Abstract
SARS-CoV-2 has caused a global health crisis and mass vaccination programmes provide the best opportunity for controlling transmission and protecting populations. Despite the impressive clinical trial results of the BNT162b2 (Pfizer/BioNTech), ChAdOx1 nCoV-19 (Oxford/AstraZeneca), and mRNA-1273 (Moderna) vaccines, important unanswered questions remain, especially in patients with pre-existing conditions. In this position statement endorsed by the British Society of Gastroenterology Inflammatory Bowel Disease (IBD) section and IBD Clinical Research Group, we consider SARS-CoV-2 vaccination strategy in patients with IBD. The risks of SARS-CoV-2 vaccination are anticipated to be very low, and we strongly support SARS-CoV-2 vaccination in patients with IBD. Based on data from previous studies with other vaccines, there are conceptual concerns that protective immune responses to SARS-CoV-2 vaccination may be diminished in some patients with IBD, such as those taking anti-TNF drugs. However, the benefits of vaccination, even in patients treated with anti-TNF drugs, are likely to outweigh these theoretical concerns. Key areas for further research are discussed, including vaccine hesitancy and its effect in the IBD community, the effect of immunosuppression on vaccine efficacy, and the search for predictive biomarkers of vaccine success.
Introduction
The COVID-19 pandemic is caused by a novel RNA coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1, 2 SARS-CoV-2 causes life-threatening pneumonia, acute respiratory distress syndrome, and multi-organ failure,1, 2 and has been responsible for a global health emergency. Effective treatment and prevention strategies are urgently needed. Vaccination is a key strategy to protect the health of the world's population from COVID-19 and is likely to be especially important in high-risk individuals, such as those with pre-existing health conditions.3 Without a vaccine, the WHO estimates that 80% of the world population will eventually become infected by this virus.
Inflammatory bowel disease (IBD), comprising Crohn's disease and ulcerative colitis, is a typical immune-mediated inflammatory disease, estimated to affect 620 000 people in the UK, and its incidence continues to increase globally.4, 5 As with other immune-mediated inflammatory diseases, patients with IBD may require immunosuppressive drugs, such as high-dose corticosteroids (≥20 mg prednisolone or equivalent), immunomodulators (thiopurines, methotrexate, and calcineurin inhibitors), anti-cytokine therapies (including anti-TNF and anti-IL-12p40 drugs), anti-integrin therapies (vedolizumab), and small-molecule inhibitors of signalling (tofacitinib), which could leave them susceptible to infection.6, 7 Concerns about the health of patients with immune-mediated inflammatory diseases during the COVID-19 pandemic has led to the introduction of radical and unprecedented health policies, including mandatory prolonged physical distancing measures, such as shielding. However, the risks associated with immunosuppression are not limited to increased susceptibility to infection. Immunosuppressive drugs may reduce the effectiveness of some vaccines, which could have major implications for the safety of immunosuppressed patients in the COVID-19 era.
Key messages.
-
1
We strongly support SARS-CoV-2 vaccination for patients with inflammatory bowel disease (IBD)
-
2
The risks of SARS-CoV-2 vaccination in patients with IBD are anticipated to be very low
-
3
In patients with IBD taking immunosuppressive drugs, including biologics and small-molecule inhibitors, the key concerns are related to the theoretical risk of suboptimal vaccine responses rather than vaccine side-effects
-
4
We recommend that patients with IBD accept whichever approved SARS-CoV-2 vaccination is offered to them, in accordance with UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency (MHRA) guidance
-
5
It is important that patients with IBD are offered consistent and unbiased advice. This will be disseminated through the British Society of Gastroenterology and Crohn's & Colitis UK
SARS-CoV-2 vaccines are an important opportunity to suppress viral transmission and protect individual patients from COVID-19. Several SARS-CoV-2 vaccines are in advanced clinical development, and currently there are three that are approved in the UK (table ; figure 1 ). It is likely that additional vaccines will become available in the future. The BNT162b2 vaccine (Pfizer/BioNTech),8 the ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca),9 and the mRNA-1273 vaccine (Moderna)10 have been given authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency (MHRA) under Regulation 174 of the Human Medicine Regulations 2012.11, 12, 13 A timeline of SARS-CoV-2 vaccine development is shown in the appendix (pp 2–3).
