Abstract
Introduction
The impact of prior therapies, especially chemotherapy, on overall survival (OS) in patients with castration-resistant prostate cancer (CRPC) receiving [177Lu]Lu-PSMA-617 therapy has been the subject of controversy. Therefore, WARMTH decided to plan a multicenter retrospective analysis (the “617 trial”) to evaluate response rate and OS as well as the impact of prior therapies on OS in more than 300 patients treated with 177Lu-PSMA-617.
Materials and methods
The data of 631 metastatic CRPC (mCRPC) patients from 11 different clinics were evaluated. According to the inclusion and exclusion criteria, all patients had to have received at least abiraterone or enzalutamide prior to [177Lu]Lu-PSMA-617 therapy. The patients were divided into three groups: patients who had received prior chemotherapy, patients who avoided chemotherapy, and patients for whom a chemotherapy was contraindicated.
Results
The analysis included the data of 416 patients, with a median age of 71.9 years. At the time of analysis, 87 patients (20,9%) were still alive. A total of 53.6% of patients had received both abiraterone and enzalutamide; 75.5% and 26.4% had a history of chemotherapy with docetaxel and cabazitaxel, respectively. A total of 20.4% had had Ra-223. The median OS was 11.1 months. Prior chemotherapy, the existence of bone and liver metastases, as well as Eastern Cooperative Oncology Group (ECOG) status, were significant prognosticators of worse overall survival in both univariate and multivariate analyses. Patients without any prior chemotherapy showed a significantly longer OS (14.6 months). The median OS in patients who received one or two lines of chemotherapy with docetaxel or docetaxel followed by cabazitaxel, respectively, was 10.9 months and 8.9 months. There was no difference in OS between patients who had not received chemotherapy and patients for whom chemotherapy was contraindicated. The other prior therapies did not have any significant impact on OS.
Conclusion
In the present multicenter analysis, chemotherapy-naïve mCRPC patients receiving [177Lu]Lu-PSMA-617 therapy had a significantly longer OS than patients with a history of chemotherapy. This remained independent in the multivariate analysis besides presence of bone and liver metastases as negative prognosticators for survival, whereas an ECOG of 0–1 is associated with a longer OS.
Electronic supplementary material
The online version of this article (10.1007/s00259-020-04797-9) contains supplementary material, which is available to authorized users.
Keywords: mCRPC, Radioligand therapy, Lu-PSMA, Chemotherapy
Introduction
Prostate-specific membrane antigen (PSMA), also known as folate hydrolase I or glutamate carboxypeptidase II, is a type II, 750-amino acid transmembrane protein (100–120 kDa), anchored in the cell membrane of epithelial prostate cells. PSMA is highly expressed in prostate epithelial cells and strongly upregulated in prostate cancer. PSMA expression levels are directly correlated to androgen independence, metastasis, and prostate cancer progression [1]. Nevertheless, PSMA is not specific to prostate cells and is expressed in other normal (e.g., salivary glands, duodenal mucosa, a subset of proximal renal tubular cells, and a subpopulation of neuroendocrine cells in the colonic crypts) and neoplastic (e.g., subtypes of breast cancer, renal cell carcinoma, hepatocellular carcinoma, colon carcinoma, and peritumoral and endotumoral endothelial cells of neovasculature) tissues [2, 3]. PSMA undergoes constitutive internalization and, as such, can serve not only as an imaging biomarker but also as a target for radioligand therapy (RLT) [4]. Thus, PSMA appears to be an appealing molecular target for theranostics in metastatic prostate cancer [5].
In the first patient cohort of ten patients, minimal early side effects and a considerable rate of prostate-specific antigen (PSA) response after one cycle of RLT with [177Lu]Lu-PSMA-617 (177Lu-PSMA-617) was demonstrated [6]. Meanwhile, several retrospective and a few phase 2 prospective studies with a limited number of patients have confirmed the efficacy and low toxicity profile of Lu-PSMA therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) [7–12]. According to the retrospective analyses, it seems that Lu-PSMA therapy prolongs overall survival (OS) at least in patients with a positive response to this therapy [12–15]. The impact of prior therapies on the overall survival of these patients has not been straightforward in different publications [12, 13, 16].
All of the studies performed so far suffer from a limited number of patients and heterogeneity regarding prior therapies. Therefore, WARMTH (World Association of Radiopharmaceutical and Molecular Therapy) decided to plan a multicenter retrospective analysis (the “617 trial”) to evaluate response rate and OS as well as the impact of prior therapies on OS in more than 300 patients treated with [177Lu]Lu-PSMA-617.
