Abstract
Background and study aims When capsule endoscopy (CE) detects a small bowel (SB) target lesion that may be manageable with enteroscopy, the selection of the insertion route is critical. Time- and progression-based CE indices have been proposed for localization of SB lesions. This systematic review analysed the role of CE transit indicators in choosing the insertion route for double-balloon enteroscopy (DBE).
Methods A comprehensive literature search identified papers assessing the role of CE on the choice of the route selection for DBE. Data on CE, criteria for route selection, and DBE success parameters were retrieved and analyzed according to the PRISMA statement. Risk of bias was assessed through the STROBE assessment. The primary outcome evaluated was DBE success rate in reaching a SB lesion, measured as the ratio of positive initial DBE to the number of total DBE.
Results Seven studies including 262 CEs requiring subsequent DBE were selected. Six studies used time-based indices and one used the PillCam Progress indicator. SB lesions were identified and insertion route was selected according to a specific cut-off, using fixed landmarks for defining SB transit except for one study in which the mouth-cecum transit was considered. DBE success rate was high in all studies, ranging from 78.3 % to 100 %. Six of seven studies were high quality.
Conclusions The precise localization of SB lesions remains an open issue, and larger studies are required to determine the most accurate index for selecting the DBE insertion route. In the future, 3 D localization technologies and tracking systems will be essential to accomplish this tricky task.
Introduction
Since its widespread adoption in clinical practice, capsule endoscopy (CE) has turned the tables in the field of accurate diagnosis and management for the majority of small bowel (SB) pathologies. Nonetheless, CE remains an endoscopic modality devoid of any interventional capabilities. CE is used, therefore, as “scout” for SB lesions. To date, several device-assisted enteroscopy (DAE) systems, such as double-balloon enteroscopy (DBE), single-balloon enteroscopy (SBE), and spiral enteroscopy (SE) are available 1 2 . DAE can be performed either through the oral or the anal orifice. The choice of anatomical route for instrument insertion depends on the lesion location, which is often based on CE data 2 3 4 . In terms of subsequent therapeutic endoscopy planning for patient management, a success-defining step is reaching the lesion/pathology in question. Therefore, an accurate decision about the route of enteroscopy insertion is essential to reduce diagnostic and therapeutic delays and the number and duration of necessary procedures; moreover, it is associated with greater patient compliance. The role of CE transit time and progression along the SB haved been evaluated in previous studies, but a relevant systematic review is absent.
The aim of this systematic review was to evaluate the role of time- and progression-based predictive indicators of CE in choosing the initial insertion route for DBE when a target SB lesion is detected.
Methods
Search strategy and inclusion criteria
Three authors (PCV, KSZ and WM) independently searched PubMed, Embase and Clinical Trials from database inception until 23 March 2020 in English/Italian/Polish for observational studies aiming to assess the predictive role of CE on the insertion route of DBE.
In PubMed and Embase, the search was performed using combinations of the following terms: enteroscopy, insertion, selection, route, cell selection, capsule endoscopy, capsule enteroscopy, capsule endoscope, wireless capsule enteroscopy, intestine endoscopy, double balloon enteroscopy, double balloon endoscopy, push and pull endoscopy, push and pull enteroscopy, capsocam, capsocam plus, capsocam sv1, endocapsule, imaging m2a capsule m2a (capsule endoscope), mirocam, mirocam green, mirocam mc1600, mirocam mc 2000, mirocam navi, mirocam system, omom, omom capsule endoscopy system, pillcam, pillcam colon, pillcam eso, pillcam sb ( Supplementary material ).
In ClinicalTrials.gov, we used the following search terms: capsule endoscopy, enteroscopy. The electronic search was supplemented by a manual review of the reference lists from eligible publications and relevant review papers.
Inclusion criteria were: (1) full-text articles, including case series; (2) papers describing patient(s) who underwent small bowel capsule endoscopy (SBCE) (single or double-headed) and subsequent DBE procedure (where the lesion(s) in question was identified); and (3) in CE, the lesion(s) SB position was determined by time-based ratios from defined anatomical landmarks or the "capsule progression index" or any other transit-based index that was subsequently used to determine the route of insertion of DBE.
We excluded studies in which SBE and/or SE only were used.
