Abstract
A 66‐year‐old man with a history of gastric pull‐up reconstruction for oesophageal cancer was hospitalized because of prolonged chest pain. Chest X‐ray demonstrated pneumopericardium. Computed tomography revealed ulceration and abscess in the gastric conduit adjacent to the heart, suggesting gastropericardial fistula. As the patient did not show tamponade physiology, he was conservatively treated with antibiotics. The pneumopericardium diminished; however, he developed effusive–constrictive pericarditis with overt heart failure symptoms. Because pericardiocentesis failed to relieve the symptoms, pericardiectomy was performed. Intraoperative exploration revealed remarkably thickened pericardium and epicardium constituting multiple layers with purulent effusion. Epicardiectomy as well as pericardiectomy were required to achieve the effective reduction of central venous pressure. Perforation of the gastric conduit into the pericardial cavity was identified and repaired. Histopathology demonstrated thickened pericardium composed of hyalinized stroma, collagenous bundles, and infiltration of inflammatory cells. Streptococcus anginosus and Candida tropicalis were identified by culture of the resected tissue.
Keywords: Effusive‐constrictive pericarditis, Pneumopericardium, Gastropericardial fistula, Oesophageal cancer, Pericardiectomy
Introduction
Effusive–constrictive pericarditis (ECP) is an uncommon manifestation of constrictive pericarditis characterized by constriction and effusion–tamponade physiology with subacute or chronic course. Common aetiologies of ECP are idiopathic, malignancy, and post‐cardiac surgery. Pneumopericardium sometimes causes acute cardiac tamponade called tension pneumopericardium; however, chronic outcome has not been well described. We herein present a case of gastropericardial fistula that circumvented tension pneumopericardium but subsequently developed subacute ECP necessitating pericardiectomy.
Case report
A 66‐year‐old man was admitted to our hospital complaining of chest pain worsened by inspiration ensuing a month. He had a history of hypertension and total esophagectomy with gastric pull‐up reconstruction for oesophageal cancer 6 years ago. He was afebrile, and his blood pressure was 168/99 mmHg and heart rate was 82 b.p.m. He did not exhibit pulsus paradoxus. Laboratory examination showed the elevation of inflammatory biomarkers: white blood cell count was 10 700/μL, and C‐reactive protein was 26.6 mg/dL. Blood cultures were all negative. Myocardial biomarkers did not significantly rise: serum troponin T 0.018 ng/mL, creatine kinase 43 U/mL, and creatine kinase‐MB 4 U/mL. Electrocardiogram showed no abnormal findings suggesting myopericardial injury and normal sinus rhythm without specific ST‐T changes. The amplitude of QRS complex was relatively low in all leads, but this finding had been unaltered for years before the present event (Supporting Information, Figure S1 ). Chest X‐ray demonstrated free air around the cardiac silhouette (Figure 1A ). Computed tomography scan revealed pneumopericardium (Figure 1B ), and ulceration with abscess formation in the lower gastric conduit placed in anterior mediastinum adjacent to the right ventricle, implying gastropericardial fistula (Figure 1B ). Endoscopy demonstrated an ulcer with white coating at the antrum of gastric conduit but failed to identify a fistula. Echocardiography showed preserved left ventricular and right ventricular (RV) wall motion without collapse of chambers. He was diagnosed with mediastinitis and pericarditis. An urgent surgical intervention was deferred because his haemodynamics was stable and it was desirable to extinguish purulent inflammation as much as possible even if a surgery would be performed. He had been hospitalized and administered with antibiotics (intravenous piperacillin/tazobactam 4.5 g three times a day). The conservative treatment initially improved his chest pain and decreased the level of inflammatory biomarkers in 3 weeks: white blood cell count 6020/μL and C‐reactive protein 1.99 mg/dL. However, he gradually developed bilateral pleural effusion and anasarca 