Abstract
Background
A high number of thrombotic complications have been reported in critically ill patients with coronavirus disease 2019 (COVID-19) and appear to be related to a hypercoagulable state. Evidence regarding detection, management, and monitoring of COVID-19–associated coagulopathy is still missing. We propose to describe the thrombus viscoelastic properties to investigate the mechanisms of hypercoagulability in patients with COVID-19.
Methods
Thromboelastography (TEG) was performed in 24 consecutive patients admitted to a single intensive care unit for COVID-19 pneumonia, and 10 had a second TEG before being discharged alive from the intensive care unit.
Results
Compared with a group of 20 healthy participants, patients with COVID-19 had significantly decreased values of reaction time, coagulation time, and lysis index and increased values of α angle, maximum amplitude, clot strength, and coagulation index. Velocity curves were consistent with increased generation of thrombin. These values persisted in surviving patients despite their good clinical course.
Discussion
In patients with COVID-19, TEG demonstrates a complex and prolonged hypercoagulable state including fast initiation of coagulation and clot reinforcement, low fibrinolysis, high potential of thrombin generation, and high fibrinogen and platelet contribution. The antithrombotic strategy in patients with COVID-19 during intensive care hospitalisation and after discharge should be investigated in further studies.
Keywords: COVID-19, Thromboelastography, Hypercoagulability, Coagulopathy, Thrombosis
1. Introduction
After China, the whole world is now facing the coronavirus disease 2019 (COVID-19) pandemic. This unprecedented outbreak has caused a global health crisis and is straining health systems, especially the intensive care units (ICUs), because around 15% of the hospitalised patients require critical care.1
A high number of both venous thromboembolism and arterial thrombotic complications have been reported in critically ill patients with COVID-19.2 Some authors found an incidence up to 69% of venous thromboembolic events in patients with COVID-19 hospitalised in ICUs; the incidence was still elevated to 56% even among patients who were given therapeutic anticoagulation.3 In another series of 100 patients with COVID-19 and severe clinical features, a 23% incidence of acute pulmonary embolism has been reported.4
These complications appear to be related to a hypercoagulable state, but its mechanism remains unclear. COVID-19 is associated with coagulopathy that correlates with disease severity.5 Abnormal coagulation parameters, especially elevated levels of D-dimer and fibrin degradation products, have been associated with poor prognosis.6 For its part, anticoagulant therapy may reduce mortality in selected patients.7 Prophylactic doses of low-molecular-weight heparin (LMWH) have been recommended for all patients requiring hospital admission for COVID-19.8 However, evidence regarding detection, management, and monitoring of COVID-19–associated coagulopathy is still missing.
We propose to describe the thrombus viscoelastic properties to investigate the mechanisms of hypercoagulability in patients hospitalised in the ICU for COVID-19 pneumonia.
2. Patients and methods
2.1. Patients
This is a monocentric retrospective study conducted in an ICU in a nonuniversity public hospital. All and consecutive patients admitted to the ICU for confirmed COVID-19 pneumonia between March 18 and April 10, 2020, were included. Patients who were given oral anticoagulant therapy at the time of admission were excluded. Owing to the high incidence of thromboembolic events reported during the COVID-19 outbreak, we decided, as a standard of care, to assess haemostasis by thromboelastography (TEG) in all the patients admitted to the ICU. On admission, one additional whole blood sample was collected into a 3-mL tube containing 0.3 mL of 3.2% (109 mM) buffered sodium citrate at the same time as the other admission blood tests.
2.2. Standard coagulation tests
Haemostasis tests included the measure of the activated partial thromboplastin time (aPTT; STA-PTT A, Diagnostica Stago, Asnières-sur-Seine, France), prothrombin time (PT; STA-NeoPTimal 10, Diagnostica Stago, Asnières-sur-Seine, France), fibrinogen (functional clotting assay according to Clauss; STA-Liquid Fib, Diagnostica Stago, Asnières-sur-Seine, France), antithrombin (colorimetric assay; STA-Stachrom AT III, Diagnostica Stago, Asnières-sur-Seine, France), and D-dimer (immunoturbidimetric assay; STA-Liatest D-DI PLUS, Diagnostica Stago, Asnières-sur-Seine, France). All tests were performed on the automated coagulometer (STA-R Evolution, Diagnostica Stago, Asnières-sur-Seine, France).