Data abstraction
Data on study design, risk of bias (ROB), patients, and procedures characteristics from each study were independently extracted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 5 standard by two of the co-authors (PCV and KSZ) who were blinded to each other’s input. Whenever data were missing for the review, authors were contacted for additional information via e-mails. Inconsistencies were resolved by consensus with the senior authors (MP, ER, WM, ET, AK).
The primary outcome was the success rate of DBE in reaching a target SB lesion detected by CE, according to the index (time- or progression-based) that was used to select the initial route of insertion. No comparison between the diagnostic yields of CE and DBE was performed, as only patients with positive CE (i. e. CE detection of a SB lesion) were included in the analysis. A meta-analytic approach was not feasible due to methodological limitations (i. e. nature of the studies and raw data not available for all the studies). For each study in which multiple DAE techniques were described, only data regarding DBE were extracted.
Quality assessment and risk of bias
Two authors (PCV and KSZ) independently assessed the ROB through the STROBE assessment 6 . However, items 6b, 14c, and 16 c were omitted as not applicable to the selected studies. Outcomes were expressed as a number (also expressed as the percentage relative to total score). When the number was below 60 % of the maximum number of points, we arbitrarily defined the quality as low. Results up to and over 80 % were considered high or very high quality, respectively. In case of any discrepancy, a third author (WM) was involved in the evaluation.
Results
Search Results
The initial search yielded 390 hits. A total 248 of screened studies were excluded for being duplicates or because they did not fit the inclusion criteria at title/abstract level. One (n = 1) additional article was identified via manual search during the revision of full-text papers. Altogether, 143 full-text articles were reviewed. Of those, a total of 136 papers were excluded. The primary reasons for exclusion were conference abstracts (n = 56) and no data on primary outcome (n = 60). We also excluded studies where no full text was available (n = 8); and language used was other than English/Italian/Polish (n = 5); and other than DBE technique was used(n = 5). An editorial (n = 1) and an article (n = 1) were excluded because the lesion was located outside the SB. Eventually, this approach yielded 7 (n = 7) studies (all in English) that were included in the present systematic review ( Fig. 1 ).
Fig. 1.
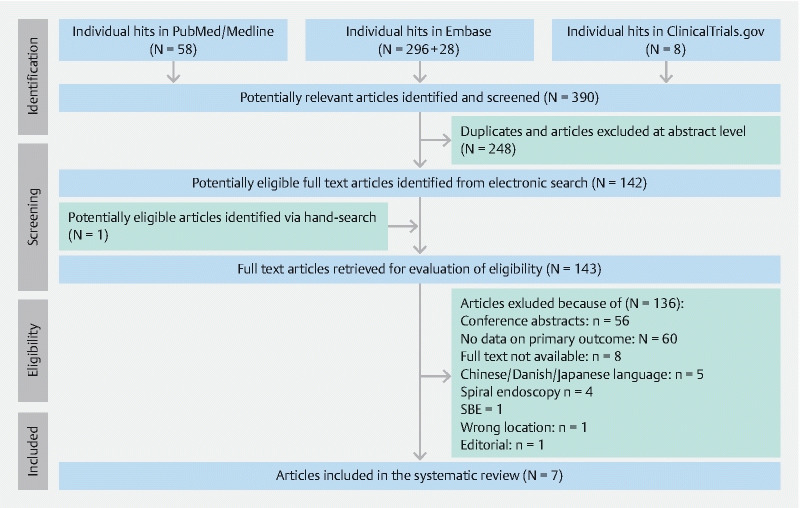
Consort diagram of this systematic review.
Study and patient characteristics
A total of seven (n = 7) single-center studies 7 8 9 10 11 12 13 , two prospective and five retrospective, were selected, comprising 460 patients ( Table 1 ). The main indication for CE evaluation was obscure gastrointestinal bleeding (OGIB). In all but two (n = 2) studies 8 12 , the aim was specifically to evaluate the utility of CE in predicting the route of insertion of subsequent DBE.
