation and PAC
We selected 10 treatment rooms located in different areas of the building and having a wide range of ventilation rates to assess the effectiveness of the mechanical ventilation and PAC (Table 4 ). Equivalent air change rates by the PAC, the ACHpac, ranged from 12 to 22 (Mean 17.5, SD 3.2) depending on the volumetric size of the rooms (Table 5 ). Accumulations of 0.3 μm aerosol particles after 5-min incense burn were significantly higher with ventilation alone (Mean 1.4 × 108/m3, SD 1.2 × 108) than with ventilation and PAC (Mean 6.9 × 107/m3, SD 5.3 × 107) (t = 3.21, p = 0.01, Table 5). Addition of the PAC reduced the aerosol accumulation by an average of 46.9 % (SD 20.8 %). As shown in Figs. 3 , concentration decay curves for the 0.3, 0.5 and 1.0 μm aerosol particles were significantly affected by the values of ACHvent and the PAC during the 30-minute measurement periods after the incenses were extinguished with the mechanical ventilation alone or with both the mechanical ventilation and the PAC turned on. Aerosol concentrations decreased faster in rooms with higher ACHvent, and the addition of the PAC further accelerated the decrease in all the rooms, especially in those with low ACHvent (Fig. 3).
Table 4.
Dental treatment rooms selected for aerosol removal study.
| RM # | Volume | ACHs | ACHe | ACHvent | Temp | RH | Floor |
|---|---|---|---|---|---|---|---|
| ft3 (m3) | oF (oC) | (%) | |||||
| 002 | 815 (23.1) | 11 | 9 | 11 | 71.0 (21.7) | 51.9 | 0 |
| 003 | 787 (22.3) | 10 | 13 | 13 | 71.4 (21.9) | 50.9 | 0 |
| 008 | 1221 (34.6) | 6 | 5 | 6 | 72.7 (22.6) | 48.5 | 2 |
| 012 | 1015 (28.7) | 8 | 3 | 8 | 73.1 (22.8) | 47.4 | 2 |
| 019 | 686 (19.4) | 8 | 32 | 32 | 72.5 (22.5) | 47.3 | 1 |
| 021 | 861 (24.4) | 3 | 3 | 3 | 71.6 (22.0) | 49.0 | 1 |
| 022 | 833 (23.6) | 4 | 0 | 4 | 73.1 (22.8) | 47.0 | 1 |
| 031 | 962 (27.2) | 19 | 15 | 19 | 71.3 (21.8) | 36.8 | 2 |
| 032 | 667 (18.9) | 26 | 16 | 26 | 73.0 (22.8) | 33.9 | 2 |
| 033 | 970 (27.5) | 13 | 15 | 15 | 72.0 (22.2) | 35.7 | 2 |
| Mean | 882 (25.0) | 11 | 11 | 14 | 72.2 (22.3) | 44.8 | – |
| SD | 166 (4.7) | 7 | 9 | 10 | 0.8 (0.4) | 6.7 | – |
ACHs –air change per hour by air supply; ACHs –air change per hour by air exhaust; ACHvent –air change per hour by ventilation. RH – relative humidity.
Table 5.
Decay constants for 0.3um particles and time needed to remove 95 % and 100 % aerosol particles accumulated from incense burns (Qe) with ventilation alone and with ventilation and portable air cleaner.
| RM # | Qe (#/m3) |
ACHvent | ACHpac | ACHtotal | Kn | Kn+pac | Time to 95 % removal |
Time to 100 % removal |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| vent | vent/pac | vent | vent/pac | vent | vent/pac | ||||||
| 002 | 3.5 × 108 | 1.5 × 108 | 11 | 18 | 29 | 10.7` | 29.4 | 17 | 6 | >30 | 9 |
| 003 | 7.6 × 107 | 5.1 × 107 | 13 | 19 | 32 | 9.5 | 27.2 | 22 | 7 | >30 | 9 |
| 008 | 2.7 × 107 | 1.1 × 107 | 6 | 12 | 18 | 7.3 | 19.3 | 25 | 7 | >30 | 8 |
| 012 | 7.4 × 107 | 6.1 × 107 | 8 | 15 | 23 | 6.7 | 16.1 | 24 | 9 | >30 | 12 |
| 019 | 4.9 × 107 | 2.4 × 107 | 32 | 22 | 54 | 29.9 | 41.5 | 7 | 5 | 10 | 8 |
| 021 | 3.0 × 108 | 1.6 × 108 | 3 | 17 | 20 | 5.0 | 23.0 | >30 | 5 | >30 | 6 |
| 022 | 6.9 × 107 | 4.8 × 107 | 4 | 18 | 22 | 1.4 | 20.5 | >30 | 5 | >30 | 5 |
| 031 | 7.3 × 107 | 5.6 × 107 | 19 | 16 | 35 | 13.1 | 32.8 | 15 | 7 | 25 | 10 |
| 032 | 6.1 × 107 | 1.3 × 107 | 26 | 22 | 48 | 13.9 | 29.0 | 11 | 3 | 19 | 4 |
| 033 | 6.7 × 107 | 1.8 × 107 | 15 | 15 | 30 | 9.0 | 19.5 | 17 | 4 | 29 | 4 |
| Mean | 1.4 × 108 | 6.9 × 107 | 14 | 17 | 31 | 10.7 | 25.8 | – | 6 | – | 8 |
| SD | 1.2 × 108 | 5.3 × 107 | 10 | 3 | 12 | 7.7 | 7.7 | – | 2 | – | 3 |
Qe– number of 0.3 μm particles per cubic meter (#/m3) accumulated from 5 min of incense burn with ventilation only (vent) and with both ventilation and portable air cleaner (pac) on. ACHvent –air change per hour by ventilation. ACHpac – equivalent air change per hour by the portable air cleaner. Kn – decay constants with ventilation only. Kn+pac – decay constants with portable air cleaner and ventilation.
