Abstract
Herlyn-Werner-Wunderlich syndrome, is a rare urogenital congenital anomaly. Coexisting Mullerian ducts anomalies and ovarian neoplasms are rarely reported. We present the first case of Herlyn-Werner-Wunderlich syndrome with borderline serous neoplasm of the ovary. A 29-year-old married female with primary infertility and elevated level of cancer antigen 125 (CA-125), underwent pelvic magnetic resonance imaging for evaluation which revealed uterus didelphys, obstructed right hemivagina, right renal agenesis as well as right ovarian cystic lesion. Ovary preserving laparoscopic cystectomy was performed with a pathological diagnosis of a serous borderline tumor. Although rarely reported, Mullerian ducts anomalies and uterine or ovarian remnant neoplasms can occur. These few case reports may suggest an underlying, yet to be discovered, genetic association of Mullerian ducts anomalies and development of ovarian neoplasms of various pathological subtypes.
Keywords: Congenital anomalies, Kidneys, Ovarian neoplasms
Introduction
Herlyn-Werner-Wunderlich syndrome (HWWS), is a rare congenital anomaly of the female urogenital tract caused by abnormalities in the development of the Mullerian and Wolffian ducts resulting in uterus didelphys, obstructed hemivagina, and associated ipsilateral renal anomaly [1]. Renal anomalies range from ectopic ureteric insertion and renal dysplasia to complete ipsilateral renal agenesis [2,3]. This syndrome is regarded as Class III Mullerian dysgenesis and constitutes around 5%-10% of the reported Mullerian duct dysgenesis cases.
Patients with this syndrome are usually diagnosed around or after puberty and present with dysmenorrhea, urine retention, endometriosis, or complicated delivery [2,4]. Patients with Mullerian dysgenesis syndrome have normal ovarian development. Few cases have been reported in the current literature about the development of uterine or ovarian tumors in patients with different classes of Mullerian dysgenesis but up to the authors’ best knowledge, there are no previous reports of ovarian tumor development in HWWS [5], [6], [7]. Thus, we report the first case of ovarian tumor development in HWWS.
Case report
We report a case of 29-year-old married female that was being evaluated in a private hospital for primary infertility where a routine gynecological ultrasound was preformed which reported a right ovarian complex lesion and uterus didelphys. She was referred to our hospital for a full evaluation.