Materials and methods
Study population
The study populations targeted in this study were mCRPC patients who underwent radioligand therapy with 177Lu-PSMA-617 with at least a 6-month follow-up from the time of the first cycle or who died within this time period.
Study design
This retrospective, multicentric study assessed response and OS and its prognostic factors in mCRPC patients who underwent 177Lu-PSMA-617 therapy according to the inclusion and exclusion criteria of this study (Table 1). The planned number of included patients for the analysis was at least 300 patients.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | |
| Castration-resistant metastatic prostate cancer | |
| Age > 18 years | |
| RLT using 177Lu-PSMA-617 | |
| Documented progressive disease prior to the first cycle according to PSA and/or imaging | |
| History of therapy with abiraterone or enzalutamide or both, or documented progressive disease under ongoing therapy with one of these agents (prior to 177Lu-PSMA) | |
| PSMA-positive metastases (in PSMA scan) | |
| ECOG 0–2 | |
| GFR > 40 mg/dl | |
| Have a follow-up at least 6 months from the time of the first cycle, or the patient died within this time | |
| Exclusion criteria | |
| Hormone-sensitive prostate cancer | |
| Active malignancy other than prostate cancer |
Methodology
First, we designed an Excel table as well as case report forms (CRF) for collecting the data. The centers were free to choose between the Excel sheet or CRF form. The anonymized data were sent to the Department of Nuclear Medicine, University Hospital Bonn, for analysis. This retrospective study was approved by the Ethical Committee of the Medical University of Innsbruck. Radiolabeled peptides were used according to the updated Declaration of Helsinki. All local regulations were observed in the participating countries. Informed consent for performing the therapy as compassionate use, according to local laws in participating countries, were obtained from each patient prior to the administration of radiopharmaceuticals. Due to the retrospective design of the study a formal consent to participate in the study was waived according to local regulations.
Inclusion criteria
The inclusion and exclusion criteria of this study are shown in Table 1. Only patients who were treated with 177Lu-PSMA-617were included in this study. Primary inclusion criteria to be considered for 177Lu-PSMA-617RLT was a PSMA-positive scan in patients with progressive disease (PSA and/or imaging). All of the patients had to have been pretreated with abiraterone or enzalutamide or both, or documented progressive disease under ongoing therapy with one of these agents. Prior chemotherapy was not part of the inclusion criteria, because in routine practice, some patients avoid getting chemotherapy, or for some, chemotherapy is contraindicated. To track the effects of chemotherapy as a prior treatment, we divided the patients into three groups: those without prior chemotherapy because of avoidance, those without chemotherapy because of contraindications, and those with a history of prior chemotherapy.
Response
Changes in the PSA level were classified as either a decrease of ≥ 50% or any percentage decrease in PSA (any PSA decline). Any increase in PSA was considered to indicate disease progression. According to our previous studies [13, 14, 17–19], responders to the first cycle of Lu-PSMA tend to live significantly longer than non-responders. Because of this fact and due to homogeneity in the group of patients regarding response, only the response to the first treatment cycle was considered as a possible predictive parameter in this study.
Overall survival
Overall survival was defined as the time from the date of the first 177Lu-PSMA-617 treatment until death from any cause or until the last follow-up.
Possible prognostic factors for overall survival
The following pre-therapeutic parameters were evaluated: age; Gleason score; prior therapies including abiraterone, enzalutamide, and first- and second-line chemotherapy; the existence of bone, lymph node, liver, and lung metastases; and the Eastern Cooperative Oncology Group (ECOG) score.
Statistical analysis
Kaplan-Meier estimators were used to compare survival times between different subgroups of the study population. p values were derived from the log-rank test. Uni- and multifactorial hazard ratios were estimated by fitting respective Cox-regression models to the data. p values in the scope of the multivariant analyses are Wald p values for the respective parameter estimates. All analyses were done with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
The data of 631 patients treated between February 2014 and December 2018 in 11 centers were collected. The list of the participating sites is shown in accessory Table 1. After the first analysis of the data, 215 patients had to be excluded from the main analysis. The main reason for this exclusion was unclear and incomplete follow-up data.
Four-hundred sixteen mCRPC patients with a median age of 71.9 years (range 43–90) were included. The patients received a total of 1493 cycles (1–12 cycles) of therapy with 177Lu-PSMA-617. In median, 3 cycles were applied. The median Gleason score (GS) was 8 (4–10). The mean and median baseline PSA value were 580 and 177 ng/ml, respectively. The age of the patients at the time of diagnosis was documented in 362 patients. The median time between the initial diagnosis and 177Lu-PSMA-617 therapy was 7.1 years (range 1–31 years). The majority of patients suffered from bone metastases (92.8%), and 20.9% of patients had liver metastases (Table 2).