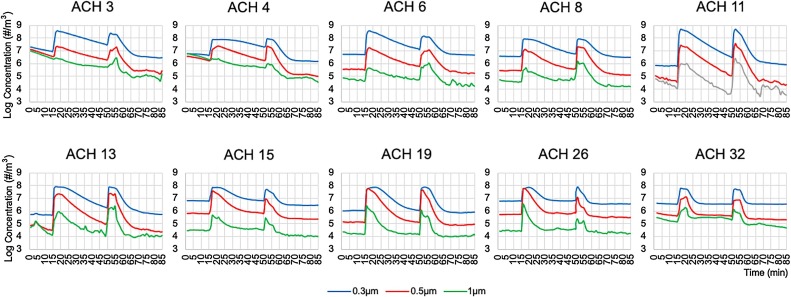
Fig. 3.
Effectiveness of aerosol particle removal by ventilation alone and by ventilation plus the portable air cleaner in dental treatment rooms with various mechanical ventilation rates measured by air change per hour (ACH). Baseline particle concentrations were measured for 15 min. Incense burns started at the 15th minutes and stopped at the 20th minutes with ventilation alone. After 30 min of observation of particle concentration decays, the portable air cleaner was turned on and the second incense burns started at the 50th minutes and stopped at the 55 min. Aerosol particle concentration decays, with the effects of both ventilation and air filtration, were observed for 30 min from the 55th and 85th minutes.
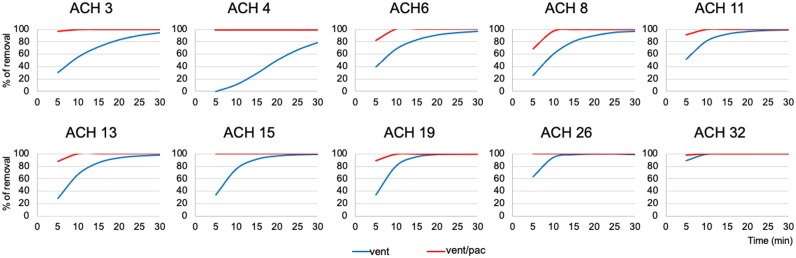
For the 0.3 μm aerosol particles, the concentration decay constants Kn ranged from 1.4–29.9 (Mean 10.7, SD 7.7) with the mechanical ventilation on (Table 5). Kn was significantly associated with ACHvent (r = 0.91, p < 0.01), signifying that the speed of aerosol removal increased with higher ventilation rate. Aerosol particle concentration decreased by 96.4 % (±6.4 %) on average after 30 min of observation, but reached the baseline level (or achieved nearly 100 % removal of the aerosol particles accumulated from incense burn) only in the 4 treatment rooms with high ventilation rate (ACHvent>15) by mechanical ventilation alone. At least 97 % of aerosol particles were removed in the treatment rooms with ACHvent ≥6 after 30 min of ventilation (Supplemental Table 4).
The particle concentration decay constants kn+pac for the 0.3 μm particles ranged from 16.1–41.5 (Mean 25.8, SD 7.7) when both ventilation and PAC were turned on. Kn+pac was significantly associated with ACHtotal (r = 0.81, p < 0.01), indicating that the speed of aerosol removal increased in general with addition of PAC and the higher combined ventilation rates. There were statistically significant differences between kn and kn+pac (t=-12.12, p < 0.01) (Table 5). Aerosol particle concentration decreased by 99.8 % on average (SD 0.7 %) within 10 min. Times need to reach 95 % removal of aerosol particles ranged from 3−9 min and nearly 100 % removal from 4−12 min when both the mechanical ventilation and the PAC were turned on. Notably, the two rooms with poor ventilation (ACHvent 3 or 4) reached nearly 100 % removal of accumulated aerosols from incense burn before the 10th minute, at a similar time with the 4 rooms with the highest ventilation rates (ACHvent 15–32) and faster than the 4 rooms with higher ventilation rates (ACHvent 6–13) (Table 5).