2.3. Thromboelastography
TEG was performed using a TEG® 5000 analyser (Haemonetics Corporation, Braintree, MA, USA). Within 30 min after the patient's citrated whole blood sample had been collected, blood coagulation was accelerated by the addition of one millilitre of blood into the manufacturer-supplied activating solution containing kaolin, phospholipids, and buffered stabilisers. From this vial, 340 μL was drawn and recalcified with 20 μL of calcium chloride (200 mM) within a specific plastic cup for TEG use. Each sample was run simultaneously with and without added heparinase to neutralise the presence of LMWH or unfractionated heparin (UFH) and determine the baseline coagulation profile. According to the manufacturer's recommendations, the selected result was the one achieved with heparinase if there was any evidence of interference with heparin.
The TEG signal is recorded with a pin suspended by a torsion wire, which is dipped into an oscillating cup containing the blood sample. Once the blood clot starts to develop, the cup's movements are gradually transmitted to the pin and the torsion wire. The TEG trace is the pin oscillation amplitude (mm) plotted as a function of time (min). It represents the clot strength evolution over time.
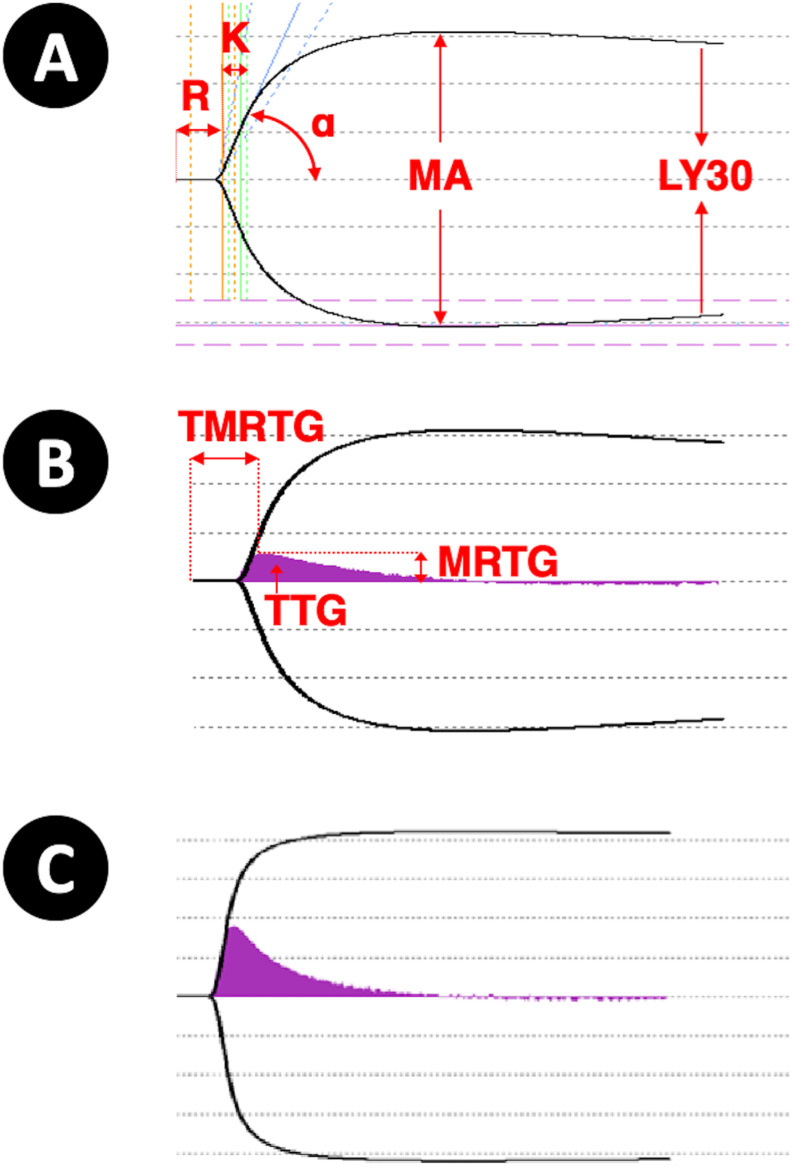
Recording and analysis were carried out using TEG analysis software, version 4.2 (TAS™; Haemonetics Corporation, Braintree, MA, USA). Some featured parameters are used to describe the TEG trace and are detailed in Fig. 1 A. The split point (minutes) is the elapsed time until the earliest resistance detected by the TEG analyser. The reaction time (R, minutes) is the elapsed time to reach a 2-mm amplitude on the TEG trace. The delta parameter (Δ, minutes) is the difference between the R and split point. The coagulation time (K, minutes) is the time interval from the R until the trace reaches a 20-mm amplitude. The α angle (α, degrees) is the slope of the line joining R and K. The maximum amplitude (MA, mm) refers to the maximum amplitude of the TEG trace. The clot strength (G, dyn/cm2) is a calculated value derived from the MA parameter (G = (5000MA/(100-MA)). The lysis index (LY30, percentage) is the amplitude reduction percentage 30 min after the MA. Finally, the coagulation index (CI) describes the patient's overall coagulation and is calculated using the following formula: CI = −0.6516R – 0.3772K + 0.1224 MA + 0.0759α −7.7922.