Table.
Overview of published phase 3 SARS-CoV-2 vaccination studies
| Pfizer/BioNTech8 | Oxford/AstraZeneca9 | Moderna10 | |
|---|---|---|---|
| Name | BNT162b2 | ChAdOx1 nCoV-19 | mRNA-1273 |
| Dosing schedule | 2 doses, 21 days apart* | 2 doses, 28 days apart* | 2 doses, 28 days apart* |
| Mechanism | mRNA encoding a genetically modified SARS-CoV-2 spike protein | Non-replicating adenovirus vector, containing SARS-CoV-2 spike protein | mRNA encoding a genetically modified SARS-CoV-2 spike protein |
| Storage (long term) | −80°C to −60°C | +2°C to +8°C | −20°C |
| Reported efficacy | 95% | 70%† | 94·5% |
| Safety | No serious concerns. Two anaphylactoid reactions since MHRA approval and roll-out | No serious concerns | No serious concerns |
| UK MHRA approval | Emergency approval granted Dec 2, 2020 | Emergency approval granted Dec 30, 2020 | Emergency approval granted Jan 8, 2021 |
In the UK, the Joint Committee on Vaccination and Immunisation has advised that the second dose of BNT162b2 can be given between 3 and 12 weeks after the first dose and the second dose of both mRNA-1273 and ChAdOx1 nCoV-19 can be given between 4 and 12 weeks after the first dose.
Pooled data from two trials: 62% efficacy in one study and 90% in another study in which first vaccination was given at half dose.
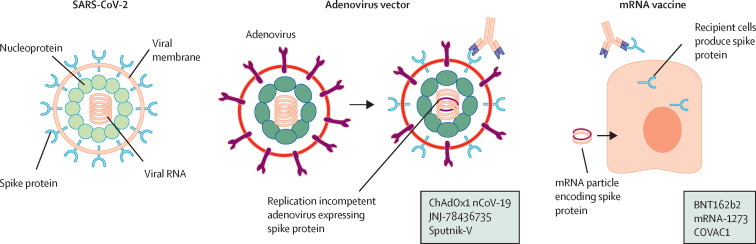
Figure 1.
SARS-CoV-2 and currently approved vaccine mechanisms
Adenovirus vector vaccines are replication-incompetent viruses that have been engineered to express the SARS-CoV-2 spike protein to which the recipient immune system responds. Examples include ChAdOx1 nCoV-19, JNJ-78436735, and Sputnik-V. mRNA vaccines are delivered in a lipid nanoparticle that is taken up by cells, which translate the mRNA to generate spike protein. Examples include BNT162b2, mRNA-1273, and COVAC1.
The effect of immunosuppression on SARS-CoV-2 vaccines
Although the MHRA does not list immunosuppression as a contraindication to the BNT162b2, ChAdOx1 nCoV-19, or mRNA-1273 vaccines, it does indicate that there is a theoretical possibility that immunosuppressive drugs could reduce the effectiveness of the vaccines, based on evidence from studies looking at the effect of immunosuppression on the immunogenicity of vaccines used for other infectious diseases (figure 2 ). Infliximab monotherapy is linked to impaired induction of protective immunity following hepatitis B virus (HBV),16 hepatitis A virus (HAV),17 pneumococcal,14, 21 and influenza18, 22, 23 vaccination, which might be more pronounced when anti-TNF therapy is combined with immunomodulators such as thiopurines or methotrexate.24, 25 Vedolizumab, which has a gut-specific mechanism of action, does not hinder HBV or influenza vaccination, but is associated with impaired antibody responses to cholera toxin, administered orally.26 There is a lack of data for some of the newer drugs used in IBD, although lessons have been learned in other immune-mediated inflammatory diseases. For instance, in psoriasis, antibody responses to pneumococcal and tetanus vaccines are preserved, and possibly even enhanced in patients treated with ustekinumab, a monoclonal antibody that blocks the p40 subunit of IL-12 and IL-23.25 In rheumatoid arthritis, tofacitinib results in diminished induction of protective immune responses to pneumococcal vaccination, but responses to influenza vaccination are maintained.27
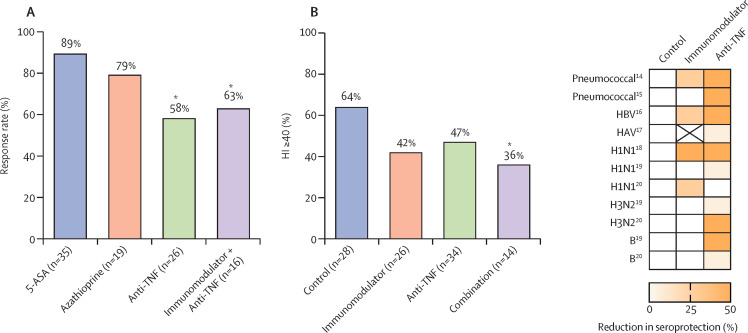
Figure 2.