Aerosol particle removal effectiveness with ventilation alone or with both ventilation and the PAC, at 5, 10, 15, 20, 25 and 30 min, are illustrated in Fig. 4 . With the PAC turned on, aerosol particle removal showed the greatest increase from those with mechanical ventilation alone in rooms with poor ventilation. The effectiveness of the PAC was especially remarkable in the room with ACHvent 4, where it reached 100 % removal of the accumulated aerosols by the 5th minute while no aerosol particles were removed by ventilation alone at the same time point. This room was located at the far end of the ventilation duct system and had only limited air supply (ACHs = 4) and a defective air exhaust return that has no air flow (ACHe = 0) (Supplemental Table 1).
Fig. 4.
Removal efficiency for the 0.3 μm aerosol particles with ventilation alone and with both ventilation and the portable air cleaner at 5, 10, 15, 20, 25 and 30 min after aerosol generations in dental treatment rooms with various mechanical ventilation rates measured by air change per hour (ACH). Only the rooms with high ventilation (ACH > 15) achieved 100 % aerosol removal by ventilation alone. The effectiveness of the portable air cleaner is especially high in rooms with poor ventilation (ACH 3 or 4).
3.4. Interactions between ACHvent and ACHpac
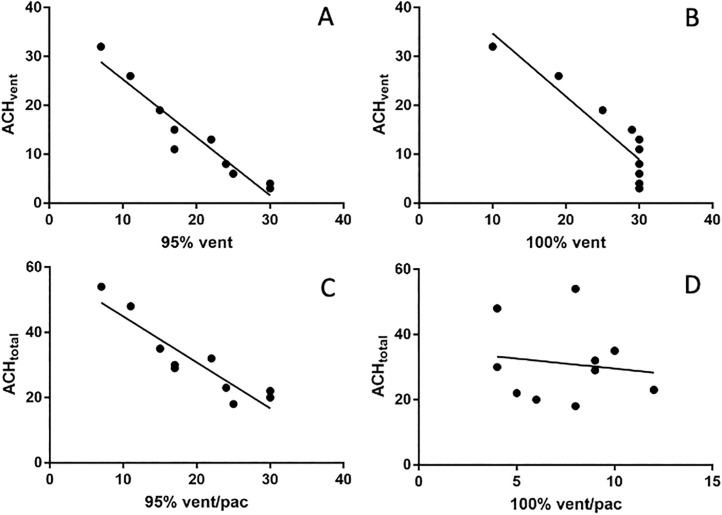
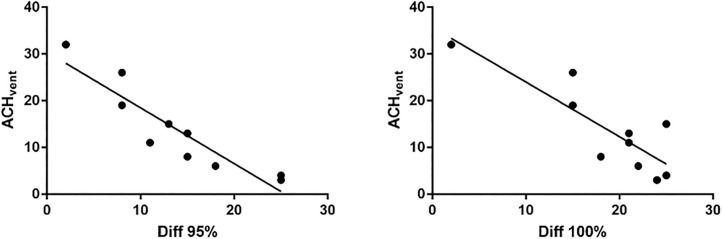
There were clear interactions between the ventilation system and the PAC. Though adding a PAC improved the effectiveness of aerosol removal in all the rooms, the effectiveness of ACHtotal, which represents the combined effects from ACHvent and ACHpac, is not a simple additive of the two components. The correlation coefficients were very high between ACHvent and times needed to reach 95 % (r=−0.97, p < 0.01) and 100 % (r=−0.90, p < 0.01) aerosol particle removals with ventilation alone (Fig. 5 A and B). Higher ACHvent invariably resulted in faster aerosol particle removal when the PAC was not turned on. However, the correlation was not as clear between ACHtotal and times needed to reach 95 % (r=−0.56, p > 0.05) and 100 % (r=−0.29, p > 0.05) aerosol removals with both ventilation and PAC turned on (Fig. 5 C, D). The two rooms with the lowest ACHvent had the most significant increase in the effectiveness of aerosol removal with both ventilation and PAC on, surpassing all but one room with much higher ACHvent and ACHtotal (Table 5). The time differentials between ACHtotal and ACHvent in reaching 95 % or 100 % aerosol removals, which represent the magnitudes of added benefit by adding a PAC, were inversely correlated with ACHvent (Fig. 6 , r=−0.91 for 95 %, and r=−0.83 for 100 %, p < 0.01). The higher the ACHvent, the lower the benefit gained from the PAC in the effectiveness of aerosol removal.
Fig. 5.
Correlations between ventilation alone (ACHvent) or ventilation plus portable air cleaner (ACHtotal) and times needed to 95 % or 100 % removals of the 0.3 μm aerosol particles. Higher ACHvent is highly correlated with shorter times needed to reach 95 % or 100 % aerosol removal (A, B), but such correlations are weak for ACHtotal (C,D).
Fig. 6.
Correlations between room ventilation rates (ACHvent) and the relative effectiveness of portable air cleaner measured by time differentials between ACHtotal and ACHvent in reaching 95 % or 100 % aerosol removals. The higher the ACHvent, the lower the benefit gained from the addition of a portable air cleaner in the overall effectiveness of aerosol removal.
3.5. Effect of PAC on noise levels in dental treatment rooms
Noise levels measured by the headrest of dental chairs was on average 56 dB (SD 8) at baseline and 70 dB (SD 9) when the PAC were turned on at high setting, an increase of 14 dB (SD 2) (t=-19.82, p < 0.01). Baseline noise levels varied between the locations of the dental treatment rooms (Supplemental Table 5).