Fig. 1.
(A) Main parameters on the TEG trace: R (reaction time), K (coagulation time), α angle, MA (maximum amplitude), and LY30 (lysis index). (B) The velocity curve (V-curve) is the first derivative of the standard TEG trace. Featured parameters are MRTG (maximum rate of thrombus generation) and TMRTG (time to maximum rate of thrombus generation), and the area under the curve gives the TTG (total thrombus generation). (C) Hypercoagulability is characterised by decreased values of R, K, LY30, and TMRTG and increased values of α, MA, MRTG, and TTG. TEG, thromboelastography; R, reaction time; K, coagulation time; MA, maximum amplitude.
TEG analysis also enables the study of thrombus generation velocity. The velocity curve (V-curve) is plotted from the first derivative of the standard TEG trace; it represents the change in clot strength per unit of time (Fig. 1B). The parameters studied on the V-curve are the maximum rate of thrombus generation (MRTG; mm/min), the time to maximum rate of thrombus generation (TMRTG; minutes), and the total thrombus generation (TTG; mm/min).
The TEG parameters measured were compared with the reference values established in our laboratory on healthy participants tested in the context of TEG laboratory accreditation (ISO 15189). A state of hypercoagulability can be characterised by decreased values of R, K, LY30, or TMRTG or increased values of α, MA, G, CI, MRTG, or TTG (Fig. 1C).
2.4. Data collection
From the medical record, we collected demographic data (age and sex), body mass index (BMI; kg/m2), and the results of other standard laboratory tests performed on admission to the ICU: platelet count, PT, aPTT, fibrinogen, D-dimer, antithrombin, and C-reactive protein.
For each patient, the disseminated intravascular coagulation (DIC) score was calculated from the International Society on Thrombosis and Haemostasis diagnostic criteria for DIC, with the D-dimer cut-off values proposed by Taylor et al[9], [10] and Dempfle et al.[9], [10] The simplified acute physiology score II was calculated using the worst value for each physiological variable within the first 24 h after ICU admission.
All patients underwent computed tomography (CT) pulmonary angiography at admission for standardised assessment of COVID-19 severity as per the recommendations of the French Thoracic Imaging Society and pulmonary embolism detection. We researched the occurrence of acute respiratory distress syndrome that required prone positioning. Based on the clinical course, the occurrence of thromboembolic events was investigated during ICU hospitalisation. In symptomatic patients, computed tomography pulmonary angiography was repeated to detect pulmonary embolism, and brain magnetic resonance imaging was performed for ischaemic stroke diagnosis. Vital status at ICU discharge was also collected.
2.5. Antithrombotic prophylaxis and follow-up
During hospitalisation in the ICU, patients were given antithrombotic prophylaxis using LMWH or UFH as per a stratified risk strategy recommended by the French Haemostasis and Thrombosis Study Group and the Perioperative Haemostasis Interest Group.11 It consisted of reinforced antithrombotic prophylaxis, for instance, 40 mg of enoxaparin twice daily, in patients receiving high-flow oxygen therapy or mechanical ventilation, and curative anticoagulation, for instance, 1 mg/kg of enoxaparin twice daily, in patients with risk factors for thromboembolic complications. Treatment was monitored via anti-Xa activity for LMWH or UFH (STA-Liquid anti-Xa, Diagnostica Stago, Asnières-sur-Seine, France). At the clinician's discretion, a second TEG analysis was carried out just before release in some patients who were discharged alive from the ICU.
2.6. Statistical analysis
Data were collected using Excel™, version 16.29.1 (Microsoft, Washington, USA), and statistical analysis was realised using R Software, version 3.5.2 (R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Vienna, Austria, http://www.r-project.org). Continuous and categorical variables are presented as median with lower and upper quartiles and number with percentage of the total, respectively. Groups were compared using the Mann–Whitney U test, and repeated measurements were compared using the Wilcoxon test for paired samples. The association between TEG parameters and standard coagulation tests was assessed using Pearson's correlation coefficient.
2.7. Ethical considerations
The patients or their trusted persons have been informed of the study. This anonymous analysis has been declared to the French National Commission on Informatics and Liberty (CNIL) under the number 2217653v0 and was performed as per its requirements (reference methodology, MR-004). The study was approved by the ethics committee of the French Society of Anaesthesia and Intensive Care Medicine (IRB 00010254 - 2020 - 068).