Summary of studies of immunogenicity of vaccines in patients taking immunosuppressive therapies
(A) Pneumococcal vaccine response rate (response measured as a two-fold increase in anti-pneumococcal antibody titre) is reduced in patients administered anti-TNF monotherapy (58%) and immunomodulator and anti-TNF combination therapy (63%) relative to 5-ASA treated controls (89%).14 Asterisks denote statistically significant difference vs controls. (B) 2009 H1N1 influenza vaccination response rate (response measured as a ≥40% haemagluttinin inhibition [HI] titre) is attenuated in patients on immunomodulator and anti-TNF combination therapy (36%) relative to non-immunosuppressed controls (64%).18 Asterisk denotes statistically significant difference vs controls. (C) Heatmap adapted from studies of responses to vaccination in patients on immunomodulator or anti-TNF therapy showing percentage reduction in seroprotection to pneumococcal,14, 15 hepatitis B virus (HBV),16 hepatitis A virus (HAV),17 and influenza H1N1, H3N2, and B.19, 20 5=ASA=5-aminosalicylic acid.
Vaccination logistics in patients with IBD
The UK Joint Committee on Vaccination and Immunisation (JCVI) is responsible for advising which sectors of society are prioritised in national vaccination programmes. As of Dec 30, 2020, priority sequencing is based on providing protection to individuals most at risk of morbidity and mortality from COVID-19.28 Since mortality from COVID-19 increases exponentially with age, the initial JCVI prioritisation is primarily age-based. The order of priority for phase one of the vaccination programme is shown in the panel .
Panel. The Joint Committee on Vaccination and Immunisation (JCVI) phase 1 priority groups28.
-
1
Residents in a care home for older adults and their carers
-
2
All those 80 years of age and over and front-line health and social care workers
-
3
All those 75 years of age and over
-
4
All those 70 years of age and over, and clinically extremely vulnerable individuals
-
5
All those 65 years of age and over
-
6
All individuals aged 16 years to 64 years with underlying health conditions which put them at higher risk of serious disease and mortality*
-
7
All those 60 years of age and over
-
8
All those 55 years of age and over
-
9
All those 50 years of age and over
In keeping with the British Society of Gastroenterology (BSG) risk grid,29 it is anticipated that patients with IBD who are younger than 65 years and in the moderate BSG risk category will fall into category 6 (individuals aged 16–64 years with underlying health conditions that put them at higher risk of serious disease and mortality). Patients with IBD in the BSG high-risk category, including those with other comorbidities such as diabetes, chronic respiratory disorders, or morbid obesity, will fall into category 4 (clinically extremely vulnerable individuals). Irrespective of their position in the BSG risk grid, patients with IBD who are resident in care homes (category 1), are front-line health and social care workers (category 2) or are aged 75 years or older (category 3) are further prioritised.
In phase 3 trials, efficacy data for the BNT162b2 vaccine were obtained based on two doses given 21 days apart. Corresponding data for the ChAdOx1 nCoV-19 vaccine and the mRNA-1273 vaccine were based on two doses given 28 days apart. However, in the context of the rapidly worsening epidemiology of COVID-19 in the UK in late 2020 and given data indicating high efficacy from the first dose of both the BNT162b2 vaccine and the ChAdOx1 nCoV-19 vaccine, the JCVI is prioritising the delivery of the first dose of vaccine to as many eligible individuals as possible. Consequently, in the UK the second dose of vaccine may be delivered between 3 and 12 weeks after the first dose of the BNT162b2 vaccine and between 4 and 12 weeks after the first dose of the ChAdOx1 nCoV-19 vaccine.28 At the time of writing, updated JCVI advice is pending for the mRNA-1273 vaccine. JCVI further advises that the same vaccine should be used for both doses and that switching between vaccines or missing the second dose is not advised.