4. Discussion
The findings of the present study indicate that mechanical ventilation and PAC are both important determinants of aerosol removal from dental treatment rooms. Rooms with poor mechanical ventilation facilitate aerosol particle accumulations in comparison to rooms with high mechanical ventilation. PAC with a HEPA filter was very effective in reducing aerosol accumulation and accelerating aerosol removal, and its effectiveness was especially prominent in dental treatment rooms with poor mechanical ventilation (ACHvent<6). The benefit gained from PAC in aerosol removal diminished with increased mechanical ventilation in the rooms.
With mechanical ventilation alone, we found that the speeds of aerosol removal from the dental treatment rooms were highly correlated with mechanical ventilation rates. For example, a room with 6 ACHvent required 25 min to remove 95 % of the 0.3 μm aerosol particles while the one with 32 ACHvent needed only 7 min. Only those rooms with ACHvent greater than 15 could completely remove the aerosols by mechanical ventilation alone within the 30 min observation period in this study. With the addition of a PAC and combined ventilation and air filtration, the speeds of aerosol removal increased in general but were not as highly correlated with the combined ventilation rates (ACHtotal) as compared to those with mechanical ventilation alone. In the two rooms with poor ventilation (ACHvent<6), addition of the PAC accelerated the aerosol particle removal to a much greater extent compared to rooms with higher mechanical ventilation rates. These findings are in agreement with a previous study that demonstrated that combined high air change rates from ventilation and air filtration did not always translate into higher aerosol particle removal efficiency, likely due to the disruption of exhaust air return by the high air flow rate [23]. The relative position of the PAC to the locations of the aerosol sources and air supply and exhaust vents might also play a role in their combined effectiveness [23,36]. Though the PAC was placed in the same location close to the footrest of the dental chair in each of the room studied, the supply and exhaust air vents in the rooms are mounted in variable locations on the ceilings or the side walls without a rational or predictable pattern, which in turn renders air flow directions and patterns inconsistent from room to room. Though airflow patterns and directions are considered important determinants of aerosol control and removal in treatment rooms [37], and CDC guidance for dental settings during COVID-19 pandemic also points to the importance of air flow directions [4], it appears that these factors had not been taken into consideration during the design, construction and recent renovations of the dental treatment rooms in the current facility. Considering the existing evidence that microbial-laden droplets and aerosols are likely present in the air spaces in the breathing zone of the DCPs after aerosol generating procedures [15,38,39], future engineering designs for dental treatment rooms may need to consider air flow directions and patterns in addition to air low rate to improve the effectiveness of aerosol removal.
Air filtration using a PAC has been shown to be an effective strategy for aerosol particle removal and improving indoor air qualities [22,24,34,40]. The most important feature of a PAC is its filtration efficiency indicated by CADR. The PAC used in this study is equipped with a HEPA filter and has a CADR of 250 for smoke, dust and pollen [41]. Using this CADR rate, we calculated that the additional equivalent air change rate provided by the PAC, the ACHpac, ranged from 12 to 22 for the 10 dental treatments rooms depending on their volumetric size. We found that the PAC could reduce aerosol accumulation as well as accelerate aerosol particle removal. Addition of a PAC reduced aerosol accumulation by nearly 50 % as compared to ventilation alone. With the ventilation turned off, the effectiveness of the PAC in removing aerosol particles was mostly associated with volumetric size of the rooms and the concentrations of aerosol particles in the rooms. Larger rooms with more aerosol particles will take longer time for the PAC to completely clean the air. Our findings suggest that the ACHpac is comparatively more effective in removing aerosols from dental treatment rooms than ACHvent as measured by the times needed to remove 100 % of the accumulated aerosols. In four rooms with mechanical ventilation turned off, PAC removed the aerosols from 5−11 min. In contrast, it took 10−30 min for the aerosols to be removed by ventilation alone in the four rooms with the highest airflow rates (ACHvent 15–32). Aerosols particle could not be completely removed within the 30 min observation period by ventilation alone if ACHvent is below 15. Considering that the PAC is all the more effective in rooms with low ventilations rates, we think that it is prudent to consider adding a PAC with a HEPA filter into a dental treatment room if the mechanical ventilation rate is low or unknown. The PAC used in this study retailed for about $229 and appeared very effective in aerosol removal in dental settings. Though the magnitude of added benefit from the PAC diminished in rooms with very high ventilation rates, it nonetheless further increased the rates of aerosol particle removals in these rooms as compared to ventilation alone.