3. Results
3.1. Patients
A total of 26 patients have been screened, and two were excluded owing to oral anticoagulant medication at the time of admission. Finally, 24 patients, 16 men and eight women, were included in the study. The median age was 69 y (61–71), and the median BMI was 28.5 kg/m2 (25.7–31.0). Nine patients (37%) had a BMI higher than 30. The CT scan found mild lesions (<25%) in four patients (17%), extensive lesions (25–50%) in six patients (25%), severe lesions (50–75%) in nine patients (37%), and critical lesions (>75%) in 5 patients (21%). The median simplified acute physiology score II was 45 (33–53). Regarding evolution, 13 patients (54%) developed severe acute respiratory distress syndrome (ARDS), requiring prone positioning. A pulmonary embolism occurred in five patients (21%), and an ischaemic stroke occurred in two patients (8%), one of whom had both. Finally, six patients (25%), died during their hospitalisation in intensive care.
3.2. Control group
The control group consisted of 20 healthy participants, nine men and 11 women. Their median age was 38 y (31–44).
3.3. Laboratory tests
Among patients with COVID-19, the median platelet count was 220 × 109/L (173–294) (reference range = 161–398), and the fibrinogen level was increased to 6.8 g/L (6.2–7.9) and was always higher than 5.0 g/L (reference range = 2.0–4.0). PT was 14.7 s (14.1–15.6) (reference range <17.6), and aPTT was 38.5 s (34.9–40.9) (reference range <40). The D-dimer level was greatly increased to 3.60 μg/mL (1.96–6.49) and higher than 1 μg/mL in 21 patients (87.5%) (reference range <0.50). The median antithrombin level was 91% (78–96) (reference range = 80–120). The median DIC score was 3 (2–3), and none of the patients scored higher than 4. All patients had a biological inflammatory syndrome with a median C-reactive protein level of 128 mg/L (101–249) (reference range <5).
3.4. TEG parameter values on admission
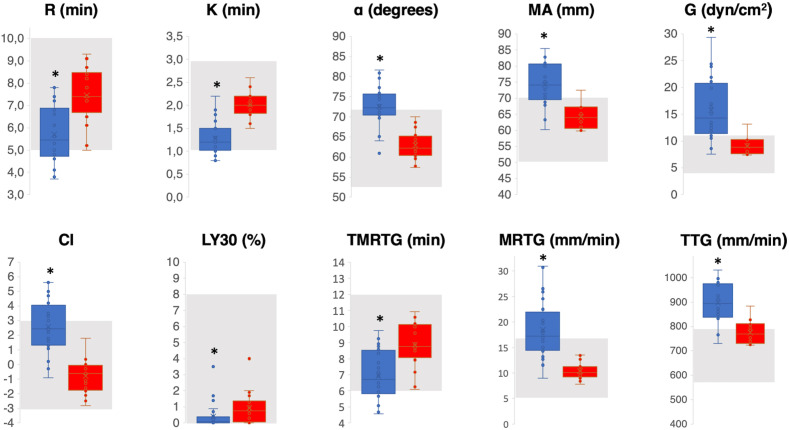
Fig. 2 shows the TEG parameter values of patients with COVID-19 (blue box plot) compared with the reference values established in our laboratory on a control group (red box plot). The grey area indicates the normal value range defined by the manufacturer. The CI was significantly higher for patients with COVID-19 than for those in the control group (p < 0.001). In patients with COVID-19, hypercoagulability was characterised by significantly decreased values of R, Δ, K, LY30, and TMRTG and significantly increased values of α, MA, G, MRTG, and TTG. The detailed results are reported in Table 1 (supplementary material).
Fig. 2.
Patients' TEG values (blue box plot) compared with the reference values established in our laboratory on a control group (red box plot). The grey area indicates the normal value range defined by the manufacturer. An asterisk indicates a statistically significant difference between the two groups. R, reaction time; K, coagulation time; MA, maximum amplitude; CI, coagulation index; TMRTG, time to maximum rate of thrombus generation; MRTG, maximum rate of thrombus generation; TTG, total thrombus generation; TEG, thromboelastography. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
TEG parameters were not different between patients with obesity (BMI >30 kg/m2) and patients without obesity and were also not different depending on the severity of CT lesions (supplementary material, Tables 2 and 3). Signs of hypercoagulability (decreased values of R, Δ, K, LY30, and TMRTG and increased values of α, MA, G, MRTG, and TTG) were more severe among the patients who developed thrombotic complications, but without significant difference among those who did not (supplementary material, Table 4). There was no difference in TEG parameters between patients who died and those who survived (supplementary material, Table 5).