Table 1. Study and patient characteristics.
| No. | Study characteristics | Sample characteristics | Indications for CE | |||||
| Reference, year, country | Study type | Study aim | ROB (STROBE score)/Ouality | Patients, n | Age, (Mean) | Male, n (%) | Main referral indication | |
| 1 | Gay et al., 2006, France | Prospective, single center | Utility of CE in predicting DBE route of insertion | 26/Very high | 164 | 54 | 90 (54.88 %) | OGIB |
| 2 | Lin et al., 2008, Taiwan | Retrospective, single center | Evaluate combined use of CE and DBE in patients with OGIB | 14/Low | 10 | 63 | 3 (30 %) | OGIB |
| 3 | Li et al., 2009, China | Prospective, single center | Utility of CE in predicting DBE route of insertion | 21//High | 60 | 49 | 31 (51.66 %) | GIB |
| 4 | Nakamura et al., 2010, Japan | Retrospective, single center | Clarification on the accuracy of the transit time of CE to the lesion as a predictive indicator for DBE insertion route | 27/Very high | 65 | 62 | 37 (56.92 %) | OGIB |
| 5 | Chalazan et al., 2012, USA | Retrospective, single center | Determination if SBTT can be used to select the enteroscopy technique | 28/Very high | 22 | 71 | 10 (33.33 %) | OGIB |
| 6 | Maeda et al., 2015, Japan | Retrospective, single center | Demonstrate the strategy of initial CE in OGIB, followed by DBE | 24/High | 89 | 70 | 48 (53.93 %) | GIB |
| 7 | Tsuboi et al., 2019, Japan | Retrospective, single center | Usefulness of RAPID indicator in choosing the DBE insertion route | 29/Very high | 50 | 69 | 33 (66 %) | OGIB |
CE, capsule endoscopy; DBE, double balloon enteroscopy; GIB, gastrointestinal bleeding; OGIB, obscure gastrointestinal bleeding; ROB, risk of bias; SBTT, small bowel transit time.
Selection of DBE insertion route
Six studies reported a CE time-based index in the choice of the initial insertion route for DBE, whereas Tsuboi et al used a progression-based index according the integrated PillCam Progress indicator. Except from Gay et al, who considered the timeframe from the mouth to the cecum, all the authors considered the small bowel transit time (SBTT), i. e. the time from the pylorus (alternatively, first duodenal image) to the cecum (or ileocecal valve).
A cut-off of 0.5 was used by Maeda et al, Nakamura et al, and Tsuboi et al. The cut-offs used by Lin et al and Li et al were 0.66 and 0.6, respectively. Conversely, data analysis with a receiver operating characteristic curve highlighted that the best cut-offs for antegrade and retrograde approach were 0.57 and 0.74, according to Chalazan et al ( Fig. 2 ).
Fig. 2.

A schematic representation of cut-offs of time- and progression-based indices and their respective papers presented in this review.
CE procedures
CE procedures were performed with PillCam capsules (Medtronic, Dublin, Ireland), with various models according to the availability at the time of each study (M2A, SB1, SB2 or SB3 model). In total, 624 CE procedures were performed, but only in 262 a SB lesion requiring a subsequent DBE approach was detected ( Table 2 ).
Table 2. CE procedures.
| Reference | Total CE, n | Complete CE with lesions requiring DBE, n | Primary lesion (n) | Index for choosing DBE route of insertion, description | Index cut-off | PillCam Progress indicator |
| Gay et al., 2006/France | 160 | 38 | Arterovenous malformation (n = 10) | Time from ingestion to the lesion, divided by the time for arrival of the capsule into the cecum, from the moment of ingestion | A: < 0.75, R: ≥ .75 | NR |
| Lin et al., 2008/Taiwan | 11 | 9 | Angiodysplasia (n = 3), SB bleeding (n = 3) | Time from pylorus to lesion, divided by the transit time from pylorus to cecum | A: < 2/3, R: > 2/3 |
NR |
| Li et al., 2009/China | 82 | 60 | Suspected tumor (n = 22) | Time from pylorus to lesion, divided by the time from pylorus to ileocecal valve | A: ≤ 0.6, R: > 0.6 | NR |
| Nakamura et al., 2010/Japan | 172 | 46 | Angiodysplasia (n = 11) | Time from duodenal bulb to lesion, divided by the time from duodenal bulb to cecum | A: < 0.5, R: > 0.5 | NR |
| Chalazan et al., 2012/USA | 60 | 22 | Angioectasia (NR) | Time from duodenal entry to lesion, divided by the SBTT | A: < 0.57, R: > 0.74 | NR |
| Maeda et al., 2015/Japan | 89 | 37 | Dieulafoy lesion (n = 9) | Time point of the lesion compared to the SBTT | A: < 0.5, R: > 0.5 | NR |
| Tsuboi et al., 2019/Japan | 50 | 50 | Angioectasia (n = 12) | % of SBTT according to PillCam Progress Indicator | A: indicator ≤ 50 %, R: % indicator > 50 % | Yes |
A, antegrade; CE, capsule endoscopy; DBE, double-balloon enteroscopy; NR, not reported; R, retrograde; SB, small bowel; SBTT, small bowel transit time.