Dental droplets or aerosols, energized by aerodynamic forces from high speed rotations or ultrasonic vibrations of dental instruments, may be spattered into air spaces in dental treatment rooms and cause contaminations of the indoor environment [38,39]. The distinction between droplets and aerosols has traditionally been described by a matter of physical size - those greater than 5 μm in diameters are droplets that fall rapidly to the ground and travel less than 2 m; and those smaller than 5 μm are droplet nuclei or aerosols that suspend in the air for an extended period time and may travel greater than 2 m in distance [42,43]. Though such distinction by size alone lacks scientific rationale as many droplets greater than 5 μm in diameters may also remain airborne for minutes or longer [44,45], it is generally agreed that the smaller the aerosol particles in sizes, the longer times it takes for them to fall into the ground, and therefore the higher the risk for them to be inhaled by individuals within the environment. In dentistry, it is those aerosol particles that reach the breathing zone of the oral health providers post true health risks as they may be inhaled by dentists or their staffs during the treatments if effective PPE was not properly used. Though low and high-volume vacuum evacuations are effective measures of droplet and aerosol controls and may reduce microbial and particulate contaminations by more than 90 % [35,[46], [47], [48], [49]], some aerosols may inevitably escape into the air spaces surrounding the DCPs and their patient. Microbial contamination and aerosol simulation studies have demonstrated that it is possible for a small fraction of the aerosols to reach the breathing zone of the DCPs [15]. Our pilot testing in dental treatment rooms also showed that aerosol particles, especially those under 1 μm in diameters, increase transiently near the breathing zones of dentists and dental assistants at approximately 18 in. away from the patient’s head following aerosol-generating procedures with high and low speed handpieces. Though the health consequences of these fine aerosol particles in dental settings remain to be elucidated, the US CDC has advised that dental professionals limit aerosol-generating procedures and take measures to improve ventilation and air filtration during the COVID-19 pandemic [4]. As aerosol particles in this size range may be suspended in the air for an extended period of time, ventilation and air filtration are the most and practical and effective ways to remove them from the indoor environment [23,50,51].
Though numerous studies have assessed the effectiveness of intra- and extra-oral vacuum evacuation devices on spatter and droplet controls by measuring microbial contaminations or particle depositions in dental treatment rooms [52,53], information is lacking on the effectiveness of mechanical ventilation and air filtration on aerosol particle removal in dental settings. The present study represents the first step towards the understanding of potential roles of engineering control through dilution ventilation and air filtration in reducing or eliminating aerosols in dental treatment rooms. We found that dental treatment rooms in the same facility and on the same air handling system had vastly different mechanical ventilation rates, with those having very low ventilation rates generally located in the distal ends of the air supply duct system. Some rooms with very high ventilation rates are dedicated as rooms for nitrous oxide sedation and have additional exhaust air returns. Rooms with poor ventilation may increase aerosol particle accumulations and increase the risks of airborne pathogen transmission [54,55]. The findings of the present study support the addition of PAC with a HEPA filter in these rooms to facilitate aerosol removals.
A previous study using computer fluidic dynamics modeling indicated that the location of the PAC relative to the patient and the DCPs was an important factor in the effectiveness of the PAC for aerosol removal [15]. A PAC placed behind the dentist would have increased the amount of aerosols reaching the breathing zone of the dentist due to an unfavorable airflow direction with the PAC turned on. The ideal location for the PAC is close to the patient’s head but away from the care providers. Considering that dentist and dental assistant occupy the areas on both sides of the patient and there are usually cabinets and equipment at the 12 o’clock position right behind the patient’s head, we chose to place the PAC at the far end of the dental chair at about 2 feet away from the footrest. Due to limited spaces in most of the dental rooms studied, we were not able to test if PAC placement in a different location would have affected its effectiveness in aerosol removal. Furthermore, the locations of air supply and exhaust vents are different in each of the room studied, which rendered it impossible to take into account of airflow directions and patterns by adjusting the relative location of the PAC in the current study setting. Future studies should consider these factors as they may also affect airborne pathogen removal in health care facilities [56,57].
Many dental equipment generate sounds and significantly increase the noise levels in the working environment for DCPs [58,59], which may contribute to hearing loss in dental professionals [60,61]. The high-volume evacuation commonly used during dental procedures raised the noise level to 77 dB [35,59]. Additional extra-oral and/or intra-oral suction devices elevated the noise levels to an average of 80–90 dB [35]. The PAC used in this study increased the noise level by an average of 14 dB. This level of increase may be significant if the background noise level is already high. The US Occupational Safety and Health Administration (OSHA) requires employers to implement a hearing conservation program when noise exposure is at or above 85 dB on average for an 8 -h period [62]. Therefore, it is important to assess the noise levels when considering additional equipment for aerosol control in dental offices.
Though the findings of the present study support the utility of PAC with a HEPA filter in aerosol removal from dental treatment rooms, its effects on risk of infectious disease transmission remain to be elucidated. Large scale prospective studies are eventually needed to assess the health impacts of improved ventilation rate and air filtration. It is also important to point out that HEPA filters are ineffective in removing gaseous contaminants such as carbon monoxide, carbon dioxide, nitrous oxide and volatile organic compounds [63]. Adequate ventilation with outside air remains to be important for improving air quality in dental treatment rooms. In addition, HEPA filters may require prudent maintenance and timely replacement when indicated [64].