Table 2.
Prior therapies
| Parameter | ||
|---|---|---|
| n of patients | 416 | |
| Age (mean) | 71.9 (range 43–90) | |
| Gleason score1 | ≤ 7 | > 7 |
| 114 (27.4%) | 239 (57.5%) | |
| Baseline PSA (ng/ml) mean; median; (range) | 580;177; (0.4–11,830) | |
| Prior therapies | Hx of n (%) | Ongoing n (%) |
| Abiraterone2 | 246 (59.1) | 76 (18.3) |
| Enzalutamide2 | 200 (48.1) | 114 (27.4) |
| Docetaxel | 314 (75.5) | |
| Cabazitaxel | 110 (26.4) | |
| Ra-223 | 85 (20.4) | |
| ECOG3 | n of patients (%) | |
| 0 | 156 (37.5) | |
| 1 | 166 (39.9) | |
| 2 | 72 (17.3) | |
| Number of cycles |
Sum; median; (range) 1493; 3; (1–12) |
|
| Extent of disease | n of patients (%) | |
| Bone metastases | 386 (92.8) | |
| Lymph node metastases | 329 (79.1) | |
| Liver metastases | 87 (20.9) | |
| Lung metastases | 68 (16.3) | |
| Brain metastases | 10 (2.4) | |
1GS of 63 patients was unknown
2223 patients (53.6%) received both abiraterone and enzalutamide
3ECOG of 22 patients (5.3%) was not reported
At the time of this analysis, 87 patients were still alive (20.9%). The majority of the patients presented with an ECOG of 0 or 1 (77.4%) (Table 2).
Prior therapies
Table 2 shows the prior therapies. According to the inclusion criteria, all patients had to have been treated with at least abiraterone or enzalutamide and had documented failure of therapy with these agents. The reason that some patients took abiraterone or enzalutamide concurrently despite progressive disease under these agents was that their urologist or oncologists did not want to stop these medications because clinical benefit was still assumed. Two hundred twenty-three patients (53.6%) had taken both abiraterone and enzalutamide. A first-line chemotherapy with docetaxel had been applied in 314 patients (75.5%).
Of the patients who did not get first-line chemotherapy, chemotherapy had only been contraindicated in 18.8%. Prior to RLT, second-line chemotherapy with cabazitaxel was given in 110 patients (26.4%). Second-line chemotherapy was contraindicated in 13.2% of patients (Table 2).
A prior treatment with radium-223 was given in 85 patients (20.4%). The median interval between the last application of radium-223 and the first cycle of 177Lu-PSMA-617 therapy was 3.9 months (range 1–36.4 months).
Response to the first cycle measured by PSA
PSA values, which were measured 2 months after the first cycle, were available in 393 patients. Two hundred eighty-two (71.8%) patients showed PSA decline, of whom 163 patients (41.5%) showed a PSA decline of ≥ 50%. One hundred eleven patients (28.2%) showed an increase in PSA.
Overall survival
The median OS was 11.1 months (95% CI 9.7–12.5 months). Table 3 shows the prognostic value of different pre-therapeutic parameters regarding OS in detail. Prior chemotherapy, the existence of bone and liver metastases, as well as ECOG status, was significant prognosticators of worse overall survival in both univariate and multivariate analyses.
Table 3.
