Abstract
The AlcR fungal protein responds to ethanol and binds to the fungal pAlcA promoter in its presence. This system was transferred to plants over twenty years ago and was claimed to function in the same manner in plants. However, never has the control experiment with plants containing the AlcR gene alone, with no downstream inducible construct, been made. In this paper, I conduct several experiments with this control, growing p35:AlcR plants in the presence or absence of ethanol. I found that when these plants were grown in the presence of ethanol, growth in several tissues and several stages of growth was retarded. This demonstrates that this system is not suitable for use in the plant sciences, and casts doubt on the conclusions of papers that have published phenotypes using this system.
Subject terms: Genetic techniques, Genetic engineering, Biological techniques, Developmental biology, Plant sciences
Introduction
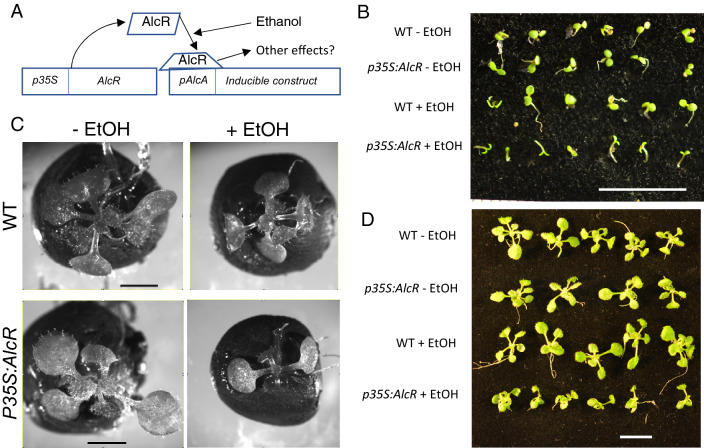
In 1998, Caddick et al.1 cloned the AlcR gene from Aspergillus nidulans along with the pAlcA promoter from the same species. Within this fungus, the AlcR protein responds to the presence of ethanol by activating an alcohol dehydrogenase gene, which is regulated by the pAlcA promoter that is bound by activated AlcR protein. It was demonstrated1 that induction of the expression of genes under the control of the pAlcA promoter occurred in plants expressing AlcR in the presence of ethanol. It was thus proclaimed that this system could be used for tissue- or developmental-stage induction of genes or other genetic objects using the simple ethanol molecule—as vapour or liquid—which is non-toxic to plants in doses well above those required for induction (Fig. 1A).
Figure 1.
Post germination growth is retarded in p35S:AlcR seedlings. (A) Model of the supposed mechanism of action of the ethanol-inducible system in plants. (B) 3 DAS seedlings grown together in the presence of ethanol since stratification. Reduced growth can be seen in the bottom row. Scale bar = 10 mm. (C) Ethanol was added to a subset of seedlings at 3 DAS and images were taken at 7 DAS. Scale bar = 3 mm. (D) Ethanol was added at 6 DAS and images taken at 12 DAS. Again, growth is retarded in the p35S:AlcR seedlings when grown in the presence of ethanol. Scale bar = 10 mm.
However, one fundamental control was missing in this publication1: plants lacking the pAlcA promoter and any downstream inducible construct were never analysed. It was therefore not shown whether or not the AlcR protein, in the presence of ethanol, had any effect on plant growth and development. This fundamental control has never been included in any publications that I have read that utilised this system. For example, shortly after the publication of the use of this system in plants, tissue-specific induction of various genes in Arabidopsis plants by placing the AlcR gene under the control of tissue-specific promoters was shown2, but again, no control with plants expressing the AlcR gene alone and grown in the presence of ethanol was shown.
ABP1 (Auxin-binding protein 1) was the first protein reported to bind auxin as a receptor in plants. Early studies on an ABP1 orthologue in Zea mays showed that this ortholog of Arabidopsis ABP1 preferentially bound to an artificial auxin equivalent3. In vitro studies contributed to the hypothesis that ABP1 was a membrane-associated auxin binding protein4. Many studies went on to identify phenotypes of abp1 knock-down mutants, establishing ABP1 as an important auxin receptor required for many growth and developmental processes. Since an initial abp1 T-DNA insertion line was embryo-lethal, other mutants and means to knock down ABP1 were sought. An alternative insertion mutant was identified4 and was used in combination with knock down mutants in several studies.
In 2015, Gao et al.5 used CRISPR-Cas9 technology to create novel abp1 null mutants. They showed that independently-derived null mutants showed none of the previously described knock-down or insertion mutant phenotypes, and that the plants grew in a manner indistinguishable from WT plants. Furthermore, the plants remained as sensitive to auxin as WT plants. Thence came the question of why all of the previous publications showing abp1 knock-down phenotypes apparently drew the wrong conclusions. Michalko et al.6 showed that embryo-lethality in early T-DNA mutants was due to an additional T-DNA insertion in the BSM gene.