The experiments on aerosol removal were limited to the single rooms with closed doors in the present study. The findings discussed above may therefore not be applicable to dental treatment spaces without doors or open bay clinics with multiple dental chairs as found in many dental school clinics. The effects of ventilation and air filtration on aerosol removals in open spaces or connected rooms deserve further investigation as a larger number of DCPs and patients is usually involved in this type of settings. To this end, collaborations among DCPs, aerosol scientists, building engineers and environmental and occupational health experts are essential. In the era of frequent, novel infectious disease pandemics, such multidisciplinary efforts are especially important for minimizing the risks to DCPS and their patients through effective engineering controls of dental droplets and aerosols.
5. Conclusions
Mechanical ventilation rates varied greatly among dental treatment rooms in a dental clinic. Aerosol accumulation occurred in rooms with poor ventilation. Addition of PAC with a HEPA filter significantly reduced aerosol accumulation and accelerated aerosol removal, especially in rooms with very low ventilation rates. PAC increased the noise levels in dental treatment rooms when on high setting.
CRediT authorship contribution statement
Yan-Fang Ren: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft. Qirong Huang: Methodology, Investigation, Formal analysis, Writing - review & editing. Tamer Marzouk: Methodology, Investigation, Writing - review & editing. Ray Richard: Methodology, Writing - review & editing. Karen Pembroke: Methodology, Writing - review & editing. Pat Martone: Methodology, Writing - review & editing. Tom Venner: Methodology, Resources. Hans Malmstrom: Conceptualization, Writing - review & editing. Eli Eliav: Conceptualization, Resources, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors declare no conflict of interests associated with this paper. We thank building engineers Dan Mateer and Kevin McLellan at Johnson Control Inc. for their technical expertise and assistance with the present study. This study is funded in part by the Eastman Institute for Oral Health Foundation, Rochester, New York.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jdent.2020.103576.
Appendix A. Supplementary data
The following is supplementary data to this article:
References
- 1.Harte J.A. Standard and transmission-based precautions: an update for dentistry. J. Am. Dent. Assoc. 2010;141(5):572–581. doi: 10.14219/jada.archive.2010.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC . 2020. How COVID-19 Spreads.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html [Google Scholar]
- 3.American Dental Association . 2020. ADA Continued Guidance for Minimizing Risk of COVID-19 Transmission.https://www.ada.org/en/press-room/news-releases/2020-archives/may/as-dental-practices-resume-operations-ada-offers-continued-guidance?utm_source=cpsorg&utm_medium=cpsalertbar&utm_content=cv-continuedguidance-statement&utm_campaign=covid-19 [Google Scholar]
- 4.CDC . 2020. Guidance for Dental Settings - Interim Infection Prevention and Control Guidance for Dental Settings During the COVID-19 Pandemic.https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html [Google Scholar]
- 5.Estrich C.G., Mikkelsen M., Morrissey R., Geisinger M.L., Ioannidou E., Vujicic M., Araujo M.W.B. Estimating COVID-19 prevalence and infection control practices among US dentists. J. Am. Dent. Assoc. 2020;151(11):815–824. doi: 10.1016/j.adaj.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng L., Ma B., Cheng Y., Bian Z. Epidemiological investigation of OHCWs with COVID-19. J. Dent. Res. 2020 doi: 10.1177/0022034520962087. 0(0) 0022034520962087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., Ma H., Chen W., Lin Y., Zheng Y., Wang J., Hu Z., Yi Y., Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Wang A., Yi B., Ding K., Wang H., Wang J., Shi H., Wang S., Xu G. The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chin. J. Epidemiol. 2020;41 doi: 10.3760/cma.j.cn112338-20200304-00251. (0) 0-0. [DOI] [PubMed] [Google Scholar]
- 9.Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., Usmani M., Shankar S.N., Mohamed K., Eiguren-Fernandez A., Stephenson C.J., Alam M.M., Elbadry M.A., Loeb J.C., Subramaniam K., Waltzek T.B., Cherabuddi K., Morris J.G., Jr., Wu C.-Y. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., Shen J., Lv Y., Pan L., Ding P., Wang X., Wang Y., MacIntyre C.R., Shi X. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106039. 106039-106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., Kurnitski J., Marr L.C., Morawska L., Noakes C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., Tanabe S.I., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.E. National Academies of Sciences . The National Academies Press; Washington, DC: 2020. Medicine, Airborne Transmission of SARS-CoV-2: Proceedings of a Workshop—in Brief. [PubMed] [Google Scholar]
- 14.Pelleu G.B., Jr., Shreve W.B., Wachtel L.W. Reduction of microbial concentration in the air of dental operating rooms. I. High-efficiency particulate air filters. J. Dent. Res. 1970;49(2):315–319. doi: 10.1177/00220345700490022001. [DOI] [PubMed] [Google Scholar]