Prognostic value of different pre-therapeutic parameters regarding OS
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Parameter | Patients (n) | mOS$ (months) (95% CI) | HR (95% CI) | p value | HR (95% CI) | p value |
| Age (years) | ||||||
|
≤ 70 > 70 |
185 231 |
11.302 (9.205–13.399) 11.105 (9.719–12.490) |
1.010 (0.812–1.256) 1 (reference) |
0.931 | ||
| Gleason score | ||||||
|
≤ 7 > 7 |
114 239 |
11.598 (9.503–13.692) 10.415 (8.645–12.185) |
1 (reference) 1.325 (0.912–1.924) |
0.132 | ||
| Abiraterone | ||||||
|
Hx1 of Ongoing No |
246 76 94 |
11.598 (9.243–13.952) 10.908 (9.123–12.692) 11.039 (8.582–13.496) |
0.913 (0.689–1.212) 1 (reference) 0.869 (0.621–1.216) |
0.420 | ||
| Enzalutamide | ||||||
|
Hx of Ongoing No |
92 48 58 |
12.287 (10.459–14.116) 10.776 (8.563–12.989) 11.302 (8.473–14.131) |
0.772 (0.598–0.997) 1 (reference) 0.859 (0.641–1.152) |
0.771* | ||
| Abi/Enza2 | ||||||
|
Both Only one of them |
223 193 |
11.269 (9.115–13.423) 11.105 (9.220–12.990) |
1.064 (0.856–1.323) 1 (reference) |
0.574 | ||
| First-line CTx3 | ||||||
|
Hx of No |
314 102 |
10.316 (0.797–8.755) 14.587 (10.331–18.844) |
1.495(1.149–1.946) 1 (reference) |
0.003 | 1.557(1.196–2.029) | 0.001 |
| First- and second-line CTx | ||||||
|
Hx of only first-line Hx of both No chemotherapy |
204 110 102 |
10.908 (9.050–12.765) 8.936 (6.925–10.948) 14.587 (10.331–18.844) |
1.440 (1.088–1.908) 1.679 (1.232–2.288) 1 (reference) |
0.01+ 0.001 |
1.495 (1.109–2.017) 1.473 (1.058–2.052) |
0.008 0.02 |
| Ra-223 | ||||||
|
Hx of No |
85 331 |
10.809 (9.544–12.994) 11.269 (9.544–12.994) |
1.132 (0.873–1.469) 1 (reference) |
0.354 | ||
| ECOG | ||||||
|
0 1 2 |
156 166 72 |
16.920 (13.921–19.919) 9.692 (7.460–11.924) 6.341 (9.788–12.619) |
0.323 (0.236–0.441) 0.553 (0.412–0.743) 1 (reference) |
< 0.0001 |
0.332 (0.127–0.461) 0.568 (0.421–0.766) |
< 0.0001 0.0002 |
| Bone metastases | ||||||
|
Yes No |
386 30 |
10.776 (9.243–12.309) 25.462 |
2.974 (1.629–5.428) 1 (reference) |
0.0004 | 3.703 (1.900–7.214) | 0.0001 |
| Liver metastases | ||||||
|
Yes No |
87 329 |
6.045 (4.744–7.346) 12.977 (11.306–14.649) |
2.506 (1.944–3.231) 1 (reference) |
< 0.0001 | 2.394 (1.818–3.153) | < 0.0001 |
| Lung metastases | ||||||
|
Yes No |
68 348 |
11.006 (9.342–12.670) 11.269 (9.613–12.925) |
1.048 (0.791–1.390) 1 (reference) |
0.743 | ||
| Lymph node metastases | ||||||
|
Yes No |
329 87 |
11.203 (9.608–12.798) 11.039 (8.685–13.393) |
0.863 (0.665–1.121) 1 (reference) |
0.275 | ||
$Median overall survival
*There was a significant difference between history of enzalutamide and ongoing usage of enzalutamide (p 0.045)
+There was no significant difference between patients with only first-line chemotherapy and both chemotherapies
1Hx: history
2Both abiraterone and enzalutamide
3CTx: chemotherapy
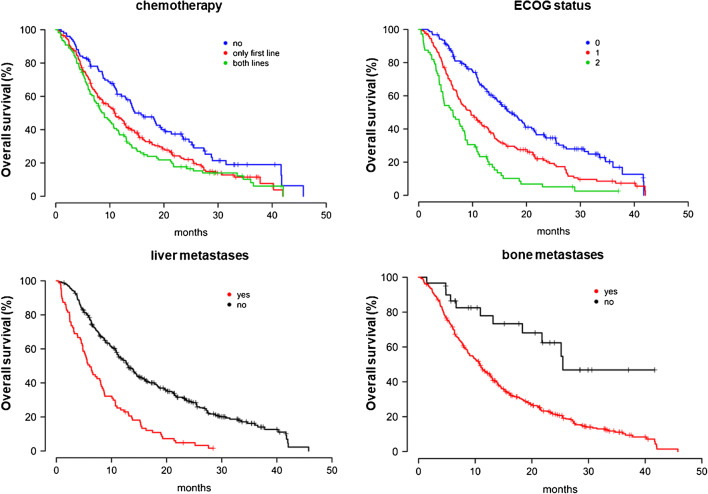
The median OS in patients who received one or two lines of chemotherapy with docetaxel or docetaxel followed by cabazitaxel, respectively, was 10.9 months (95% CI 9.05–12.76) and 8.9 months (95% CI 6.9–10.9). The OS was significantly shorter than in patients without any prior chemotherapy with a median OS of 14.6 months (95% CI 10.3–18.4) (Fig. 1). Out of 102 patients without chemotherapy, 83 patients avoided chemotherapy despite lacking contraindications, and for 19, it was contraindicated. The median OS in the first group was 15.8 months (95% CI 11.4–20.1) and in the second group was 14.0 months (95% CI 3.7–24.4), p = 0.29.