3 weeks after the hospitalization, both of which were suggestive of right heart failure. Plasma BNP level was high at 1039.3 pg/mL, and serum troponin T level rose up to 0.244 ng/mL at this stage. He was administered with diuretics—bolus injection followed by continuous infusion of furosemide, but the symptoms become refractory. His blood pressure declined to ~100/80 mmHg and heart rate increased to ~100 b.p.m. He exhibited jugular vein distention but not pulsus paradoxus. Echocardiography revealed the increase of pericardial effusion (Supporting Information, Figure S2 ), and Doppler flow patterns showed the inspiratory decrease and expiratory increase of early transmitral left ventricular inflow, suggesting constrictive physiology. Follow‐up computed tomography scan demonstrated increased pericardial effusion, diminishing pneumopericardium, and thickened pericardium (Figure 1C ). Pericardiocentesis failed to relieve the symptom. Invasive haemodynamics demonstrated the following findings compatible with constrictive pericarditis 1 : (i) increased right atrial pressure (RAP) with deep x and y descents (Figure 1D ), (ii) diminished inspiratory dRAP, (iii) increased RV end‐diastolic pressure relative to RV systolic pressure and dip–plateau morphology (Figure 1D ), (iv) equalization of RV and left ventricular end‐diastolic pressure (Figure 1E ), and (v) exaggerated interventricular dependence—discordance of RV and left ventricular pressure with respiratory variation (Figure 1F ). He was diagnosed with ECP based on the earlier findings, and urgent pericardiectomy was performed. Intraoperative exploration revealed remarkably thickened pericardium and epicardium (~5 mm) constituting millefeuille‐like multiple layers with purulent effusion (Figure 2A ). Because the central venous pressure was still high at 18 mmHg even after pericardiotomy, epicardiectomy was additionally performed under cardiopulmonary bypass. After pericardiectomy and epicardiectomy, central venous pressure declined and arterial pressure recovered, indicating the successful relief of pericardial constriction. Perforation of the gastric conduit into the pericardial cavity near the acute margin of the right ventricle was identified and surgically repaired. Histopathology of the resected tissue demonstrated thickened pericardium composed of hyalinized stroma, collagenous bundles, microcalcifications, and infiltration of neutrophils and lymphocytes, accompanied by foreign materials with multinucleated giant cells (Figure 2B and 2C ). The culture of the resected pericardial tissue identified Streptococcus anginosus and Candida tropicalis, which are indigenous gastrointestinal microbes.
Figure 1.
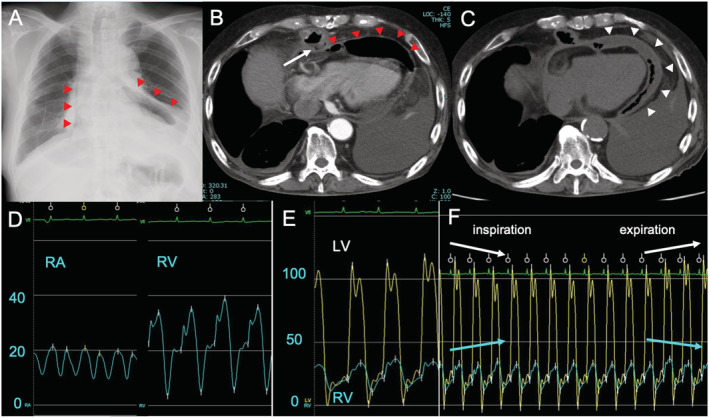
Images and invasive haemodynamic findings. Chest X‐ray (A) and computed tomography scans (B) at admission revealed pneumopericardium (red arrowheads) and abscess formation in the gastric conduit adjacent to the heart (arrow). Computed tomography scans 4 weeks after the initiation of conservative treatment exhibited diminished pneumopericardium, thickened pericardium (white arrowheads), and increased pericardial effusion (C). Right heart catheterization demonstrated deep x and y descents of right atrial (RA) pressure and dip–plateau right ventricular (RV) pressure morphology (D), equalization of RV and left ventricular (LV) end‐diastolic pressure (E), and increase of RV pressure (blue line) and decrease of LV pressure (yellow line) in inspiration and reverse change in expiration (F). These findings were compatible with constrictive pericarditis.
Figure 2.

Intraoperative findings and histopathology of the resected pericardial tissue. Markedly thickened and rigid pericardium (arrowheads) and purulent effusion (arrows) were observed (A). The resected pericardium tissue comprised hyalinized stroma, collagenous bundles, aggregation of neutrophils and lymphocytes (arrows), and microcalcifications (arrowheads) (B). Higher magnification revealed foreign materials (red arrows) within the pericardial tissue accompanied by inflammatory cell infiltration with multinucleated giant cells (white arrow) (C).