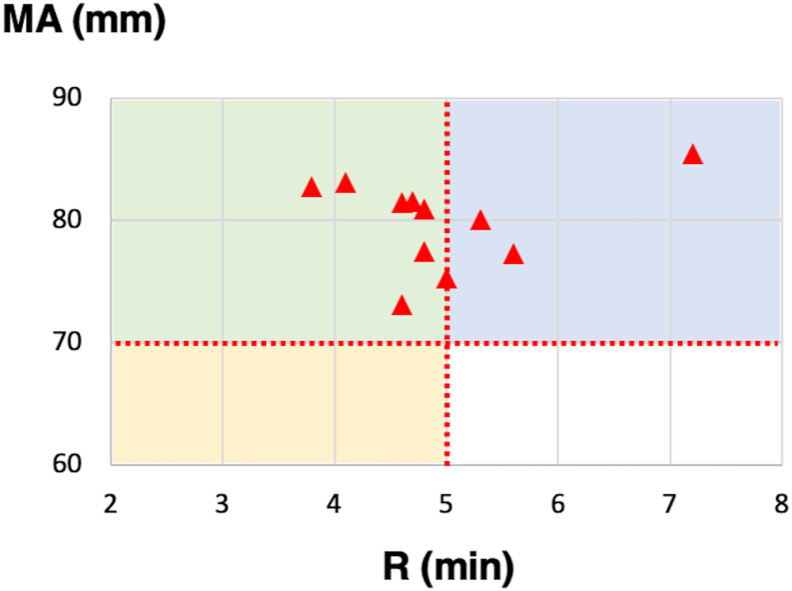
According to the manufacturer, TEG can classify hypercoagulability into three types: enzymatic hypercoagulability (CI > 3, R ≤ 5 min, MA ≤ 70 mm), platelet hypercoagulability (CI > 3, R > 5 min, MA > 70 mm), and mixed hypercoagulability (CI > 3, R < 5 min, MA > 70 mm). Among patients with COVID-19, 11 (46%) had hypercoagulability defined by CI >3. Fig. 3 presents the R and MA values of these 11 patients; four (36%) could be classified into platelet hypercoagulability, and seven could be classified (64%) into mixed hypercoagulability.
Fig. 3.
Eleven patients had hypercoagulability defined by CI >3, that could be classified into enzymatic hypercoagulability (R ≤ 5 min, MA ≤70 mm, yellow area), platelet hypercoagulability (R > 5 min, MA >70 mm, blue area), and mixed hypercoagulability (R < 5 min, MA >70 mm, green area). CI, coagulation index; R, reaction time; MA, maximum amplitude. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Correlations between TEG parameters and standard coagulation tests
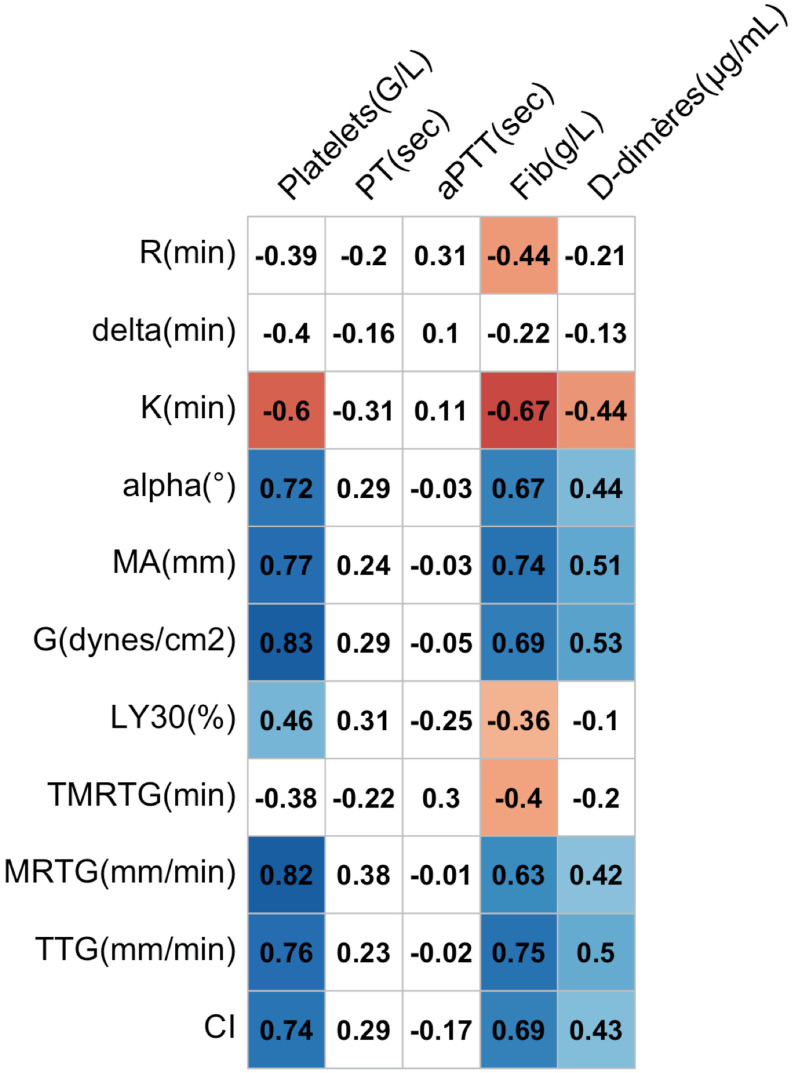
Pearson's correlation coefficients between the TEG parameters and the standard coagulation tests in the 24 patients with COVID-19 are reported in Fig. 4 . MA, G, MRTG, and TTG were highly correlated with platelet count, and TTG was highly correlated with the fibrinogen level.