Fig. 1.
Overall survival of patients for chemotheraphy, liver metastases, ECOG status, and bone metastases
Thirty patients without bone metastases (Table 3; supp. Fig. 1) only had lymph node metastases, and these patients showed the longest median OS.
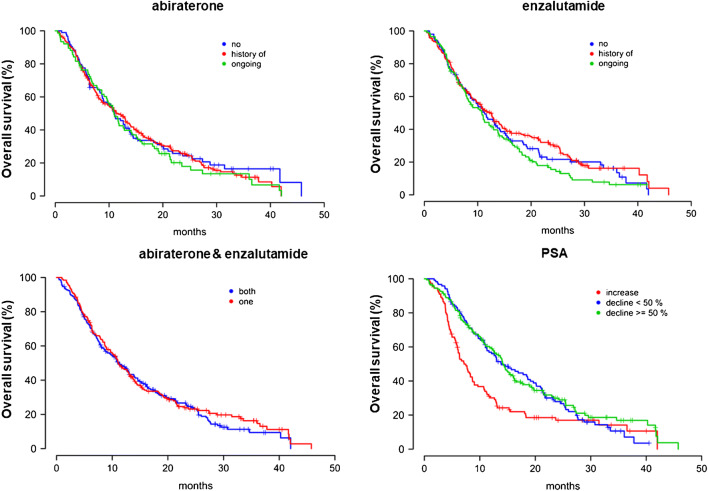
Although a prior anti-hormonal therapy with either abiraterone or enzalutamide or both was not a significant predictive factor, there was a significant difference between the OS of patients with a history of enzalutamide and patients who were under concurrent usage of enzalutamide during 177Lu-PSMA-617 treatment (12.3 vs 10.8 months, respectively; p = 0.045) (Fig. 2).
Fig. 2.
Overall survival of patients who undergone abiraterone, enzalutamide, abiraterone and enzalutamide, and PSA medications
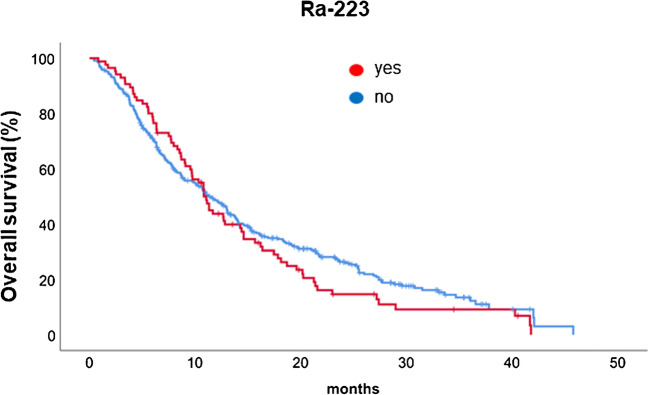
Patients with prior radium-223 therapy showed a median OS of 10.8 months (95% CI 9.8–11.9 months) vs a median of OS of 11.3 months (95% CI 9.5–13.0 months) in patients without prior radium-223 (p = 0.34) (Fig. 3).
Fig. 3.
Overall survival of patients who undegone Ra-223 treatment
The median OS of patients with a PSA decline of less than 50% as well as with a decline ≥ 50% was significantly longer than patients with a rising PSA after the first cycle. The median OS of patients with rising PSA, decline < 50%, and decline ≥50% was 7.2 months (95% CI 5.6–8.7), 13.9 months (95% CI 10.1–17.7), and 14.3 months (95% CI 12.6–15.9), respectively (p < 0.0001). There was no significant difference between patients with more or less than 50% PSA decline regarding OS (p = 0.6) (Fig. 2).
Discussion
The approved therapies for patients with mCRPC that can significantly improve OS are next-generation anti-hormonal therapies (abiraterone and enzalutamide), first- and second-line chemotherapies with docetaxel and cabazitaxel, and alpha radionuclide therapy with radium-223 [20]. Currently, several novel agents, such as immunotherapeutics or therapies targeting poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, are under advanced clinical investigation [20]. Radionuclide imaging and therapy using PSMA as a target have been investigated for more than two decades beginning with monoclonal antibodies that were later replaced by low molecular weight PSMA ligand inhibitors [21]. Since 2015, there have been increasing numbers of published data showing promising efficacy and a low toxicity profile of these agents in mCRPC patients [6, 7, 11, 22].
Although this therapy is still experimental and is mainly given in compassionate-use scenarios only, different societies have published guidelines or recommendations trying to standardize this therapy for patients with mCRPC [23–26]. The most important limitations of the published data are the limited number of included patients and the heterogeneity of patients regarding prior therapies.