Upon evaluation, the patient was asymptomatic with regular normal flow menses. Her general physical exam was unremarkable. Speculum examination showed a normal single vagina and single cervix. No abnormal discharge. A repeat gynecological ultrasound was performed by the treating gynecologist and reported a right ovarian complex lesion and uterus didelphys; hence a pelvic magnetic resonance imaging was ordered for evaluation. CA-125 level was elevated at 384 U/mL (Normal range: 0-35).
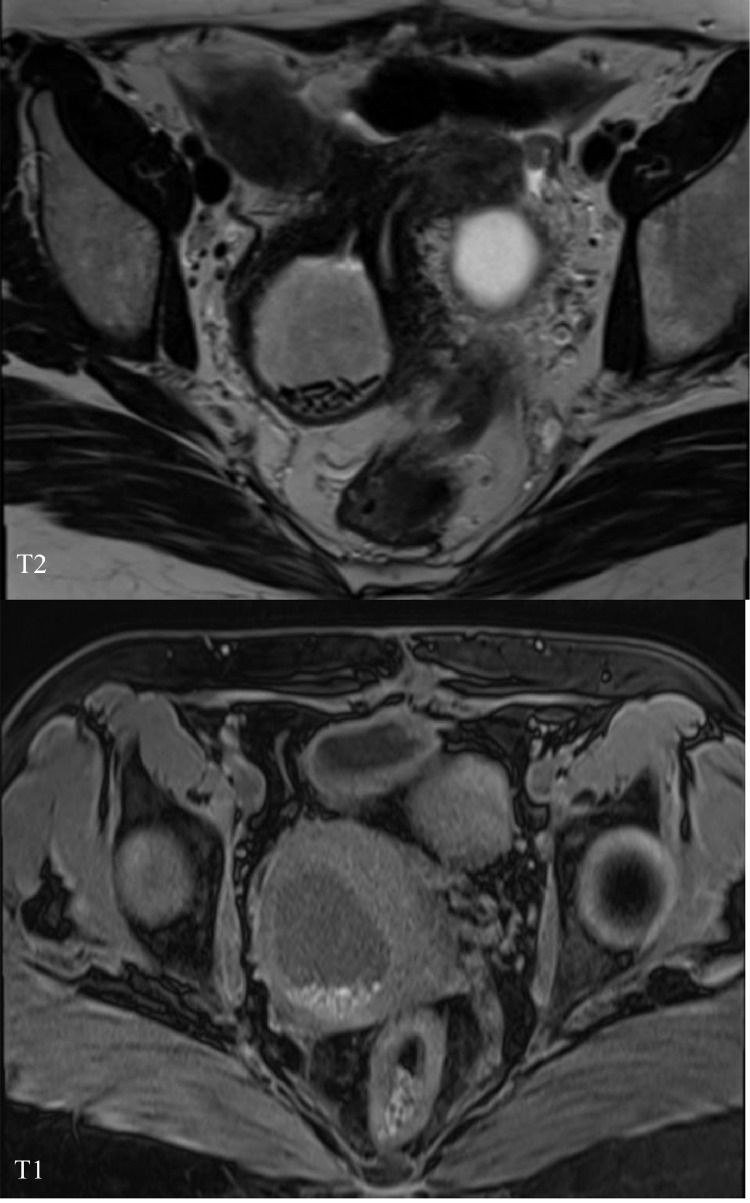
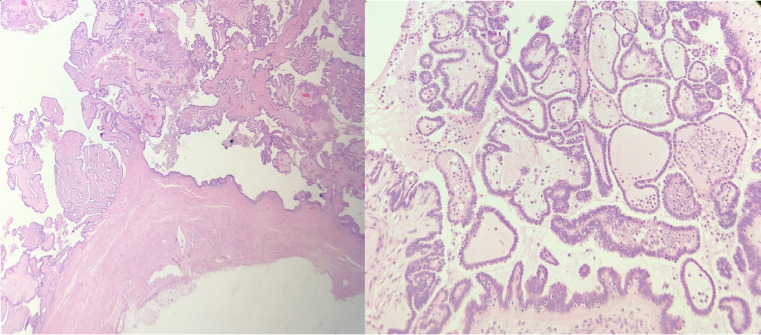
Pelvic magnetic resonance imaging revealed 2 normal uterine horns, 2 cervices, and 2 vaginas, in keeping with uterus didelphys, and an absent right kidney (Fig. 1). The right hemivagina was distended suggestive of obstructing vaginal septum and filled with T1 and T2 intermediate fluid as well as dependent high T1 and low T2 contents implicative of a repetitive hemorrhagic process (Fig. 2). There was also a large right ovarian cystic lesion showing intermediate T1 and T2 internal signals with enhancing papillary soft tissue components (Fig. 3). There was no evidence of peritoneal disease, lymph nodal involvement, or distant metastasis. The left ovary and left kidney were normal. Therefore, a diagnosis of HWWS with right ovarian neoplasm was made. The patient underwent a laparoscopic organ-preserving right ovarian cystectomy and the pathology report was of a serous borderline tumor (Fig. 4). The patient therefore did not require postoperative chemotherapy and was advised for annual clinical, radiological, and CA-125 level follow-up.
Fig. 1.
Coronal high resolution T2 of the pelvis and coronal HASTE of the abdomen demonstrate 2 separate uterine cavities, right ovarian cystic lesion and congenitally absent right kidney.
Fig. 2.
Axial T2 and axial nonenhanced T1 fat sat demonstrating 2 separate cervices and vaginas. The right hemivagina is obstructed with internal blood products.
Fig. 3.
Axial T2 at the level of the ovaries showing large right cystic lesion with internal frond like projections.
Fig. 4.
Postresection histopathology specimen of the resected serous borderline tumor: Left image (Hematoxylin and eosin stain, × 4), right image (Hematoxylin and eosin stain, × 20).
Concerning the uterine anomaly, the patient was not a candidate for a unification surgery. She underwent a laparoscopic right hemivaginal drainage for hematocolpos and vaginal septotomy. Fertility issues were discussed with the patient and she was a candidate for future in vitro fertilization.
Discussion
Mullerian duct anomalies are rare with varying reported worldwide prevalence ranging between 1% and 5.5% [8]. There are 7 classes of Mullerian duct anomalies according to the American Society of Reproductive Medicine classification with HWWS regarded as Class III Mullerian dysgenesis [9].