Fig. 4.
Correlation matrix between TEG parameters and standard coagulation tests in 24 patients with COVID-19. Positive correlations are indicated in a blue cell, and negative correlations are indicated in a red cell. Correlation coefficients on a white field are not statistically significant. PT, prothrombin time; aPTT, activated partial thromboplastin time; MA, maximum amplitude; TMRTG, time to maximum rate of thrombus generation; TTG, total thrombus generation; MRTG, maximum rate of thrombus generation; CI, coagulation index; TEG, thromboelastography; COVID-19, coronavirus disease 2019.
3.6. TEG analyses at discharge
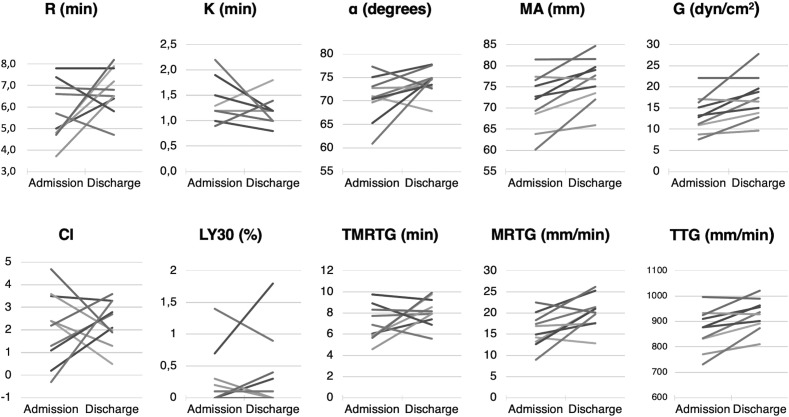
A total of 10 patients had a second TEG analysis before being discharged alive from the ICU. Their median length of stay in the ICU was 18 d (12–24). Fig. 5 illustrates the evolution of the TEG parameters for each patient.
Fig. 5.
Evolution of the individual TEG values between ICU admission and before being discharged alive. R, reaction time; K, coagulation time; MA, maximum amplitude; TMRTG, time to maximum rate of thrombus generation; TTG, total thrombus generation; MRTG, maximum rate of thrombus generation; CI, coagulation index; TEG, thromboelastography; ICU, intensive care unit.
Among these 10 patients, the median (IQR) CI was 2.3 (1.1–3.2) at admission and 2.4 (1.9–3.2) at discharge (p = 0.769). The median R value increased from 5.4 min (4.8–6.8) to 6.7 min (6.4–7.6) (p = 0.193). K values did not change significantly, from 1.3 min (1.2–1.5) to 1.2 min (1.1–1.2) (p = 0.361). The α angle increased from 71° (70–73) to 75° (73–75) (p = 0.106). MA and G values increased significantly from 72 mm (69–76) and 13.1 dyn/cm2 (11.1–16.1) to 77 mm (74–80) (p = 0.006) and 17.0 dyn/cm2 (14.2–19.4) (p = 0.013), respectively. The lysis rate remained unchanged at 0.1%.
Concerning the V-curve parameters, TMRTG increased from 6.5 min (5.9–8.2) to 8.0 min (7.5–9.1) at discharge (p = 0.202). TTG increased from 878 mm/min (834–923) to 933 mm/min (895–963) (p = 0.009). MRTG increased from 16.0 mm/min (13.5–18.1) to 20.6 mm/min (18.2–21.3) (p = 0.019).
4. Discussion
The main finding of our study is the complex hypercoagulable state in critical patients with COVID-19 pneumonia, which persists even in case of favourable clinical evolution.