Many of the studies that had analyzed abp1 knock-down phenotypes used the ethanol-inducible system to induce ABP1-antisense RNA and antibody peptide fragments that were assumed to immobilise ABP1 and prevent it from functioning. In 2016 it was shown that abp1 null mutants demonstrated phenotypes only when the antisense RNA or antibody fragments were induced7. It was concluded that the phenotypes were due to off-target effects of the antisense RNA and antibody fragments, and not due to knock-down of the ABP1 gene. However, it was not considered that the ethanol inducible system itself might be causing artefacts.
Here, growth phenotypes of plants expressing the AlcR gene under the control of the constitutively active Cauliflower Mosaic virus p35S promoter in the absence of any downstream inducible construct are observed.
Results
Post-germination growth is retarded in p35S:AlcR seedlings in the presence of ethanol vapour
p35S:AlcR plants were PCR genotyped to confirm that no pAlcA promoter was present (Supp. Fig. 1). Initially, post-germination growth was observed in WT and p35S:AlcR seedlings in the presence and absence of 50% ethanol vapour (Fig. 1B). Growth proceeded equally in WT seedlings in the presence and absence of ethanol, whereas post-germination growth was retarded in p35S:AlcR seedlings in the presence of ethanol. In the absence of ethanol, p35S:AlcR seedlings grew normally. Since a retarded growth phenotype was observed in p35S:AlcR seedlings in the presence of ethanol, ethanol was added at later stages of growth in all further experiments.
Seedling growth is retarded in p35S:AlcR seedlings in the presence of ethanol vapour
Tubes containing 50% v/v ethanol were added to WT and p35S:AlcR seedling-containing plates at 7 DAS (days after stratification). At 12 DAS, WT seedlings in the presence and absence of ethanol, as well as p35S:AlcR seedlings, grew at similar rates, showing the growth of true leaves (Fig. 1C). p35S:AlcR seedlings grown in the presence of ethanol were retarded in seedling growth, with only the appearance of small juvenile leaves, while other seedlings had already grown several true leaves (Fig. 1C). To confirm these findings, seedlings were grown for 5 days without ethanol, following which 50% ethanol was added. Images of seedlings were then taken after 12 days of growth (7 days in the presence of ethanol) (Supp. Fig. 2). These images confirm that pAlcR seedlings display retarded growth in the presence of ethanol.
Rosette growth is retarded in p35S:AlcR seedlings in the presence of ethanol
To observe the effect of the AlcR protein in the presence of ethanol, WT and p35S:AlcR seedlings were grown in the absence of ethanol for 7 days, after which ethanol was added to ca. half of the population of seedlings. Since the 50% v/v ethanol vaporises, ethanol was replenished every two days during further growth. While WT rosettes in the presence and absence of ethanol, as well as p35S:AlcR rosettes in the absence of ethanol, grew normally, p35S:AlcR rosettes in the presence of ethanol demonstrated retarded growth (Fig. 1D).
Root growth is retarded in p35S:AlcR seedlings in the presence of ethanol
To analyse the effects of ethanol vapour on p35S;AlcR seedling roots, ethanol was added (or not) to 6 DAS WT and p35S:AlcR seedlings. Ethanol was again replenished every two days to counteract vaporisation and subsequent loss of ethanol. While root growth proceeded normally in both WT and p35S:AlcR plants until 6 DAS, growth slowed dramatically in p35S:AlcR plants in the presence of ethanol after 6 DAS (Fig. 2A,B).
Figure 2.