Discussion
Pneumopericardium mostly results from chest trauma, thoracic procedure, endoscopy, and positive pressure ventilation. Rare causes include gastropericardial fistula associated with ulcer, carcinoma, and spontaneous rupture and less commonly intrapericardial perforation of lung abscess or tuberculosis cavity. 2 Pneumopericardium does not always require invasive intervention; however, one‐third of cases have been reported to result in tension pneumopericardium that mimics cardiac tamponade and can be fatal. 3 Management of tension pneumopericardium involves decompression by pericardiocentesis or surgical drainage. 3 , 4
Effusive–constrictive pericarditis is characterized by the constriction of the heart by pericardium with tense pericardial effusion, and typically, RAP fails to decrease even after pericardial pressure is lowered by removal of pericardial fluid. 5 Sagristà‐Sauleda et al. reported that ECP accounted for 1.3% of the entire pericarditis population, and they were mostly associated with idiopathic and neoplastic aetiologies. 5 Kim et al. reported that 16% of patients developed ECP following pericardiocentesis, and major causes were procedure‐related hemopericardium, post‐cardiac surgery, and idiopathic. 6 Less frequent are infection‐related aetiologies. 5 , 6 The necessity of pericardiectomy ranges from 6% to 47% in the literature. 5 , 6 , 7 In advanced pericardial constriction like the present case, pericardiectomy alone might not relieve constrictive physiology and requires additional procedures including epicardiectomy or pericardial waffle procedure. 8 Therapeutic strategy should be individualized depending on underlying causes and haemodynamics. 1
The present report is the first describing a case of pneumopericardium leading to subacute ECP that necessitated pericardiectomy and epicardiectomy. A latent long‐standing inflammation caused by gastropericardial fistula was thought to underlie the disease progression, and pneumopericardium developed as an early phenotype in the present case. Uncontrolled inflammation finally led to ECP. Although the initial conservative care apparently palliated inflammatory response and prevented the patient from developing life‐threatening tension pneumopericardium, the pericardium and epicardium were being exposed to microorganisms, foreign materials, and gastric juice due to unrepaired gastric conduit–pericardium fistula, leading to prolonged chemical injuries and smouldering infection. These pathobiological processes were considered to induce inflammatory pericardial hyperplasia with increasing purulent pericardial fluid after all in the present case. Pneumopericardium secondary to gastropericardial fistula is at high risk of microbial infection because of the communication of pericardial cavity with gastrointestinal tract. Some cases with pneumopericardium, especially in traumatic or idiopathic cases, can be treated with conservative management; however, infection‐associated pneumopericardium should require early surgical repair even without tension pneumopericardium in acute phase, considering the high risk of developing purulent ECP.
Conflict of interest
None declared.
Funding
This work was supported by the JSPS KAKENHI grant (JP19K17567) awarded to T.H.
Supporting information
Figure S1. Electrocardiogram at admission.
Figure S2. Echocardiographic images 4 weeks after the admission. Two‐dimensional imaging in the apical view (A) and in the parasternal long‐axis view (B). Pericardial effusion (arrows) with thickened pericardium (arrowheads) are noticeable.
Ono, Y. , Hashimoto, T. , Sakamoto, K. , Matsushima, S. , Higo, T. , Sonoda, H. , Kimura, Y. , Mori, M. , Shiose, A. , and Tsutsui, H. (2021) Effusive–constrictive pericarditis secondary to pneumopericardium associated with gastropericardial fistula. ESC Heart Failure, 8: 778–781. 10.1002/ehf2.13135.
References
- 1. Talreja DR, Nishimura RA, Oh JK, Holmes DR. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol 2008; 51: 315–319. [DOI] [PubMed] [Google Scholar]
- 2. Grandhi TM, Rawlings D, Morran CG. Gastropericardial fistula: a case report and review of literature. Emerg Med J 2004; 21: 644–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nachi S, Okada H, Kato H, Suzuki K, Nakano S, Yoshida T, Yoshida S, Ushikoshi H, Toyoda I, Ogura S. Simple pneumopericardium due to blunt trauma progressing to tension pneumopericardium during transportation. Am J Emerg Med 2016; 34: 933 e3–933.e5. [DOI] [PubMed] [Google Scholar]
- 4. Misenheimer JA, Liu Y, Means G, Kaul P, Yeung M. Tension pneumopericardium secondary to gastropericardial fistula presenting as acute pericarditis with cardiac tamponade physiology. JACC Cardiovasc Interv 2016; 9: 1748–1749. [DOI] [PubMed] [Google Scholar]
- 5. Sagristà‐Sauleda J, Angel J, Sanchez A, Permanyer‐Miralda G, Soler‐Soler J. Effusive–constrictive pericarditis. N Engl J Med 2004; 350: 469–475. [DOI] [PubMed] [Google Scholar]
- 6. Kim KH, Miranda WR, Sinak LJ, Syed FF, Melduni RM, Espinosa RE, Kane GC, Oh JK. Effusive‐constrictive pericarditis after pericardiocentesis: incidence, associated findings, and natural history. JACC Cardiovasc Imaging 2018; 11: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Syed FF, Ntsekhe M, Mayosi BM, Oh JK. Effusive‐constrictive pericarditis. Heart Fail Rev 2013; 18: 277–287. [DOI] [PubMed] [Google Scholar]
- 8. Kiamanesh O, Luk A, Nesbitt GC, Badiwala M, Mak S. Pericardial waffle for effusive‐constrictive pericarditis. ESC Heart Fail 2020; 7: 3213–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Electrocardiogram at admission.
Figure S2. Echocardiographic images 4 weeks after the admission. Two‐dimensional imaging in the apical view (A) and in the parasternal long‐axis view (B). Pericardial effusion (arrows) with thickened pericardium (arrowheads) are noticeable.