DBE procedures
DBE procedures were performed with EN-450 or EN-580 Fujinon enteroscopes (Fujinon, Saitama, Japan). DBE procedures were performed after a SB lesion was detected with CE. In total, 268 DBE procedures were performed; 180 with the antegrade approach. In the study by Chalazan et al, 28 DBE procedures were performed to investigate 22 preceding positive CEs. DBE success rate was calculated as the ratio of positive DBEs during the first approach to the number of total DBEs, resulting as it follows: Maeda et al, 78.3 %; Gay et al, 94.7 %; Lin et al, 100 %; Li et al, 100 %; Tsuboi et al, 96 %.
The number of positive DBEs was not directly described by Nakamura et al and Chalazan et al, but overall success parameters according to the specific cut-offs were reported by the authors: Nakamura et al, sensitivity 90 % and positive predictive value (PPV) 97 %; Chalazan et al, sensitivity 75 % and PPV 75 % (for antegrade approach), sensitivity 88 % and PPV 78 % (for retrograde approach) ( Table 3 ).
Table 3. DBE procedures.
| Reference | Model of enteroscope (Brand) | Procedures after positive CE, n | Antegrade DBE, n (lesion found) | Retrograde DBE, n (lesion found) | DBE Success Rate (%) |
| Gay et al., 2006/France | EN-450 P5/20 (Fujinon) | 38 | 30 (28) | 8 (8) | 94.7 % |
| Lin et al., 2008/Taiwan | EN-450 P5 and EN-450 T5 (Fujinon) | 9 | 9 (9) | 0 | 100 % |
| Li et al., 2009/China | EN-450 P5/20 and EN-450 P5/28 (Fujinon) | 60 | 41 (41) | 19 (19) | 100 % |
| Nakamura et al., 2010/Japan | EN-450 P5 and EN-450 T5 (Fujinon) | 46 | 27 (NR) | 19 (NR) | Sensitivity 90 %, PPV 97 % 1 |
| Chalazan et al., 2012/USA | NR (Fujinon) | 28 | 17 (NR) | 11 (NR) | A: sensitivity 75 %, PPV 75 %; R: sensitivity 88 %, PPV 78 % 1 |
| Maeda et al., 2015/Japan | EN-450 T5/w or EN-580 T (Fujinon) | 37 | 26 (21) | 11 (8) | 78.3 % |
| Tsuboi et al., 2019/Japan | EN-450 P5, EN-450 T5 or EN-580 T5 (Fujinon) | 50 | 30 (28) | 20 (20) | 96 % |
A, antegrade; DBE, double-balloon enteroscopy; NR, not reported; PPV, positive predictive value; R, retrograde.
As reported in the paper
Assessment of quality of included studies
The subjective ROB assessment by means of the STROBE tool has shown that the mean number of points was 24.14 ± 5.21 with the highest score 29 and the lowest 14. Qualitatively, all studies were considered to be of high quality with mean percentages of points respective to a maximum score of 77.8 %. Only one study was evaluated as of low quality 8 . The sum of points from STROBE assessment is presented in Table 1 . The scoring in particular domains of a tool is available in Supplementary Table 1
Discussion
CE has become a well-established technology for identifying SB pathology and is the pivotal modality in investigation pathways for midgut bleeding, Crohn’s disease, and SB polyposis syndromes 3 14 15 . Moreover, the recent COVID-19 pandemic and the mature emergence of artificial intelligence has renewed interest among healthcare providers about wire-free, distance-respecting modalities 16 17 . However, despite advances in technology 18 19 , the challenge of accurate lesion localization within the featureless structure of the SB tract remains very much alive. Currently, CE lacks the capability of providing diagnosis and biopsy/therapy in a single session 18 . Managing relevant SB findings, therefore, relies on the use of DAE modalities such as DBE, SBE and SE.