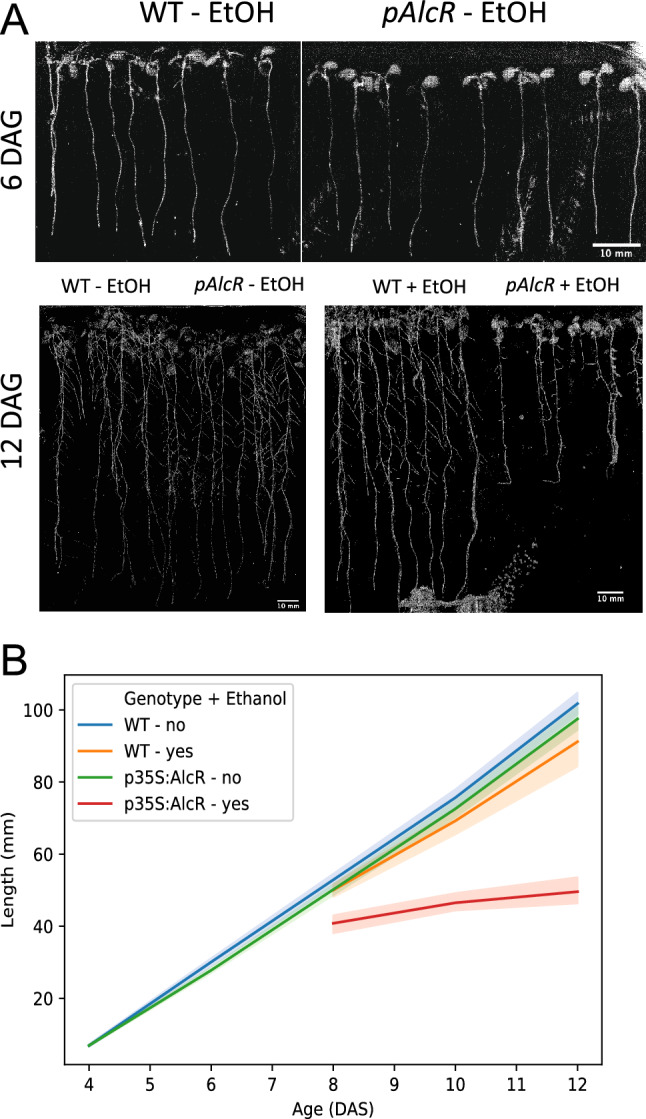
Root growth is retarded in p35S:AlcR seedlings in the presence of ethanol, while WT seedling growth proceeds normally (A, B). Ethanol was added at 6 DAS. (A) Scans of roots at the end of the experiment. Scale bar = 10 mm. (B) Line plots showing the progression of growth under all conditions. At least ten roots were measured per data point. Shaded areas surrounding lines lines represent 95% confidence intervals.
Discussion
The results described here demonstrate that the AlcR-pAlcA ethanol inducible system used in many plant science projects over the last two decades has a gross growth artefact phenotype. Since the phenotype is only present when ethanol is present, it would appear that the phenotype has nothing to do with the insertion site of the construct but is rather solely due to the presence of activated AlcR protein.
Many of the projects utilizing the system were studies of ABP1 function in Arabidopsis thaliana1, 2, 4, 8. For example, reduced cell division activity and retarded post embryonic shoot development was described following induction of ABP1 antisense RNA and ABP1-sequestering antibody-derived peptide fragments8. Chen et al.9 analysed this cellular growth phenotype at a cellular level, but again employed the ethanol inducible system. They observed inverted microtubule orientation following ethanol induction, the reversed orientation explaining the reduced cell elongation phenotype. As show in Fig. 1, this phenotype could easily be due to the presence of activated AlcR protein. However, the system has been used to study the function of various other genes. For example, Zürcher et al.10 used the system to knock down genes involved in the establishment of cytokinin sinks. Phenotypes in root cells were observed; these phenotypes could result from the p35S:AlcR phenotype, since root growth was retarded in the presence of activated AlcR (Fig. 2). Since the artefact phenotype seems to be global and strong, it is likely that most if not all of the phenotypes reported in these publications arise from the effects of activated AlcR protein, and thus the conclusions of these publications will most likely be false.
To prevent any further waste of resources, including researchers’ work input, this system should cease to be used in plants. Furthermore, publications that used this system to demonstrate phenotypes11–18 should be heavily revised or removed from the literature, since their remaining presence will lead some researchers to plan projects based on false information. Macro phenotypes arise from micro phenotypes, and thus publications investigating micro-cellular effects when the ethanol-inducible system is used to induce some gene or other genetic object should just as importantly be reviewed.
One should also beware of other versions of the ethanol inducible system that have been developed and still utilise the AlcR protein19–21.
Materials and methods
Plant lines and media
P35S:AlcR (N67789) and p35S:AlcR pAlcA:LHY (N67791) lines were obtained from NASC, Loughborough, UK. Seeds used, including WT Col-0, were obtained from plants grown side-by-side prior to experiments using the harvested seeds. ½ MS media containing Gamborg B5 Vitamins was used for growth. Light intensity for all experiments was ca. 100 µE. Temperature was maintained at 22 °C.
Ethanol induction
200 mL tubes containing 100 µL 50% v/v were mobilised in plates by partially embedding them in the growth media. Ethanol was replenished every two days following initial addition to counteract evaporation.
Images of seedlings
Colour seedling images were taken using the Nikon D7000 DSLR camera. Grey scale images were taken using the Leica M60 ergo dissecting microscope.
Root growth measurements
Root growth was manually measured in Fiji. For plotting, Python Pandas (https://pandas.pydata.org) and Seaborn https://seaborn.pydata.org) packages were used.