- 15.Chen C., Zhao B., Cui W., Dong L., An N., Ouyang X. The effectiveness of an air cleaner in controlling droplet/aerosol particle dispersion emitted from a patient’s mouth in the indoor environment of dental clinics. J. R. Soc. Interface. 2010;7(48):1105–1118. doi: 10.1098/rsif.2009.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.New York State Department of Health . L. Center for Health Care Facility Planning, and Finance. Division of Palnning and Licensure. Bureau of Architecture & Engineering Review, (Ed.) 2017. Design submission requirements: dental facilities; pp. 1–5. [Google Scholar]
- 17.Ninomura P., Bartley J. New ventilation guidelines for health-care facilities. ASHRAE J. 2001;43(6):29–32. [Google Scholar]
- 18.Ninomura P.T., Byrns G. Dental ventilation theory and applications. ASHRAE J. 1998;40(2):48–52. [Google Scholar]
- 19.Chinn R.Y.W., Sehulster L. 2003. Guidelines for Environmental Infection Control in Health-care Facilities; Recommendations of CDC and Healthcare Infection Control Practices Advisory Committee (HICPAC) [PubMed] [Google Scholar]
- 20.Waring M.S., Siegel J.A., Corsi R.L. Ultrafine particle removal and generation by portable air cleaners. Atmos. Environ. 2008;42(20):5003–5014. [Google Scholar]
- 21.Offermann F.J., Sextro R.G., Fisk W.J., Grimsrud D.T., Nazaroff W.W., Nero A.V., Revzan K.L., Yater J. Control of respirable particles in indoor air with portable air cleaners. Atmos. Environ. 1967;19(11):1761–1771. (1985) [Google Scholar]
- 22.Ward M., Siegel J.A., Corsi R.L. The effectiveness of stand alone air cleaners for shelter-in-place. Indoor Air. 2005;15(2):127–134. doi: 10.1111/j.1600-0668.2004.00326.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Leiden S., Lobascio C., Nazaroff W.W., Macher J.M. Effectiveness of in-room air filtration and dilution ventilation for tuberculosis infection control. J. Air Waste Manag. Assoc. 1996;46(9):869–882. doi: 10.1080/10473289.1996.10467523. [DOI] [PubMed] [Google Scholar]
- 24.Shaughnessy R.J., Sextro R.G. What is an effective portable air cleaning device? A review. J. Occup. Environ. Hyg. 2006;3(4):169–181. doi: 10.1080/15459620600580129. [DOI] [PubMed] [Google Scholar]
- 25.United States Environmental Protection Agency; 2020. Indoor Air Quality (IAQ): What Is a MERV Rating?https://www.epa.gov/indoor-air-quality-iaq/what-merv-rating-1 (Accessed Nov. 22, 2020. [Google Scholar]
- 26.Noh K.-C., Hwang J. The effect of ventilation rate and filter performance on indoor particle concentration and fan power consumption in a residential housing unit. Indoor Built Environ. 2010;19(4):444–452. [Google Scholar]
- 27.Institutional Consultation Services . Francis J Curry National Tuberculosis Center; 1999. What Does Air Change Mean? Isolation Rooms: Design, Assessment, and Upgrade. pp. 43–44. [Google Scholar]
- 28.Cheng Y.S., Bechtold W.E., Yu C.C., Hung I.F. Incense smoke: characterization and dynamics in indoor environments. Aerosol Sci. Technol. 1995;23(3):271–281. [Google Scholar]
- 29.Sotiriou M., Ferguson S.F., Davey M., Wolfson J.M., Demokritou P., Lawrence J., Sax S.N., Koutrakis P. Measurement of particle concentrations in a dental office. Environ. Monit. Assess. 2007;137(1):351. doi: 10.1007/s10661-007-9770-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu M.-H., Chen C.-T., Chuang L.-C., Lin W.-M., Wan G.-H. Removal efficiency of central vacuum system and protective masks to suspended particles from dental treatment. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0225644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polednik B. Aerosol and bioaerosol particles in a dental office. Environ. Res. 2014;134:405–409. doi: 10.1016/j.envres.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Perry J.L., Agui J.H., Vijayakimar R. NASA Science and Technical Information NASA Marshall Space Flight Center; Huntsville, AL, United States: 2016. Submicron and Nanoparticulate Matter Removal by HEPA-Rated Media Filters and Packed Beds of Granular Materials. [Google Scholar]
- 33.AHAM . 2014. ANSI/AHAM AC-1: Method for Measuring the Performance of Portable Household Electric Room Air Cleaners - Understanding its Scope and the Related AHAM Industry Certification Program.https://ahamverifide.org/wp-content/uploads/2019/07/Scope-of-Air-Cleaner-Certification.pdf Available at. [Google Scholar]
- 34.Medical Advisory Secretariat Air cleaning technologies: an evidence-based analysis. Ont. Health Technol. Assess. Ser. 2005;5(17):1–52. [PMC free article] [PubMed] [Google Scholar]
- 35.Ravenel T.D., Kessler R., Comisi J.C., Kelly A., Renne W.G., Teich S.T. Evaluation of the spatter-reduction effectiveness and aerosol containment of eight dry-field isolation techniques. Quintessence Int. 2020;51(8):660–670. doi: 10.3290/j.qi.a44919. [DOI] [PubMed] [Google Scholar]