The main priority with timing is administration of the vaccine at the earliest opportunity. This is especially important with health-care systems under considerable strain during vaccine roll out and it is recommended that patients with IBD accept the first available vaccine appointment offered. For most patients, active IBD should not be a barrier to vaccination, although in patients with severe IBD flares or those requiring hospitalisation, it may be preferable to consider a short delay pending recovery to prevent confusion arising from incorrect attribution of vaccine-related adverse effects to complications of acute illness, and vice versa.
Maintenance immunosuppression should not be withheld for vaccination and the timing of subcutaneous or intravenous IBD medications should not delay vaccination. There is some evidence with annual influenza vaccination that the timing of anti-TNF administration does not significantly affect vaccination immunogenicity.30 High-dose systemic corticosteroids, particularly in combination with other immunosuppressants, may reduce vaccine immunogenicity, as has been observed with annual influenza vaccination.25 When possible, SARS-CoV-2 vaccination should be administered while patients are taking the lowest dose of systemic corticosteroid. These considerations should be interpreted in the context of individual cases and discussed with patients. In patients participating in IBD clinical trials, provisions for vaccination should be included in trial protocols and discussed with the sponsor.
Serology testing for SARS-CoV-2 is not necessary before administering vaccination, even in people with suspected or proven prior infection. Patients with IBD should receive both doses of SARS-CoV-2 vaccination, even if they have previously been infected with SARS-CoV-2, because data on whether individuals acquire sufficient immunity following COVID-19 are lacking, as are data on the duration and strength of acquired immunity. It is advised that vaccination is done at least 4 weeks after onset of COVID-19 symptoms or 4 weeks from the first PCR-positive specimen in asymptomatic patients.31 Patients with IBD should be encouraged to have both the annual influenza and the SARS-CoV-2 vaccinations, although co-administration at the same visit is not advised. Other vaccines such as the influenza and pneumococcal vaccines should be scheduled at intervals of at least 7 days from SARS-CoV-2 vaccination.31 Following SARS-CoV-2 vaccination, patients with IBD are currently advised to continue to follow existing guidance on social distancing or shielding, determined by their position in the BSG risk grid.29 Tips on SARS-CoV-2 vaccination in patients with IBD can be found in the appendix (p 4).
SARS-CoV-2 vaccination safety in patients with IBD
Patients with IBD will have many questions regarding vaccination and specific data to inform many of these concerns are not yet available. However, there are several factors that can offer some reassurance. There is a robust and comprehensive regulatory process in place and approval is only given when there are compelling safety data. Standards for testing and monitoring of vaccines are generally higher than for most other medical interventions due to their intended use in healthy individuals, in whom the level of acceptable risk is lower. The approved SARS-CoV-2 vaccines have been tested in tens of thousands of patients with safety profiles comparable to other vaccines commonly used in patients with IBD, and have been appraised by multiple independent regulators, including the MHRA, the European Medicines Agency, and the US Food and Drug Administration. The BNT162b2, ChAdOx1 nCoV-19, and mRNA-1273 vaccines have received regulatory approval, which applies to patients with IBD. Moreover, for all three vaccines, immunosuppression is not a contraindication. To date there are no studies reporting the effect of any SARS-CoV-2 vaccine specifically in patients with IBD, as these patient groups have been excluded from the phase 3 vaccination studies. Nevertheless, insights into how different IBD therapies affect host immunity can be inferred from serological responses to other vaccination programmes. Notably, other commonly used vaccines (eg, influenza, HBV, HAV, etc) are also very low risk in patients with IBD, although they were never specifically trialled in patients with IBD before approval.