Supplementary Information
Acknowledgements
I thank Célia Baroux for allowing me to conduct this project as a side project, and Ueli Grossniklaus for infrastructure support.
Author contributions
The author planned and carried out all experiments and prepared all figures. The author wrote the manuscript alone.
Competing interests
The author declares no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80903-z.
References
- 1.Caddick MX, et al. An ethanol inducible gene switch for plants used to manipulate carbon metabolism. Nat. Biotechnol. 1998;16:177–180. doi: 10.1038/nbt0298-177. [DOI] [PubMed] [Google Scholar]
- 2.Deveaux Y, et al. The ethanol switch: A tool for tissue-specific gene induction during plant development. Plant J. Cell Mol. Biol. 2004;36:918–930. doi: 10.1046/j.1365-313X.2003.01922.x. [DOI] [PubMed] [Google Scholar]
- 3.Hertel R, Thomson KS, Russo VEA. In-vitro auxin binding to particulate cell fractions from corn coleoptiles. Planta. 1972;107:325–340. doi: 10.1007/BF00386394. [DOI] [PubMed] [Google Scholar]
- 4.Tromas A, Paponov I, Perrot-Rechenmann C. AUXIN BINDING PROTEIN 1: Functional and evolutionary aspects. Trends Plant Sci. 2010;15:436–446. doi: 10.1016/j.tplants.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, et al. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl. Acad. Sci. USA. 2015;112:2275–2280. doi: 10.1073/pnas.1500365112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalko J, Dravecká M, Bollenbach T, Friml J. Embryo-lethal phenotypes in early abp1 mutants are due to disruption of the neighboring BSM gene. F1000Res. 2015;4:1104. doi: 10.12688/f1000research.7143.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalko J, Glanc M, Perrot-Rechenmann C, Friml J. Strong morphological defects in conditional Arabidopsis abp1 knock-down mutants generated in absence of functional ABP1 protein. F1000Res. 2016;5:86. doi: 10.12688/f1000research.7654.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun N, et al. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell. 2008;20:2746–2762. doi: 10.1105/tpc.108.059048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, et al. Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature. 2014;516:90–93. doi: 10.1038/nature13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zürcher E, Liu J, di Donato M, Geisler M, Müller B. Plant development regulated by cytokinin sinks. Science. 2016;353:1027. doi: 10.1126/science.aaf7254. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia R, Brambilla V, Colombo L, Stuitje AR, Kater MM. Functional analysis of MADS-box genes controlling ovule development in Arabidopsis using the ethanol-inducible alc gene-expression system. Mech. Dev. 2006;123:267–276. doi: 10.1016/j.mod.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Maizel A, Weigel D. Temporally and spatially controlled induction of gene expression in Arabidopsis thaliana. Plant J. 2004;38:164–171. doi: 10.1111/j.1365-313X.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 13.Paque S, et al. AUXIN BINDING PROTEIN1 links cell wall remodeling, auxin signaling, and cell expansion in arabidopsis. Plant Cell. 2014;26:280–295. doi: 10.1105/tpc.113.120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert S, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143:111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, et al. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat. Commun. 2019;10:5093. doi: 10.1038/s41467-019-13074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tromas A, et al. The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE. 2009;4:e6648. doi: 10.1371/journal.pone.0006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tromas A, et al. Auxin-binding protein 1 is a negative regulator of the SCFTIR1/AFB pathway. Nat. Commun. 2013;4:2496. doi: 10.1038/ncomms3496. [DOI] [PubMed] [Google Scholar]
- 18.Landrein B, et al. Mechanical stress contributes to the expression of the STM homeobox gene in Arabidopsis shoot meristems. Elife. 2015;4:e07811. doi: 10.7554/eLife.07811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakvarelidze L, et al. Coupling the GAL4 UAS system with alcR for versatile cell type-specific chemically inducible gene expression in Arabidopsis. Plant Biotechnol. J. 2007;5:465–476. doi: 10.1111/j.1467-7652.2007.00254.x. [DOI] [PubMed] [Google Scholar]
- 20.Roberts GR, et al. The alc-GR system: A modified alc gene switch designed for use in plant tissue culture. Plant Physiol. 2005;138:1259–1267. doi: 10.1104/pp.105.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia H, Van Loock B, Liao M, Verbelen JP, Vissenberg K. Combination of the ALCR/alcA ethanol switch and GAL4/VP16-UAS enhancer trap system enables spatial and temporal control of transgene expression in Arabidopsis. Plant Biotechnol. J. 2007;5:477–482. doi: 10.1111/j.1467-7652.2007.00255.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.