Ahmadzadehfar et al. reported a median OS of 60 weeks in 100 patients who received a median of 3 cycles of 177Lu-PSMA-617 (total 347 cycles). All of the patients had a history of therapy with either enzalutamide or abiraterone, or both. At least one line of chemotherapy had been performed in 70% of the patients, and 36% had a history of radionuclide therapy with radium-223. Here, a PSA decline after the first RLT, as well as a decline ≥ 50%, was significant prognosticators of longer OS. Rahbar et al. [14] reported an OS of 56 weeks in 104 patients treated with 351 cycles of 177Lu-PSMA-617. All of these patients had a history of therapy with at least one line of chemotherapy, as well as either abiraterone or enzalutamide. Both studies showed that patients who respond to PSMA therapy live longer than those who do not. In these studies, prior therapies, such as chemotherapy, had no impact on OS. Heck et al. [11] reported the results of therapies using [177Lu]Lu-PSMA-I&T in 100 patients with mCRPC who received a total number of 319 cycles (median 2 cycles). Here, the median OS was 12.9 months (95% CI 9.9–15.9). The included patients were comparable with those of our cohort regarding prior therapies.
Heck et al. also showed a significant correlation between PSA response under RLT and survival. They analyzed the PSA changes within 12 weeks of RLT. According to their analysis, a maximum PSA decline of ≥ 50% was associated with longer OS (median 16.7 (n = 32) vs 6.2 (n = 60) months, p = 0.007). In our current study, the median OS of patients with rising PSA, decline < 50%, and decline ≥50% were 7.2 months, 13.9 months, and 14.3 months, respectively (p < 0.0001); however, there was no significant difference between patients with more or less than 50% decline regarding OS (p = 0.6) by measuring PSA 8 weeks after the first cycle. This difference may be due to the measuring time point of PSA. The best response, within 12 weeks, means that the majority of patients got two cycles of therapy; on the other hand, it seems that Heck et al. did not differentiate between rising PSA and a decline of less than 50%.
Despite PCWG3’s suggestion to measure PSA decline after 12 weeks in clinical trials, prior studies as well as the present study found that a good response to the first cycle (a PSA decline, measured 2 months after the first cycle) is associated with a favorable response to further cycles in more than 90% of patients [13, 27].
Barber et al. reported the results of 167 mCRPC patients who were treated with 177Lu-PSMA-617 or 177Lu-PSMA-I&T [12]. The patients were divided into two groups according to prior therapy with taxane-based chemotherapy. The median OS in 83 patients in the taxane-pretreated group was 10.7 months; it was 27.1 months in 84 patients in the taxane-naïve group [12]. In the taxane-pretreated group, 76% and 14% of patients prior to RLT had received abiraterone or enzalutamide and Ra-223 treatments, respectively, while only 38% and 2% of patients in the taxane-naïve group had received abiraterone or enzalutamide and Ra-223 therapies, respectively [12]. This likely means that the long OS of 27.1 months in the taxane-naïve group was caused by the fact that about 60% of the patients in this group had received an RLT as a first-line therapy, thus the natural course of their prostate cancer was associated with a significantly longer OS than that of the patients who had been treated with various mCRPC-approved compounds prior to 177Lu-PSMA-RLT. Thus, despite longer OS in the taxane-naïve group, in the multivariate analysis, prior chemotherapy was not a significant prognosticator of overall survival [12].
In our multicenter analysis, all of the patients had received abiraterone or enzalutamide, and 53.6% had been treated with both agents. A total of 75.5% had at least a history of first-line chemotherapy with docetaxel. A therapy with Ra-223 had been done in 20.4% of the patients. Altogether, the included patients in this retrospective multicenter analysis were heavily pretreated. Despite prior therapies and advanced disease (92.8% and 20.9% bone and liver involvement, respectively), the median OS was 11.1 months (95% CI 9.7–12.5 months) and was comparable with the taxane-pretreated group of Barber et al. and other prior studies [11–14].
In terms of treatment planning, having predictive parameters for a favorable response and prognosticators of longer OS is of importance for us as clinicians, first, to decide on the indication of a therapy, and second, to accurately inform patients about the treatment response rate and their prognosis for survival. In the current study, age, GS, prior therapies with abiraterone or enzalutamide as well as Ra-223, and the existence of lymph node as well as lung metastases were not significant prognosticators of OS, while prior chemotherapy, ECOG status and the existence of bone and liver metastases were prognosticators of OS in both univariate and multivariate analysis.