HWWS, is a rare congenital anomaly of the female urogenital tract caused by abnormalities in the development of the Mullerian and Wolffian ducts [1,2]. It has been frequently recognized as Mullerian dysgenesis over the past few decades, but this theory fails to explain the complexity of the vaginal abnormalities in this syndrome. To understand the complexity of this syndrome, it's important to review the current postulated theory on its embryogenesis. The mesonephric ducts on each side out-pouch to form the mesonephric diverticulum by the fifth week of gestation [10]. Soon after, the diverticulum will become the ureteric bud that triggers the kidney formation by the mesonephric blastema. The paramesonephric ducts will begin to develop by the seventh week of gestation lateral to the mesonephric ducts then migrate medially to it. It has been stated that the development of the paramesonephric ducts as well as their positioning and fusion depends largely on the factors released by the mesonephric ducts [11]. Subsequent fusion of the mesonephric ducts occurs and resorption of the midline septum results in normal uterine formation. The Acines hypothesis postulates that the vagina is derived from the Wolffian ducts instead of the classical theory of the upper two-thirds derived from the Mullerian ducts and lower third from the sinovaginal bulb, however, the vaginal lining epithelium is derived from the Mullerian ducts [11,12]. Hence, any developmental abnormality of the Mullerian ducts will affect the ipsilateral hemivagina, ureteric bud, and uterine fusion.
Patients with HWWS usually present around puberty with symptoms related to mass effect due to enlarging obstructed hemivagina, for example; vague cyclical lower abdominal pain, urine retention, lower abdominal mass, and pelvic inflammatory disease [2,4]. A retrospective cohort analysis reported 43 prepubertal patients with HWWS, most of which were diagnosed at birth or soon afterward by investigating concomitant renal anomalies [3].
Patients who were asymptomatic in the peripubertal period would present later in life with problems related to endometriosis or infertility [13]. Historically, The main treatment of those patients is usually symptomatic and was delivered in forms of vaginal drainage, vaginal septotomy with or without marsupialization [14]. A retrospective analysis of 14 patients with HWWS performed by Troncon et al (2018) [15], reported that most of the patients presented by after menarche with dysmenorrhea and are usually offered symptomatic treatment with removal of the obstructing vaginal septum. Also, 11 of 14 patients did not report natural successful pregnancy during 10 years of follow up. Few case reports exist of successful pregnancy outcomes in patients with uterus didelphys after intrauterine insemination or in vitro fertilization [16].
The development of uterine remnants neoplasms such as leiomyomas or adenomyosis has been reported in patients with Mullerian ducts anomalies [17]. Ovarian remnants neoplasms are rarely reported in these patients as they have normal ovarian development and function. A literature review by Miao et al (2018) [5], collected few case reports of Mullerian ducts anomalies with various types of ovarian neoplasms, the majority of which were found to be benign after histopathological evaluation. Huepenbecker et al (2017) [6], reported a rare occurrence of ovarian cystadenocarcinoma in two sisters affected by MRKH syndrome with normal 46XY karyotype and negative ovarian cancer genetic panel screening. Also, Villa et al (2019) [7], described a case of a 33-year-old patient with MRKH syndrome, ovarian low-grade serous carcinoma, and normal karyotype as well as negative screening for the breast cancer gene 1 and 2 (BRCA1/BRCA2).
Up to our best knowledge, no case report of HWWS with ovarian neoplasm exists to date. The authors are aware of the great possibility that the co-occurrence of those 2 entities is incidental as our patient had no family history of ovarian, uterine, or breast cancers with negative BRCA1/BRCA2 screening and a normal karyotype. However, the aforementioned few case reports may open an underlying genetic research window for such patients with complex multiorgan involvement.
Patient Consent Statement
Written informed consent from the patient is not required by the IRB of our institute for radiology case reports publications as there is no exposure of patient's identifying information.
Acknowledgment
All authors have nothing to acknowledge.
Footnotes
Case report location: Department of Medical Imaging, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
Support: No financial support was obtained.
Conflict of Interests: The authors have no conflicts of interest to report.
Contributor Information
Johara AlMulhim, Email: dr.johara1988@gmail.com.
Mohannad Rasheed AlRasheed, Email: mrrasheed@psmmc.med.sa.