Many authors have already reported severe disturbances of haemostasis and their association with poor prognosis in patients with COVID-19.6 The most frequently described disorder is the increased D-dimer level, which we also found in our series.12 It has been reported that elevation higher than 1 μg/L was a risk factor for fatal outcomes: this is not relevant in our experience because nearly 90% of patients had a D-dimer level higher than this threshold.13 Our findings are not consistent with the hypothesis of DIC that has been widely proposed.14 The D-dimer level was clearly increased, but we also observed that the platelet count was maintained, the fibrinogen level was greatly increased, and PT, aPTT, and antithrombin were only slightly altered. Although it has been recommended to check for the occurrence of DIC, none of our patients reached a DIC score of 5, and the highest scores were only due to isolated increase in the D-dimer level.8 COVID-19–related coagulopathy seems to be mainly a severe hypercoagulable state.15 This may explain why the rate of venous and arterial thrombotic complications we observed was so high, but consistent with the other data in the literature.4
Our study highlights the relevance of TEG for exploring hypercoagulable profiles. Routine clotting assay provides limited information regarding the overall haemostatic competency of a patient. Standard tests do not include the participation of platelets in the coagulation process, and the end point of clotting assays is the formation of fibrin, that occurs whereas less than 5% of the total thrombin has been generated.16 TEG provides a comprehensive view of coagulation and hypercoagulability mechanisms.17 Enzymatic contribution to clot formation is assessed using the R value; it has also been reported that a Δ value lower than 0.6, as we found in our study, might reveal an enzymatic hypercoagulability.18 K values and the α angle explore the rate of clot strengthening and are representative of thrombin cleaving of the available fibrinogen into fibrin.18 Contribution of fibrinogen and platelet to clot strength is assessed by MA and G parameters.19 Finally, TEG is also a simple method for fibrinolysis assay.20
Our findings suggest that COVID-19 coagulopathy is a hypercoagulable state related to all of these parameters, even if fibrinogen and platelet participation appears to be paramount. These results are consistent with recent research on the viscoelastic characteristics of the clot in patients with COVID-19, based on TEG or rotational thromboelastometry, which describe a similar prothrombotic state with supranormal clot firmness.21 , 22 The hypofibrinolytic state has also been reported.23 However, our study is to our knowledge the first including velocity curves. These curves allow evaluating the profile of thrombus generation in whole blood under in vivo conditions. It was previously demonstrated that TTG was significantly correlated with the generation of thrombin assessed by the thrombin–antithrombin complex.24 We observed greatly increased values of TTG and MRTG, as well as a decreased TMRTG, which reveal high potential for thrombin generation.
Other hypotheses must also be considered to explain the COVID-19 hypercoagulable state. All of our patients had a marked inflammatory syndrome, which may contribute to an increased risk of thrombosis.25 Increase in interleukin 6 levels has been demonstrated in patients with COVID-19, which stimulates tissue factor expression, fibrinogen synthesis, and platelet production.26 , 27 Endothelial dysfunction should also be discussed as in other septic states.
Our results raise the issue of the antithrombotic strategy in patients with COVID-19. As an easy, real-time, and widely available point-of-care technique, TEG may help to identify at-risk patients and to provide tailored antithrombotic prophylaxis.28 Because of a lack of statistical significance, we did not find any association between the TEG values and the occurrence of thromboembolic events. However, in other studies, elevated MA values29 or fibrinolysis shutdown23 was associated with a high rate of thrombotic events. Concerning high platelet involvement, some authors reported the addition of antiplatelet drugs when the platelet count was higher than 400 × 109/L.26 Hyperfibrinogenemia, for its part, could lead to heparin resistance.30 Some authors advocate for prolonged systemic anticoagulation using UFH.15 While one author reported a return to normal of the viscoelastic parameters in COVID-19–survived patients, we are concerned about the persistence of a hypercoagulable state in surviving patients despite their good clinical course.26 Given this finding, the question of the continuation and duration of antithrombotic prophylaxis after ICU discharge should be evaluated.
Our study has several limitations. The number of complications may have been underestimated because they were not specifically investigated for the study but only when the patient's condition required it. The clinical relevance of the hypercoagulability we observed remains unknown because we could not link it to the occurrence of thrombotic events. Finally, we do not know if hypercoagulability is related to disease severity. Further studies would be useful to compare TEG values in critical and noncritical patients with COVID-19.