As mentioned above, a prior therapy with enzalutamide did not have a significant impact on OS; however, there was a significant difference between the OS of patients with a history of enzalutamide and the OS of patients who were under ongoing usage of enzalutamide (12.3 vs 10.8 months, respectively; p = 0.045). These findings should be further explored in future studies. One explanation could be the negative impact of enzalutamide on PSMA expression, reducing the tumor-absorbed dose, or an agonistic effect of enzalutamide on patients who do not respond any more to this agent. The potential impact of enzalutamide and abiraterone on the efficacy of PSMA-RLT when used concurrently is therefore very interesting, even more so since the current phase III registrational trial, VISION, tests PSMA-RLT in combination with enzalutamide or abiraterone only. Thus, in the future, depending on the results of VISION [28] and subgroup analyses, a PSMA monotherapy may have to be compared with a combination therapy including abiraterone or enzalutamide. For some patients, PSMA-RLT alone may be sufficient.
Patients who had received a prior therapy of Ra-223 showed a longer OS during the first 10 months as compared with patients without any Ra-223 (Fig. 3). Ra-223 is a bone-seeking alpha-emitting radionuclide acting in reactive bone forming cells adjacent to cancer cells. This therapy has demonstrated a median OS of 12.4 months alone in 150 patients [29]. The early improvement of OS is thus possible due to existing long-acting synergistic Ra-223 effect; this effect lasts typically 4–5 months, and thus an average of 7–9 months benefit is expected. After 10 months, the 177Lu-PSMA-617 showed better OS in those patients who did not receive Ra-223.
The existence of visceral metastases is a negative prognostic factor, as other studies have shown [11, 12, 30]. Although in our multicenter study patients without prior chemotherapy showed a significantly longer OS, we should take into consideration that some of these patients who had initially avoided chemotherapy received it after having progressed on PSMA treatment. Future trials will have to elucidate the ideal position of PSMA-RLT within the ever-growing armamentarium of therapies for patients with mCRPC. This issue cannot be analyzed in a retrospective setting, and it should thus be evaluated in a prospective setting.
Since we included patients from different departments, we did not analyze baseline laboratory parameters such as blood count, alkaline phosphatase, LDH, and different tumor markers other than PSA, first, because different laboratories have different ranges of normal, and second, because apart from blood counts, the other parameters were not checked routinely in all clinics.
Limitations
One of the most important limitations of this study is its retrospective design; however, we tried to exclude all patients with unclear follow-up or documentation from the analysis. The high proportion of patients who were excluded in this analysis is a major drawback of the analysis and is due to the retrospective design and recorded data mostly in clinical routine and different countries.
Additional limiting factor is the timepoint of calculation of survival rates, especially according to performed chemotherapy, this might represent a lag-time bias. This might be overcome with results of the prospective trials, which are currently running [28, 31].
Conclusion
In the present multicenter analysis, the median OS was 11.1 months, whereas the patients without prior chemotherapy showed a significantly longer OS, 14.6 months. This remained independent in the multivariate analysis besides presence of bone and liver metastases as negative prognosticators for survival, whereas an ECOG of 0–1 is associated with a longer OS. Results of the phase III VISION trial are eagerly awaited to bring this effective therapy to approval.
Electronic supplementary material
(PNG 246 kb)
(DOCX 13 kb)
Funding Information
Open Access funding provided by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Oncology - Genitourinary
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hojjat Ahmadzadehfar and Kambiz Rahbar contributed equally to this work.