Regulatory approval is based on safety data generated from monitoring over 19 000 vaccine recipients for at least 2 months after their second dose (serious side-effects from vaccines are very rare beyond this point). Side-effect profiles from SARS-CoV-2 vaccines are in line with events observed with other commonly used vaccines. Data from the BNT162b2 phase 3 trial, which enrolled more than 40 000 individuals, the ChAdOx1 nCoV-19 phase 3 trials, which enrolled more than 23 000 participants, and the mRNA-1273 phase 3 trial, which enrolled more than 30 000 individuals, demonstrated that mild local injection site reactions (eg, pain, redness, and swelling) and systemic features (fatigue, headache, chills) were common, but serious adverse events were rare.8, 9, 10 For instance, there were four serious adverse events in BNT162b2 recipients, 79 in ChAdOx1 nCoV-19 recipients, and 71 in mRNA-1273 recipients.8, 9, 10 Very rare events of neuroinflammatory disorder have been reported with the ChAdOx1 nCoV-19 vaccine, but a causative role has not been established.9 In the BNT162b2 phase 3 trial, there were two deaths among BNT162b2 recipients (one from arteriosclerosis, one from cardiac arrest) and four deaths in placebo recipients (two from unknown causes, one from haemorrhagic stroke, and one from myocardial infarction), which were considered to be unrelated to the vaccine or placebo.8 There were four non-COVID-19 related deaths in ChAdOx1 nCoV-19 trials, three in controls and one in the vaccine recipients (one death each from road traffic accident, blunt force trauma, homicide, and fungal pneumonia).9 All were considered unrelated to vaccination. Five deaths occurred in the mRNA-1273 trial, three in the placebo group (one from intra-abdominal perforation, one from cardiopulmonary arrest, and one from severe systemic inflammatory syndrome in a participant with chronic lymphocytic leukaemia and diffuse bullous rash) and two in the vaccine group (one from cardiopulmonary arrest and one by suicide); none were considered related to vaccination.10 In the event of patients experiencing significant side-effects related to vaccination, these should be reported through the Coronavirus Yellow Card reporting website. If common side-effects such as pain or fever are troublesome, they can be treated with analgesia or anti-pyretic medication such as paracetamol.
There are certain groups of patients with IBD in whom vaccination is not advised or should be considered on the basis of a benefit-versus-risk analysis. Vaccination is not currently approved in those younger than 16 years. This is because almost all children will have asymptomatic or very mild disease if affected by COVID-1932, 33 and there are currently no data on the safety and efficacy of SARS-CoV-2 vaccination in children. In pregnancy, the routine use of SARS-CoV-2 vaccines is not recommended due to the lack of safety data in this population.28 There is some evidence that severe COVID-19 may be associated with premature birth, but evidence currently suggests that infection during pregnancy poses no additional risk of fetal developmental problems, nor increased risk of miscarriage.34, 35 The JCVI advises that vaccination should be considered in pregnant women in whom the risk of exposure to SARS-CoV-2 is high and unavoidable, or in women with underlying health conditions that put them at very high risk of serious complications of COVID-19. In these cases, risk and benefit should be discussed with individual patients.28, 36 The JCVI advises that breastfeeding is not a contraindication to vaccination. The clinical need for immunisation should be considered and women informed about the absence of safety data for the vaccine in the context of breastfeeding.28, 35 The BNT162b2, ChAdOx1 nCoV-19, and mRNA-1273 vaccines are contraindicated if there is a history of hypersensitivity to the active substance or any of the vaccine excipients. A history of an adverse reaction to other medications, including patients with IBD who have had severe immediate-onset anaphylaxis following biologic treatment, is not a contraindication to the BNT162b2, ChAdOx1 nCoV-19, or mRNA-1273 vaccines. Guidance on allergies for other SARS-CoV-2 vaccines will be issued by the MHRA as the vaccines are approved.