References
- 1.Mehra S, Chamaria K, Garga UC, Kataria A, Ahuja A. Imaging diagnosis of Herlyn-Werner-Wunderlich syndrome- an extremely rare urogenital anomaly. J Clin Diagn Res. 2015;9(5):TD06–TTD8. doi: 10.7860/JCDR/2015/11123.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz S., Yildiz A.E., Fitoz S. Herlyn-Werner-Wunderlich syndrome: sonographic and magnetic resonance (MR) imaging findings of this rare urogenital anomaly. Polish J Radiol. 2017;82:216–219. doi: 10.12659/PJR.899889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han JH, Lee YS, Im YJ, Kim SW, Lee MJ, Han SW. Clinical implications of Obstructed Hemivagina and Ipsilateral Renal Anomaly (OHVIRA) Syndrome in the prepubertal age group. PloS one. 2016;11(11) doi: 10.1371/journal.pone.0166776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sleiman Z, Zreik T, Bitar R, Sheaib R, Al Bederi A, Tanos V. Uncommon presentations of an uncommon entity: OHVIRA syndrome with hematosalpinx and pyocolpos. Facts Views Vision ObGyn. 2017;9(3):167–170. [PMC free article] [PubMed] [Google Scholar]
- 5.Miao Y, Wen J, Huang L, Wu J, Zhao Z. Diagnosis and Management of Ovarian Tumor in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome. BioMed Res Int. 2018;2018 doi: 10.1155/2018/2369430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huepenbecker SP, Divine L, Chu CS, Mutch DG. Two sisters with Mayer-Rokitansky-Küster-Hauser syndrome and serous adenocarcinoma of the ovary. Gynecol Oncol Rep. 2017;22:13–15. doi: 10.1016/j.gore.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villa R, Azzollini J, Peissel B, Manoukian S. Co-occurrence of Mayer-Rokitansky-Küster-Hauser syndrome and ovarian cancer: a case report and review of the literature. Gynecol Oncol Rep. 2019;28:68–70. doi: 10.1016/j.gore.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behr S.C., Courtier J.L., Qayyum A. Imaging of Müllerian duct anomalies. RadioGraphics. 2012;32(6):E233–E250. doi: 10.1148/rg.326125515. [DOI] [PubMed] [Google Scholar]
- 9.The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955. doi: 10.1016/s0015-0282(16)59942-7. [DOI] [PubMed] [Google Scholar]
- 10.Aswani Y, Varma R, Choudhary P, Gupta RB. Wolffian origin of vagina unfolds the embryopathogenesis of OHVIRA (Obstructed Hemivagina and Ipsilateral Renal Anomaly) syndrome and places OHVIRA as a female counterpart of zinner syndrome in males. Polish J Radiol. 2016;81:549–556. doi: 10.12659/PJR.898244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acién P. REVIEW: embryological observations on the female genital tract. Hum Reproduc. 1992;7(4):437–445. doi: 10.1093/oxfordjournals.humrep.a137666. [DOI] [PubMed] [Google Scholar]
- 12.Asha B., Manila K. An unusual presentation of uterus didelphys with obstructed hemivagina with ipsilateral renal agenesis. Fertil Steril. 2008;90(3):849.e9–849.e10. doi: 10.1016/j.fertnstert.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Dhar H., Razek Y.A., Hamdi I. Uterus didelphys with obstructed right hemivagina, ipsilateral renal agenesis and right pyocolpos: a case report. Oman Med J. 2011;26(6):447–450. doi: 10.5001/omj.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yakıştıran Betül, Şükür Yavuz Emre, Turgay Batuhan, Atabekoğlu Cem. True management of obstructed hemi-vagina and ipsilateral renal anomaly syndrome. Turkish J Pbstetr Gynecol. 2016;13(4):208–211. doi: 10.4274/tjod.23434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troncon JK, Rosa-e-Silva JC, Miranda R, Candido-dos-Reis FJ, Poli-Neto OB, Nogueira AA. Diagnosis and treatment in a tertiary hospital of a series of complex genital malformations corresponding to double uterus with obstructed hemivagina and ipsilateral renal agenesis. Int J Reproduc Med. 2018;2018 doi: 10.1155/2018/3806856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carranza F, González-Ravina A, Blasco V, Fernández-Sánchez M. Different endometrial receptivity in each hemiuterus of a woman with uterus didelphys and previous failed embryo transfers. J Hum Reproduc Sci. 2018;11(3):297–299. doi: 10.4103/jhrs.JHRS_113_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girma W., Woldeyes W. Leiomyoma arising from mullerian remnant, mimicking ovarian tumor in a woman with MRKH syndrome and unilateral renal agenesis. Ethiop J Health Sci. 2015;25(4):381–384. doi: 10.4314/ejhs.v25i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]