References
- 1.Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Cascinu S, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014;28:555–563. [PubMed] [Google Scholar]
- 2.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 3.Tolkach Y, Gevensleben H, Bundschuh R, Koyun A, Huber D, Kehrer C, et al. Prostate-specific membrane antigen in breast cancer: a comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res Treat. 2018;169:447–455. doi: 10.1007/s10549-018-4717-y. [DOI] [PubMed] [Google Scholar]
- 4.Bouchelouche K, Choyke PL, Capala J. Prostate specific membrane antigen-a target for imaging and therapy with radionuclides. Discov Med. 2010;9:55–61. [PMC free article] [PubMed] [Google Scholar]
- 5.Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22:9–15. doi: 10.1158/1078-0432.CCR-15-0820. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. doi: 10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadzadehfar H, Rahbar K, Essler M, Biersack HJ. PSMA-based theranostics: a step-by-step practical approach to diagnosis and therapy for mCRPC patients. Semin Nucl Med. 2020;50:98–109. doi: 10.1053/j.semnuclmed.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Aghdam RA, Amoui M, Ghodsirad M, Khoshbakht S, Mofid B, Kaghazchi F, et al. Efficacy and safety of (177)lutetium-prostate-specific membrane antigen therapy in metastatic castration-resistant prostate cancer patients: first experience in west Asia-a prospective study. World J Nucl Med. 2019;18:258–265. doi: 10.4103/wjnm.WJNM_66_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 10.Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of (177)Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17:15–22. doi: 10.1016/j.clgc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for Radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–926. doi: 10.1016/j.eururo.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of (177)Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60:955–962. doi: 10.2967/jnumed.118.216820. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kurpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–1454. doi: 10.1007/s00259-017-3716-2. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schafers M, Essler M, et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45:12–19. doi: 10.1007/s00259-017-3848-4. [DOI] [PubMed] [Google Scholar]
- 15.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57:97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadzadehfar H, Zimbelmann S, Yordanova A, Fimmers R, Kurpig S, Eppard E, et al. Radioligand therapy of metastatic prostate cancer using (177)Lu-PSMA-617 after radiation exposure to (223)Ra-dichloride. Oncotarget. 2017;8:55567–55574. doi: 10.18632/oncotarget.15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahbar K, Bogeman M, Yordanova A, Eveslage M, Schafers M, Essler M, et al. Delayed response after repeated (177)Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:243–246. doi: 10.1007/s00259-017-3877-z. [DOI] [PubMed] [Google Scholar]
- 19.Brauer A, Grubert LS, Roll W, Schrader AJ, Schafers M, Bogemann M, et al. (177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–1670. doi: 10.1007/s00259-017-3751-z. [DOI] [PubMed] [Google Scholar]
- 20.Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99. doi: 10.1016/j.eururo.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Vahidfar N, Fallahpoor M, Farzanehfar S, Divband G, Ahmadzadehfar H. Historical review of pharmacological development and dosimetry of PSMA-based theranostics for prostate cancer. J Radioanal Nucl Chem. 2019;322:237–248. doi: 10.1007/s10967-019-06800-6. [DOI] [Google Scholar]
- 22.Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benesova M, Mier W, et al. [(1)(7)(7)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:987–988. doi: 10.1007/s00259-014-2978-1. [DOI] [PubMed] [Google Scholar]
- 23.Vorster M, Warwick J, Lawal IO, Du Toit P, Vangu M, Nyakale NE, et al. South African guidelines for receptor radioligand therapy (RLT) with Lu-177-PSMA in prostate cancer. S Afr J Surg. 2019;57:45–51. doi: 10.17159/2078-5151/2019/v57n4a3107. [DOI] [PubMed] [Google Scholar]
- 24.Kratochwil C, Fendler WP, Eiber M, Baum R, Bozkurt MF, Czernin J, et al. EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT) Eur J Nucl Med Mol Imaging. 2019;46:2536–2544. doi: 10.1007/s00259-019-04485-3. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadzadehfar H, Aryana K, Pirayesh E, Farzanehfar S, Assadi M, Fallahi B, et al. The Iranian Society of Nuclear Medicine practical guideline on radioligand therapy in metastatic castration-resistant prostate cancer using 177Lu-PSMA. Iranian J Nuclear Med. 2018;26:2–8. [Google Scholar]
- 26.Fendler WP, Kratochwil C, Ahmadzadehfar H, Rahbar K, Baum RP, Schmidt M, et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin. 2016;55:123–128. doi: 10.1055/s-0037-1616480. [DOI] [PubMed] [Google Scholar]
- 27.Kratochwil C, Giesel FL, Stefanova M, Benesova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–1176. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 28.Rahbar K, Bodei L, Morris MJ. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase III VISION trial and its importance for the future of theranostics. J Nucl Med. 2019;60:1504–1506. doi: 10.2967/jnumed.119.234054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kairemo K, Milton DR, Etchebehere E, Rohren EM, Macapinlac HA. Final outcome of 223Ra-therapy and the role of 18F-fluoride-PET in response evaluation in metastatic castration-resistant prostate cancer-a single institution experience. Curr Radiopharm. 2018;11:147–152. doi: 10.2174/1874471011666180629145030. [DOI] [PubMed] [Google Scholar]
- 30.Kessel K, Seifert R, Schafers M, Weckesser M, Schlack K, Boegemann M, et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving (177)Lu-PSMA-617. Theranostics. 2019;9:4841–4848. doi: 10.7150/thno.35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofman MS, Emmett L, Violet J, Zhang AY, Lawrence NJ, Stockler M, et al. TheraP: a randomized phase 2 trial of (177) Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603) BJU Int. 2019;124(Suppl 1):5–13. doi: 10.1111/bju.14876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 246 kb)
(DOCX 13 kb)