IBD disease activity following SARS-CoV-2 vaccination
Since SARS-CoV-2 vaccines have not been tested in patients with IBD it is not possible to judge whether vaccination will have an effect on IBD disease activity. However, no serious gastrointestinal side-effects to SARS-CoV-2 vaccinations have yet been reported. Furthermore, there are reassuring data from studies of patients with IBD receiving other commonly used vaccinations. In a trial of 96 patients with IBD administered 23-valent polysaccharide pneumococcal vaccine, there were no serious adverse events and no reported deteriorations in IBD disease activity.14 In a study of influenza H1N1 vaccination, just 12 (11%) of 108 individuals enrolled had an increase of more than two points in either the Harvey Bradshaw Index (HBI) or Simple Colitis Clinical Activity Index during 6 months of follow up.18 Only three patients required consequent alteration in their IBD medication. There was no unvaccinated control group, making it difficult to determine whether these findings were truly associated with vaccination. In another study of trivalent influenza vaccination given to 255 patients with IBD, no significant variations in HBI or Mayo scores were seen during 2 years of follow-up following vaccination.37
Research opportunities
We have identified some key research priorities with regard to SARS-CoV-2 vaccination in patients with IBD. In light of data showing impaired responses to pneumococcal, influenza, and other vaccines in patients with IBD on immunosuppression, there is a pressing need to understand whether different immunosuppressive regimens impair the development of anti-SARS-CoV-2 immunity in this high-risk population. Although routine serological testing of vaccine immunogenicity may not be available in all IBD units, where available this information could help to guide future studies regarding the need to implement mitigation strategies, such as administration of further booster doses of vaccine. This information might also help to inform advice given about physical distancing strategies, including shielding in the event of future surges in COVID-19. The emerging field of precision vaccination seeks to define how baseline features can predict which individual patients will respond to vaccination and to what extent.38 Baseline characteristics that have been investigated as predictors of vaccination response to other vaccines include germline genetics, host transcriptional responses, microbiome, metabolome, and particular immune features.38, 39, 40, 41, 42, 43 Future research should use multi-platform approaches to study biomarkers predicting vaccination outcome. Finally, the uptake of recommended vaccines amongst patients with IBD has historically been suboptimal.44 There is a necessity for qualitative research on attitudes towards SARS-CoV-2 vaccination in patients with IBD. Rates of acceptance of vaccination should also be tracked and reasons for non-uptake explored.
Acknowledgments
Acknowledgments
CAL acknowledges support from the NIHR Newcastle Biomedical Research Centre. JLA and NP acknowledge funding from the National Institute of Health Research (NIHR) Biomedical Research Centre based at Imperial College London and Imperial College Healthcare NHS Trust. JLA is the recipient of a NIHR Academic Clinical Lectureship. JLA receives funding for his Clinical Lectureship from Imperial College London and The Freed Foundation.
Contributors
JLA, GWM, DRG, IA, and NP researched and wrote the original draft of the article. All authors reviewed, edited, and approved the manuscript.
Declaration of interests
DRG, RA, MP, PJS, JPS, AD, RW, and THI have nothing to disclose. JLA reports sponsorship from Vifor Pharma for accommodation/travel to BSG 2019, outside the submitted work. GWM has received educational support from AbbVie, Janssen, NAPP, Takeda Pharmaceuticals, Merck Sharp & Dohme Ltd, Ferring, Phebra, and Dr Falk. GWM has received speaker honoraria from Merck Sharp & Dohme Ltd, AbbVie, Janssen, Ferring, and Takeda Pharmaceuticals. GWM attended advisory boards for AbbVie, Takeda Pharmaceuticals, Janssen, Medtronic, Phebra Pharmaceuticals, Servertus Associates Ltd, and Dr Falk. GWM works as a consultant with Alimentiv. NAK reports personal fees from Dr Falk, Janssen, Takeda, and Tillotts, and grants and personal fees from Pharmacosmos, outside the submitted work. CAL reports grants from Genentech, Eli Lilly, Pfizer, Roche, UCB Biopharma, Sanofi Aventis, Biogen IDEC, Orion OYJ, AstraZeneca, and AbbVie, grants and personal fees from Janssen and Takeda, and personal fees from Ferring and Dr Falk Pharma, outside the submitted work. SrS reports personal fees from Celltrion, Janssen, Takeda, Vifor Pharma, and Boehringer-Ingelheim, outside the submitted work. TR reports grants and personal fees from AbbVie, personal fees from BMS, Celgene, Ferring, Gilead, GSK, LabGenius, Janssen, Mylan, MSD, Novartis, Pfizer, Sandoz, Takeda, Galapagos, and Arena, outside the submitted work. CPS reports grants and personal fees from