- 36.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian H., Zheng X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J. Thorac. Dis. 2018:S2295–S2304. doi: 10.21037/jtd.2018.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrel S.K., Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J. Am. Dent. Assoc. 2004;135(4):429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zemouri C., de Soet H., Crielaard W., Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian H., Li Y., Sun H., Nielsen P.V., Huang X., Zheng X. Particle removal efficiency of the portable HEPA air cleaner in a simulated hospital ward. Build. Simul. 2010;3(3):215–224. [Google Scholar]
- 41.Honeywell International Inc.; 2020. Honeywell True HEPA 50250: Description.https://www.honeywellstore.com/store/products/honeywell-50250-true-hepa-germ-fighting-allergen-reducer-air-purifier.htm (Accessed Nov. 20, 2020) [Google Scholar]
- 42.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109819. 109819-109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., the Healthcare Infection Control Practices Advisory Committee . Part I-Review of Scientific Data Regarding Transmission of Infectious Agents in Healthcare Settings; 2007. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8(9):914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gralton J., Tovey E., McLaws M.L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62(1):1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bentley C.D., Burkhart N.W., Crawford J.J. Evaluating spatter and aerosol contamination during dental procedures. J. Am. Dent. Assoc. 1994;125(5):579–584. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 47.Harrel S.K., Barnes J.B., Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J. Periodontol. 1996;67(1):28–32. doi: 10.1902/jop.1996.67.1.28. [DOI] [PubMed] [Google Scholar]
- 48.Jacks M.E. A laboratory comparison of evacuation devices on aerosol reduction. J. Dent. Hyg. 2002;76(3):202–206. [PubMed] [Google Scholar]
- 49.Micik R.E., Miller R.L., Mazzarella M.A., Ryge G. Studies on dental aerobiology: I. Bacterial aerosols generated during dental procedures. J. Dent. Res. 1969;48(1):49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 50.Aliabadi A.A., Rogak S.N., Bartlett K.H., Green S.I. Preventing airborne disease transmission: review of methods for ventilation design in health care facilities. Adv. Prev. Med. 2011;2011:124064. doi: 10.4061/2011/124064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciuzas D. Indoor air quality management by combined ventilation and air cleaning: an experimental study. Aerosol Air Qual. Res. 2016;16(10):2550–2559. [Google Scholar]
- 52.Koletsi D., Belibasakis G.N., Eliades T. Interventions to reduce aerosolized microbes in dental practice: a systematic review with network meta-analysis of randomized controlled trials. J. Dent. Res. 2020;99(11):1228–1238. doi: 10.1177/0022034520943574. [DOI] [PubMed] [Google Scholar]
- 53.Kumbargere Nagraj S., Eachempati P., Paisi M., Nasser M., Sivaramakrishnan G., Verbeek J.H. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst. Rev. 2020;10 doi: 10.1002/14651858.CD013686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolashikov Z.D., Melikov A.K. Methods for air cleaning and protection of building occupants from airborne pathogens. Build. Environ. 2009;44(7):1378–1385. doi: 10.1016/j.buildenv.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen P.V. Control of airborne infectious diseases in ventilated spaces. J. R. Soc. Interface. 2009;6(Suppl 6):S747–55. doi: 10.1098/rsif.2009.0228.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Memarzadeh F., Xu W. Role of air changes per hour (ACH) in possible transmission of airborne infections. Build. Simul. 2012;5(1):15–28. doi: 10.1007/s12273-011-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mousavi E.S., Grosskopf K.R. Ventilation rates and airflow pathways in patient rooms: a case study of bioaerosol containment and removal. Ann. Occup. Hyg. 2015;59(9):1190–1199. doi: 10.1093/annhyg/mev048. [DOI] [PubMed] [Google Scholar]
- 58.Burk A., Neitzel R.L. An exploratory study of noise exposures in educational and private dental clinics. J. Occup. Environ. Hyg. 2016;13(10):741–749. doi: 10.1080/15459624.2016.1177646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorainen E., Rytkönen E. High-frequency noise in dentistry. AIHA J. (Fairfax, Va) 2002;63(2):231–233. doi: 10.1080/15428110208984709. [DOI] [PubMed] [Google Scholar]
- 60.Al-Omoush S.A., Abdul-Baqi K.J., Zuriekat M., Alsoleihat F., Elmanaseer W.R., Jamani K.D. Assessment of occupational noise-related hearing impairment among dental health personnel. J. Occup. Health. 2020;62(1) doi: 10.1002/1348-9585.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Rawi N.H., Al Nuaimi A.S., Sadiqi A., Azaiah E., Ezzeddine D., Ghunaim Q., Abbas Z. Occupational noise-induced hearing loss among dental professionals. Quintessence Int. 2019;50(3):245–250. doi: 10.3290/j.qi.a41907. [DOI] [PubMed] [Google Scholar]
- 62.OSHA, Occupational Noise Exposure. https://www.osha.gov/noise. (Accessed Nov. 22, 2020 2020).
- 63.Guieysse B., Hort C., Platel V., Munoz R., Ondarts M., Revah S. Biological treatment of indoor air for VOC removal: potential and challenges. Biotechnol. Adv. 2008;26(5):398–410. doi: 10.1016/j.biotechadv.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 64.White E. HEPA and ULPA filters. J. Validat. Technol. 2009;15(3):48–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.