The use of proprietary software solutions to position the capsule (and consequently the lesion) in the SB lumen has changed over time with consecutive upgrades of the reading software 20 . The PillCam Progress Indicator, operating on the RAPID 6.5 and following software program versions, graphically demonstrates the progress of a capsule through the SB ( Fig. 3 ). The % CE progress represents a percentage of the entire SB images. The percentage of SB time represents a percentage of the entire SBTT. Although triangulation of radiofrequency signals 21 22 23 allows a more accurate approximation of CE position in a 3 D space, there is still a lot of uncertainty about the actual position of a lesion in the SB. Another possible solution, the odocapsule, was described in a conceptual proof-of-principle study to calculate the distance traveled by the capsule and offer accurate distance measurements for SB lesions 24 . In the majority of Western centers, expertise (and resources) are lacking for one to go straight to DAE. However, CE is now widely available and alongside lesion detection, localization is required. Ultimately, translation of CE info in clinical practice is condensed in a simple question: “Shall I take the oral or rectal approach to get to the lesion?”
Fig. 3.
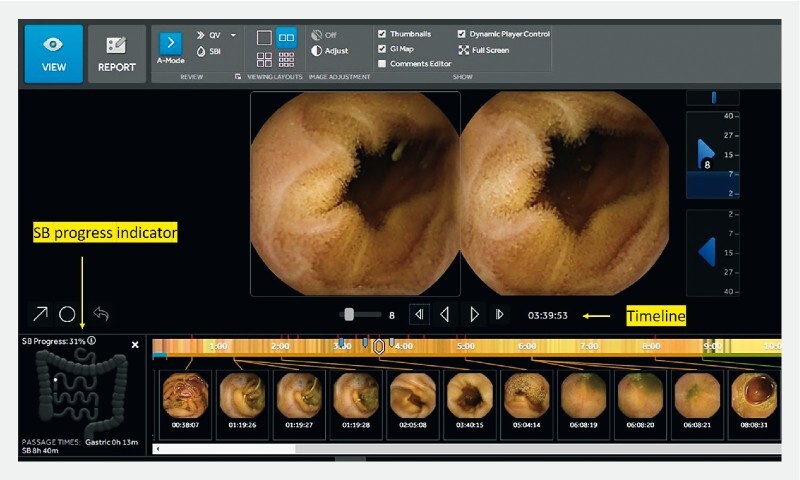
The current software for RAPID with the progress indicator.
Accurate selection of the insertion route for DBE provides session-efficient and cost-effective treatment and also allows streamlining of the post-CE treatment pathways. A recent meta-analysis showed that DBE was superior to SBE concerning complete SB visualization 25 . Another one confirmed that balloon enteroscopy and spiral can achieve a similar depth of insertion 26 . However, because DBE is anecdotally considered the best approach to providing combined antegrade and retrograde (complete) SB inspection and as available literature favors DBE, we decided to focus our systematic search on this form of deep enteroscopy as a follow-up procedure for biopsy and/or treatment of CE findings. Notwithstanding, a recent study confirmed the usefulness of CE transit index with SE 27 .
Our results confirm that the clinical query for the appropriate route of insertion remains an open topic, with attempts made over the years by several groups to provide the best index-predictor for the insertion route. However, irrespective of the index used as a decision-making tool, the successful outcomes (defined as reaching the lesion by the selected route of insertion) were consistently high. Although this may simply reflect the strengths of the DBE technique and/or the determination of individual endoscopists to provide management/therapy, there is still a group of patients in which the lesion is not reached on the first attempt and for whom another DAE (via the alternative route) needs to be performed, thus increasing costs and potential complications.
Furthermore, things can get even more complicated in some cases: what if the lesion is located in the 0.57–0–74 interval, as in the study by Chalazan et al, or it is located spot on the 0.5 of SBTT?