AbbVie, and Janssen, and personal fees from Takeda, Galapagos, and Arena, outside the submitted work. CWL reports grants and personal fees from Gilead and personal fees from AbbVie, Takeda, Janssen, Ferring, Trellus Health, Pfizer, Galapagos, and Iterative Scopes, outside the submitted work. LD reports grants from Amgen, Biogen, Janssen, Takeda, Galapagos, Tillotts, and Gilead outside the submitted work. TA reports grants from F Hoffmann-La Roche AG, Janssen, and Galapagos, grants and personal fees from Biogen and Celltrion, non-financial support from AbbVie and Tillotts, personal fees from ARENA, Adcock Ingram, Gilead, Pfizer, and Genentech, grants and personal fees from Celgene, and grants, personal fees, and non-financial support from Takeda, outside the submitted work. IA has received speaking fees from Vifor Pharma and Takeda. NP reports he has served as a speaker for Allergan, Bristol Myers Squibb, Falk, Ferring, Janssen, Pfizer, Tillotts, and Takeda, and as a consultant and/or an advisory board member for AbbVie, Allergan, Celgene, Bristol Myers Squibb, Ferring, and Vifor Pharma. JOL reports serving as an advisory board member for Atlantic Health, Abbvie UK/Global, MSD UK, Shire UK, Ferring International, Celltrion, Takeda, Pfizer, Janssen, GSK, Cellgene, Lilly, and Bristol Myers Squibb, has consulted on the development of treatment algorithms/publication strategy/clinical trial for AbbVie UK/Takeda/BMS, has received investigator led research grants from Takeda, Hospira, Pfizer, Abbvie, and Gilead, has received lecture fees from AbbVie UK/International, Ferring UK/International, MSD UK, Shire UK, Allergan, Shire UK, Takeda, Cornerstones, Janssen, and Pfizer, and received travel/accommodation/meeting expenses from AbbVie UK, Warner Chilcott UK, Takeda, and Pfizer, outside the submitted work. AH reports she has served as consultant, advisory board member, or speaker for AbbVie, Arena, Atlantic, Bristol-Myers Squibb, Celgene, Celltrion, Falk, Ferring, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire, and Takeda, and also serves on the Global Steering Committee for Genentech. ShS reports grants from Takeda, Tillotts Pharma, Pfizer, and Amgen, personal fees from Cellgene, Tillots Pharma, Falk Pharma, AbbVie, Amgen, Takeda, Pfizer, and Pharmacocosmos, and personal fees from Takeda, Ferring Pharma, Tillotts Pharma, Pfizer, and Celltrion, outside the submitted work. JM reports grants and personal fees from Takeda Pharmaceuticals and Biogen, personal fees and non-financial support from AbbVie and Janssen, personal fees from Grifols, Sandoz, Celltrion, Vifor Pharmaceuticals, Predictimmune, and Bristol Myers Squibb, and non-financial support from Ferring Pharmaceuticals, outside the submitted work.
Footnotes
The ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine is only authorised for use in those aged 18 years of age and over; however, JCVI is of the view that this vaccine may be used in those 16–17 years of age where there is no access or availability to an alternative approved COVID-19 vaccine.
Contributor Information
Inflammatory Bowel Disease section of the British Society of Gastroenterology and the the Inflammatory Bowel Disease Clinical Research Group:
Christine Norton, Shahida Din, Jackie Glatter, Jochen Kammermeier, Madhoor Ramdeen, Nabil Quraishi, Peter Sagar, Shellie Radford, R. Alexander Speight, Helen Steed, Michael Mcfarlane, and A. Barney Hawthorne
Supplementary Material
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2–10. doi: 10.3899/jrheum.091461. [DOI] [PubMed] [Google Scholar]
- 4.Jones GR, Lyons M, Plevris N, et al. IBD prevalence in Lothian, Scotland, derived by capture-recapture methodology. Gut. 2019;68:1953–1960. doi: 10.1136/gutjnl-2019-318936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 6.Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346. doi: 10.1053/j.gastro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin K, et al. for the C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2035389. published online Dec 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medicines & Healthcare products Regulatory Agency Information for healthcare professionals on Pfizer/BioNTech COVID-19 vaccine. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/information-for-healthcare-professionals-on-pfizerbiontech-covid-19-vaccine
- 12.Medicines & Healthcare products Regulatory Agency Information for healthcare professionals on COVID-19 vaccine AstraZeneca. https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-healthcare-professionals-on-covid-19-vaccine-astrazeneca
- 13.Medicines & Healthcare products Regulatory Agency Information for healthcare professionals on COVID-19 vaccine Moderna. https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna/information-for-healthcare-professionals-on-covid-19-vaccine-moderna
- 14.Fiorino G, Peyrin-Biroulet L, Naccarato P, et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18:1042–1047. doi: 10.1002/ibd.21800. [DOI] [PubMed] [Google Scholar]