5. Conclusion
In patients with COVID-19, TEG demonstrates a hypercoagulable state from multiple causes including high potential of thrombin generation and high fibrinogen and platelet contribution. Hypercoagulability observed with TEG parameters persists despite a good clinical course. The specific contribution of this hypercoagulability to the pathophysiology of COVID-19 remains uncertain, but the antithrombotic strategy in patients with COVID-19 during intensive care hospitalisation and after discharge should be investigated in further studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Pierre-Yves Cordier: Methodology, performed the research, analysed the data, Writing - original draft, approved the submitted version of the manuscript. Candice Pierrou: Methodology, performed the research, analysed the data, Writing - original draft, approved the submitted version of the manuscript. Alexandre Noel: Methodology, performed the research, analysed the data, Writing - original draft, approved the submitted version of the manuscript. Raphaël Paris: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Eliott Gaudray: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Edouard Martin: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Claire Contargyris: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Frédérik Bélot-De Saint Léger: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Arthur Lyochon: approved the submitted version of the manuscript. Hélène Astier: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Florian Desmots: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Hélène Savini: performed the research, Writing - review & editing, approved the submitted version of the manuscript. Corinne Surcouf: Methodology, performed the research, analysed the data, Writing - original draft, approved the submitted version of the manuscript.
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aucc.2020.11.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. Jama. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020:1–4. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemostasis. 2020:14869. doi: 10.1111/jth.14869. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020:201544. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin Loeches I., Browne P. COVID-19 coagulopathy in caucasian patients. Br J Haematol. 2020:16749. doi: 10.1111/bjh.16749. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis. 2020:1–15. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemostasis. 2020:1–8. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor F.B., Toh C.H., Hoots W.K., Wada H., Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemostasis. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 10.Dempfle C.-E., Wurst M., Smolinski M., Lorenz S., Osika A., Olenik D. Use of soluble fibrin antigen instead of D-dimer as fibrin-related marker may enhance the prognostic power of the ISTH overt DIC score. Thromb Haemostasis. 2004;91:812–818. doi: 10.1160/TH03-09-0577. [DOI] [PubMed] [Google Scholar]
- 11.Albaladejo P. Anticoagulant treatment for the prevention of thrombotic risk in a hospitalized patient with covid-19 and hemostasis monitoring. GIHP and GFHT proposals. SiteGehtorg. 2020:1–5. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippi G., Favaloro E.J. D-Dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemostasis. 2020 doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemostasis. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett C.D., Moore H.B., Yaffe M.B., Moore E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a Comment. J Thromb Haemostasis. 2020 doi: 10.1111/jth.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brummel K.E., Paradis S.G., Butenas S., Mann K.G. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 17.Wesley B., Matthew L., Michael M., Alexandra E., Christopher S., Richard J. The ability of thromboelastography to detect hypercoagulability. J Orthop Trauma. 2019:1–29. doi: 10.1097/BOT.0000000000001714. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez E., Kashuk J.L., Moore E.E., Silliman C.C. Differentiation of enzymatic from platelet hypercoagulability using the novel thrombelastography parameter delta (Δ) J Surg Res. 2010;163:96–101. doi: 10.1016/j.jss.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harr J.N., Moore E.E., Chin T.L., Ghasabyan A., Gonzalez E., Wohlauer M.V. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74:756–765. doi: 10.1097/TA.0b013e3182826d7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemostasis. 2018;16:652–662. doi: 10.1111/jth.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemostasis. 2020:jth:14850. doi: 10.1111/jth.14850. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collett L.W., Gluck S., Strickland R.M., Reddi B.J. Evaluation of coagulation status using viscoelastic testing in intensive care patients with coronavirus disease 2019 (COVID-19): an observational point prevalence cohort study. Aust Crit Care. 2020 doi: 10.1016/j.aucc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright F.L., Vogler T.O., Moore E.E., Moore H.B., Wohlauer M.V., Urban S. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivard G.E., Brummel-Ziedins K.E., Mann K.G., Fan L., Hofer A., Cohen E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J Thromb Haemostasis. 2005;3:2039–2043. doi: 10.1111/j.1538-7836.2005.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemostasis. 2020:14849. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemostasis. 2020:14854. doi: 10.1111/jth.14854. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr R., Stirling D., Ludlam C.A. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary R., Kreutz R.P., Bliden K.P., Tantry U.S., Gurbel P.A. Personalizing antithrombotic therapy in COVID-19: role of thromboelastography and Thromboelastometry. Thromb Haemostasis. 2020 doi: 10.1055/s-0040-1714217. [DOI] [PubMed] [Google Scholar]
- 29.Mortus J.R., Manek S.E., Brubaker L.S., Loor M., Cruz M.A., Trautner B.W. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harr J.N., Moore E.E., Chin T.L., Ghasabyan A., Gonzalez E., Wohlauer M.V. Postinjury hyperfibrinogenemia compromises efficacy of heparin-based venous thromboembolism prophylaxis. Shock. 2014;41:33–39. doi: 10.1097/SHK.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.