In the first case, as stated by the authors, a combined DBE approach may be necessary, due to the lower potential yield of the first approach, as well as alternative solutions (i. e. surgical or radiological). In the latter case, Nakamura et al suggest performing an initial antegrade enteroscopy: The half point of SB is usually more proximal to the middle position of SBTT, due to variable speed of CE in the intestine. In such cases, we recommend performing antegrade enteroscopy upfront: it is generally associated with a higher diagnostic yield 28 29 and it is favored for technical reasons 2 .
Essentially, our systematic review confirms that although 3 D localization is considered essential for future remote therapeutic platforms, current CE software tools can successfully work on the basis of SB transit time-based indices that relie on excluding variability related to esophageal transit and gastric emptying times. Despite being retrospective, the included studies were of high quality but heterogeneous in terms of results reporting. Furthermore, the proposed cut-offs slightly differ in terms of SB percentage and in terms of performance. Attempts were made to contact all corresponding authors to obtain raw data for a more in-depth synthesis/analysis of the results; however, as the majority of studies are more than a decade old, the responses were understandably limited.
This study has some limitations. It was not possible to perform a meta-analysis due to absence of data concerning time measurements of CE procedures. The cohorts were relatively small. Analysis of management after negative DBE was not performed, as data were not always reported.
Conclusion
The localization of SB lesions, which is important for patient management, still remains an open issue. Prospective large studies are needed to verify which time-based index is accurate for predicting lesion location and which route of insertion is preferable because no strong recommendation can be made based on current evidence and currently, each center is advised to the use the route with which they feel more comfortable ( Fig. 2 ). New software and hardware features, such as magnetic capsule tracking systems, may be helpful in the near future for better locating SB lesions.
Acknowledgments
The authors are grateful to all corresponding authors and specifically, Dr. Masanao Nakamura, Dr. Arthur J. Kaffes, and Professor Elizabeth Rajan.
Footnotes
Competing interests Dr. Emanuele Rondonotti received speaker honoraria from Fujifilm co.
Supplementary material :
References
- 1.Pennazio M, Venezia L, Cortegoso Valdivia P et al. Device-assisted enteroscopy: An update on techniques, clinical indications and safety. Dig Liver Dis. 2019;51:934–943. doi: 10.1016/j.dld.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Rondonotti E, Spada C, Adler S et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2018;50:423–446. doi: 10.1055/a-0576-0566. [DOI] [PubMed] [Google Scholar]
- 3.Pennazio M, Spada C, Eliakim R et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352–386. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Cuadrado Robles E, Pinho R, González-Suárez B et al. Small bowel enteroscopy - A joint clinical guideline by the Spanish and Portuguese small-bowel study groups. Rev Esp Enferm Dig. 2020;112:309–318. doi: 10.17235/reed.2020.7020/2020. [DOI] [PubMed] [Google Scholar]
- 5.Liberati A, Altman D G, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Vandenbroucke J P, von Elm E, Altman D G et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49–58. doi: 10.1055/s-2005-921176. [DOI] [PubMed] [Google Scholar]
- 8.Lin T-N, Su M-Y, Hsu C-M et al. Combined use of capsule endoscopy and double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Chang Gung Med J. 2008;31:450–456. [PubMed] [Google Scholar]
- 9.Li X, Chen H, Dai J et al. Predictive role of capsule endoscopy on the insertion route of double-balloon enteroscopy. Endoscopy. 2009;41:762–766. doi: 10.1055/s-0029-1215009. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M, Ohmiya N, Shirai O et al. Route selection for double-balloon endoscopy, based on capsule transit time, in obscure gastrointestinal bleeding. J Gastroenterol. 2010;45:592–599. doi: 10.1007/s00535-010-0202-z. [DOI] [PubMed] [Google Scholar]