- 15.Lee CK, Kim HS, Ye BD, et al. Patients with Crohn's disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis. 2014;8:384–391. doi: 10.1016/j.crohns.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Pratt PK, Jr, David N, Weber HC, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24:380–386. doi: 10.1093/ibd/izx001. [DOI] [PubMed] [Google Scholar]
- 17.Park SH, Yang SK, Park SK, et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:69–74. doi: 10.1097/01.MIB.0000437736.91712.a1. [DOI] [PubMed] [Google Scholar]
- 18.Cullen G, Bader C, Korzenik JR, Sands BE. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385–391. doi: 10.1136/gutjnl-2011-300256. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Jacobson DL, Ashworth LA, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol. 2009;104:444–453. doi: 10.1038/ajg.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagihara Y, Ohfuji S, Watanabe K, et al. Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J Crohns Colitis. 2014;8:223–233. doi: 10.1016/j.crohns.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–154. doi: 10.1038/ajg.2009.523. [DOI] [PubMed] [Google Scholar]
- 22.Caldera F, Hillman L, Saha S, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26:593–602. doi: 10.1093/ibd/izz164. [DOI] [PubMed] [Google Scholar]
- 23.Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082–1091. doi: 10.1093/ibd/izx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelinck LB, van der Bijl AE, Visser LG, et al. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine. 2008;26:3528–3533. doi: 10.1016/j.vaccine.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Andrisani G, Frasca D, Romero M, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: effects of combined therapy with immunosuppressants. J Crohns Colitis. 2013;7:301–307. doi: 10.1016/j.crohns.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77–83. doi: 10.1136/gutjnl-2014-307127. [DOI] [PubMed] [Google Scholar]
- 27.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–695. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Joint Committee on Vaccination and Immunisation Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination. 30 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020
- 29.Kennedy NA, Jones GR, Lamb CA, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984–990. doi: 10.1136/gutjnl-2020-321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkayam O, Bashkin A, Mandelboim M, et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2010;39:442–447. doi: 10.1016/j.semarthrit.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Public Health England COVID-19: the green book, chapter 14a. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/948757/Greenbook_chapter_14a_v4.pdf
- 32.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.5052. Published online: November 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Public Health England The safety of COVID-19 vaccines when given in pregnancy. https://www.gov.uk/government/publications/safety-of-covid-19-vaccines-when-given-in-pregnancy/the-safety-of-covid-19-vaccines-when-given-in-pregnancy#fnref:1
- 36.Public Health England COVID-19 vaccination: a guide for women of childbearing age, pregnant or breastfeeding. https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-for-women-of-childbearing-age-pregnant-planning-a-pregnancy-or-breastfeeding
- 37.Launay O, Abitbol V, Krivine A, et al. Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: a two-year prospective study. J Crohns Colitis. 2015;9:1096–1107. doi: 10.1093/ecco-jcc/jjv152. [DOI] [PubMed] [Google Scholar]
- 38.Tsang JS, Dobano C, VanDamme P, et al. Improving vaccine-induced immunity: can baseline predict outcome? Trends Immunol. 2020;41:457–465. doi: 10.1016/j.it.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynn MA, Tu DJ, Choo JM, et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23:653–660. doi: 10.1016/j.chom.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Ovsyannikova IG, Ryan JE, Vierkant RA, et al. Influence of host genetic variation on rubella-specific T cell cytokine responses following rubella vaccination. Vaccine. 2009;27:3359–3366. doi: 10.1016/j.vaccine.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ovsyannikova IG, Vierkant RA, Pankratz VS, O'Byrne MM, Jacobson RM, Poland GA. HLA haplotype and supertype associations with cellular immune responses and cytokine production in healthy children after rubella vaccine. Vaccine. 2009;27:3349–3358. doi: 10.1016/j.vaccine.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connor D, Png E, Khor CC, et al. Common genetic variations associated with the persistence of immunity following childhood immunization. Cell Rep. 2019;27:3241–3253. doi: 10.1016/j.celrep.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 43.Hagan T, Cortese M, Rouphael N, et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178:1313–1328. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439–444. doi: 10.1093/ecco-jcc/jjv064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.