- 11.Chalazan B, Gostout C J, Song L M et al. Use of capsule small bowel transit time to determine the optimal enteroscopy approach. Gastroenterology Res. 2012;5:39–44. doi: 10.4021/gr404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda Y, Moribata K, Deguchi H et al. Video capsule endoscopy as the initial examination for overt obscure gastrointestinal bleeding can efficiently identify patients who require double-balloon enteroscopy. BMC Gastroenterol. 2015;15:132. doi: 10.1186/s12876-015-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuboi A, Oka S, Tanaka S et al. The clinical usefulness of the PillCam progress indicator for route selection in double balloon endoscopy. Intern Med. 2019;58:1375–1381. doi: 10.2169/internalmedicine.2043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khashab M A, Pasha S F, Muthusamy V R et al. The role of deep enteroscopy in the management of small-bowel disorders. Gastrointest Endosc. 2015;82:600–607. doi: 10.1016/j.gie.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto H, Ogata H, Matsumoto T et al. Clinical Practice Guideline for Enteroscopy. Dig Endosc. 2017;29:519–546. doi: 10.1111/den.12883. [DOI] [PubMed] [Google Scholar]
- 16.Koulaouzidis A, Marlicz W, Wenzek H. Returning to digestive endoscopy normality will be slow and must include novelty and telemedicine. Dig Liver Dis. 2020 doi: 10.1016/j.dld.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koulaouzidis G, Charisopoulou D, Wojakowski W et al. Telemedicine in cardiology in the time of coronavirus disease 2019: a friend that everybody needs. Pol Arch Intern Med. 2020;130:559–561. doi: 10.20452/pamw.15432. [DOI] [PubMed] [Google Scholar]
- 18.Vasilakakis M, Koulaouzidis A, Yung D E et al. Follow-up on: optimizing lesion detection in small bowel capsule endoscopy and beyond: from present problems to future solutions. Expert Rev Gastroenterol Hepatol. 2019;13:129–141. doi: 10.1080/17474124.2019.1553616. [DOI] [PubMed] [Google Scholar]
- 19.Steiger C, Abramson A, Nadeau P et al. Ingestible electronics for diagnostics and therapy. Nat Rev Mater. 2019;4:83–98. [Google Scholar]
- 20.Eliakim R, Spada C, Lapidus A et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease - feasibility study. Endosc Int Open. 2018;06:E1235–E1246. doi: 10.1055/a-0677-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marya N B, Jawaid S, Foley A et al. A randomized controlled trial comparing efficacy of early video capsule endoscopy with standard of care in the approach to nonhematemesis GI bleeding (with videos) Gastrointest Endosc. 2019;89:33–430000. doi: 10.1016/j.gie.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iakovidis D K, Koulaouzidis A. Software for enhanced video capsule endoscopy: challenges for essential progress. Nat Rev Gastroenterol Hepatol. 2015;12:172–186. doi: 10.1038/nrgastro.2015.13. [DOI] [PubMed] [Google Scholar]
- 23.Ciuti G, Caliò R, Camboni D et al. Frontiers of robotic endoscopic capsules: a review. J Micro-Bio Robot. 2016;11:1–18. doi: 10.1007/s12213-016-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karargyris A, Koulaouzidis A. OdoCapsule: next-generation wireless capsule endoscopy with accurate lesion localization and video stabilization capabilities. IEEE Trans Biomed Eng. 2015;62:352–360. doi: 10.1109/TBME.2014.2352493. [DOI] [PubMed] [Google Scholar]
- 25.Wadhwa V, Sethi S, Tewani S et al. A meta-analysis on efficacy and safety: single-balloon vs. double-balloon enteroscopy. Gastroenterol Rep (Oxf) 2015;3:148–155. doi: 10.1093/gastro/gov003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baniya R, Upadhaya S, Subedi S C et al. Balloon enteroscopy versus spiral enteroscopy for small-bowel disorders: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:997–1005. doi: 10.1016/j.gie.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Mandaliya R, Korenblit J, O’Hare B et al. Spiral enteroscopy utilizing capsule location index for achieving high diagnostic and therapeutic yield. Diagn Ther Endosc. 2015;2015:1–7. doi: 10.1155/2015/793516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanaka M, Navaneethan U, Kosuru B et al. Antegrade is more effective than retrograde enteroscopy for evaluation and management of suspected small bowel disease. Clin Gastroenterol Hepatol. 2012;10:910–916. doi: 10.1016/j.cgh.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Davie M, Yung D E, Douglas S et al. Mapping the distribution of small bowel angioectasias. Scand J Gastroenterol. 2019;54:597–602. doi: 10.1080/00365521.2019.1608293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


